Taurine Supplementation in Low-Fishmeal of Golden Pompano (Trachinotus ovatus) Diets: Improving Intestinal Health and Alleviation of Inflammatory Response
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Diet Preparation
2.3. Fish Rearing and Experimental Conditions
2.4. Calculations
2.5. Sample Collection
2.6. Parameters Measurement and Analysis
2.6.1. Proximate Composition Analysis
2.6.2. Enzyme Activity Assay Analysis
2.6.3. Gene Expression Level Analysis
2.6.4. Intestinal Microbiota Communities
2.7. Statistical Analysis
3. Results
3.1. Effects of Taurine Supplementation on Growth Performance and Feed Utilization of T. ovatus
3.2. Effects of Taurine Supplementation on Serum Biochemical Indexes of T. ovatus
3.3. Effects of Taurine Supplementation on Hepatic Antioxidant Indices of T. ovatus
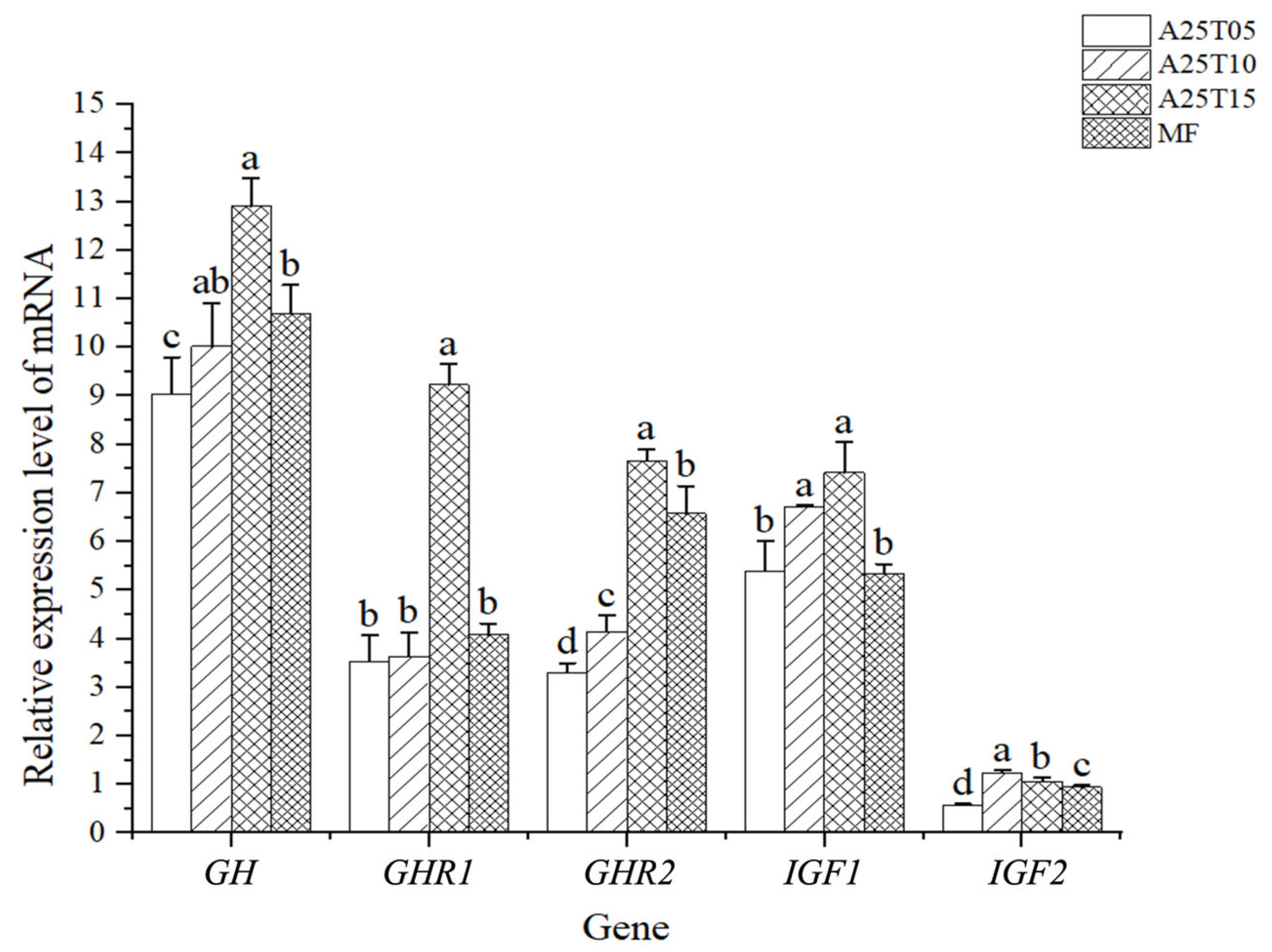
3.4. Effects of Taurine Supplementation on the Expression of Antioxidant-Related Genes in Liver of T. ovatus
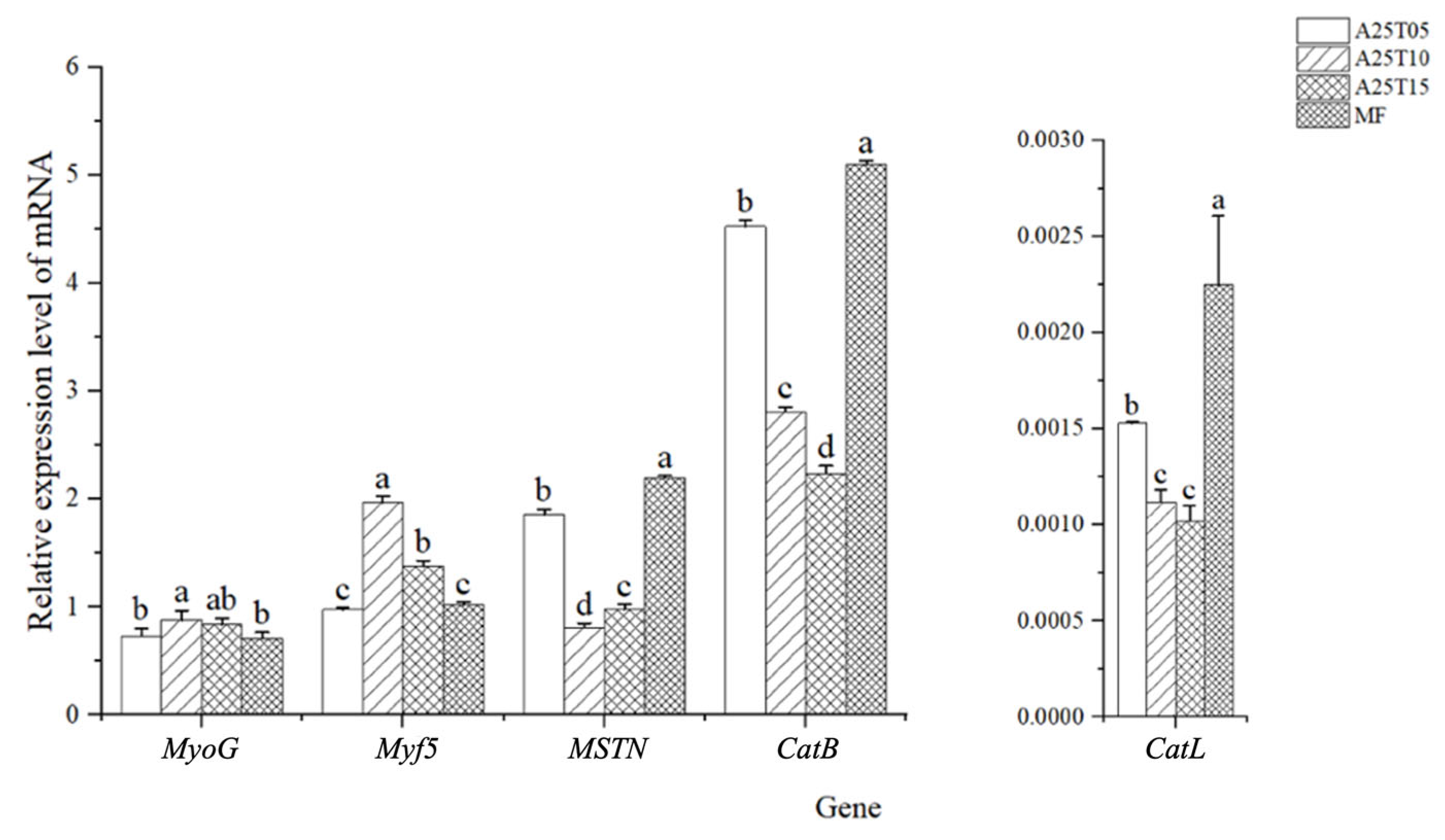
3.5. Effects of Taurine Supplementation on the Expression of Growth and Quality-Related Genes in Muscle of T. ovatus
3.6. Effects of Taurine Supplementation on Intestinal Enzymatic Activity of T. ovatus
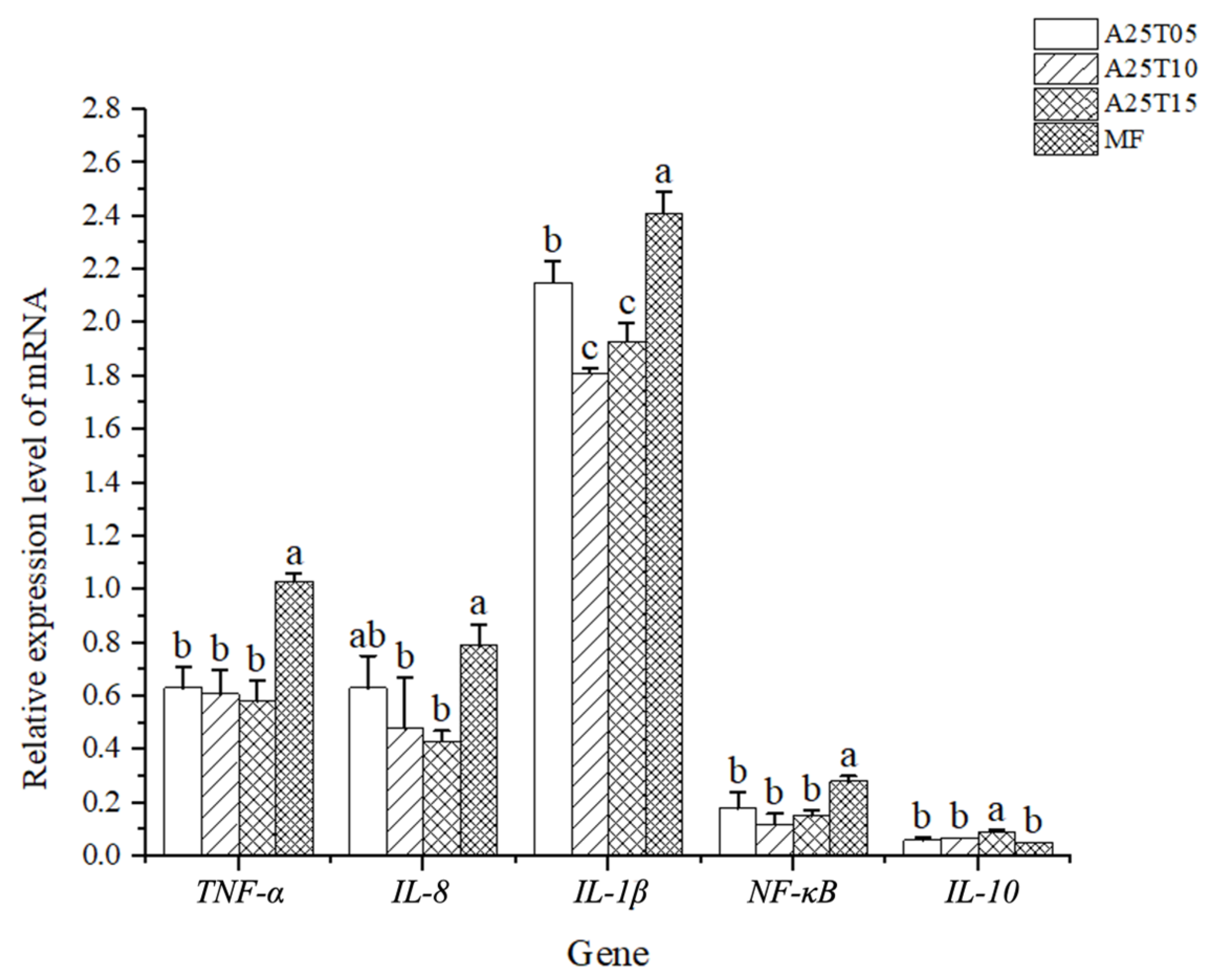
3.7. Effects of Taurine Supplementation Intestinal Immune-Related Gene Expression of T. ovatus
3.8. Effects of Taurine Supplementation on Intestinal Microbiota of T. ovatus
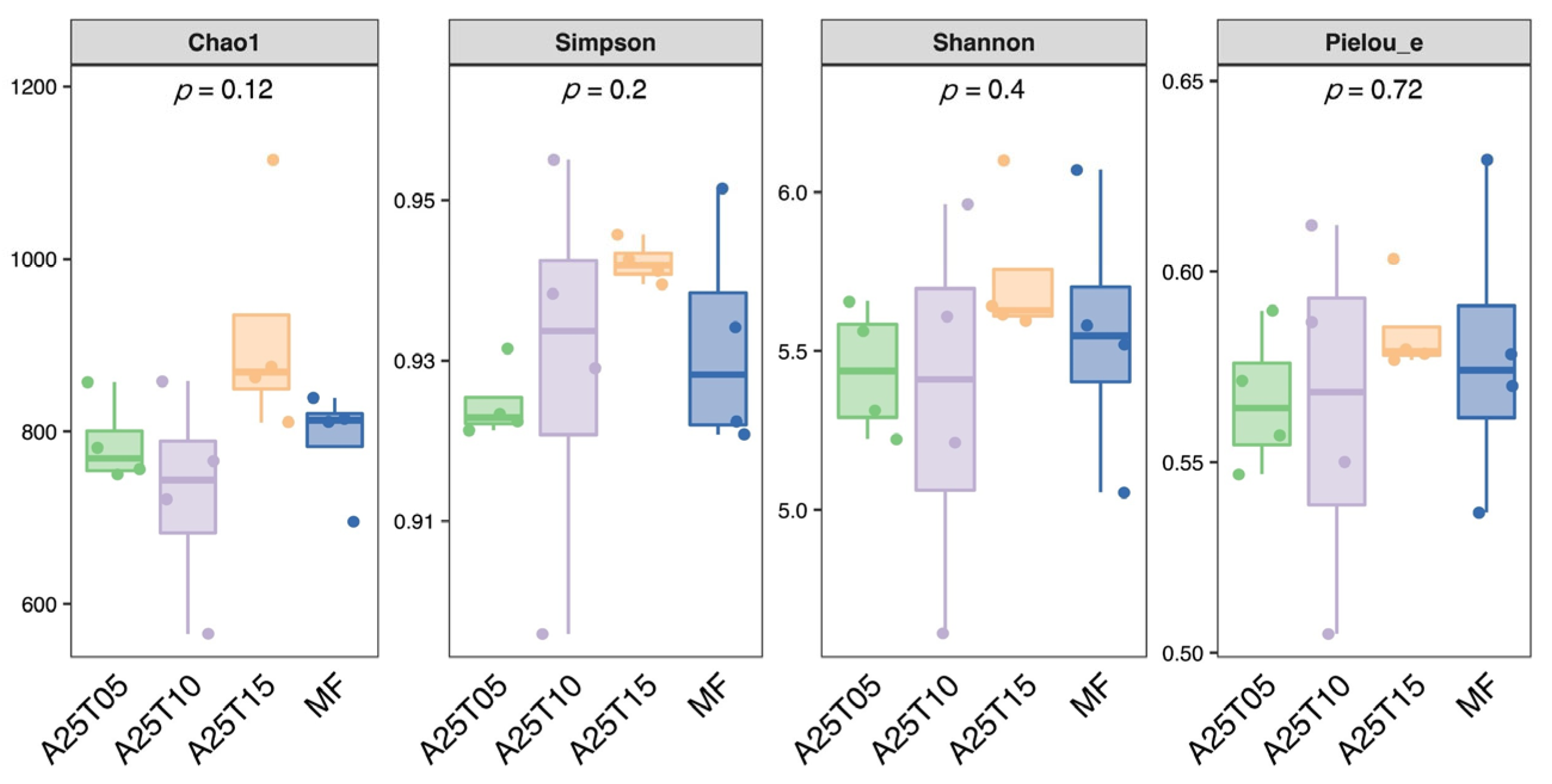
3.8.1. Analysis of Microbial and Alpha Diversity of Intestinal Flora
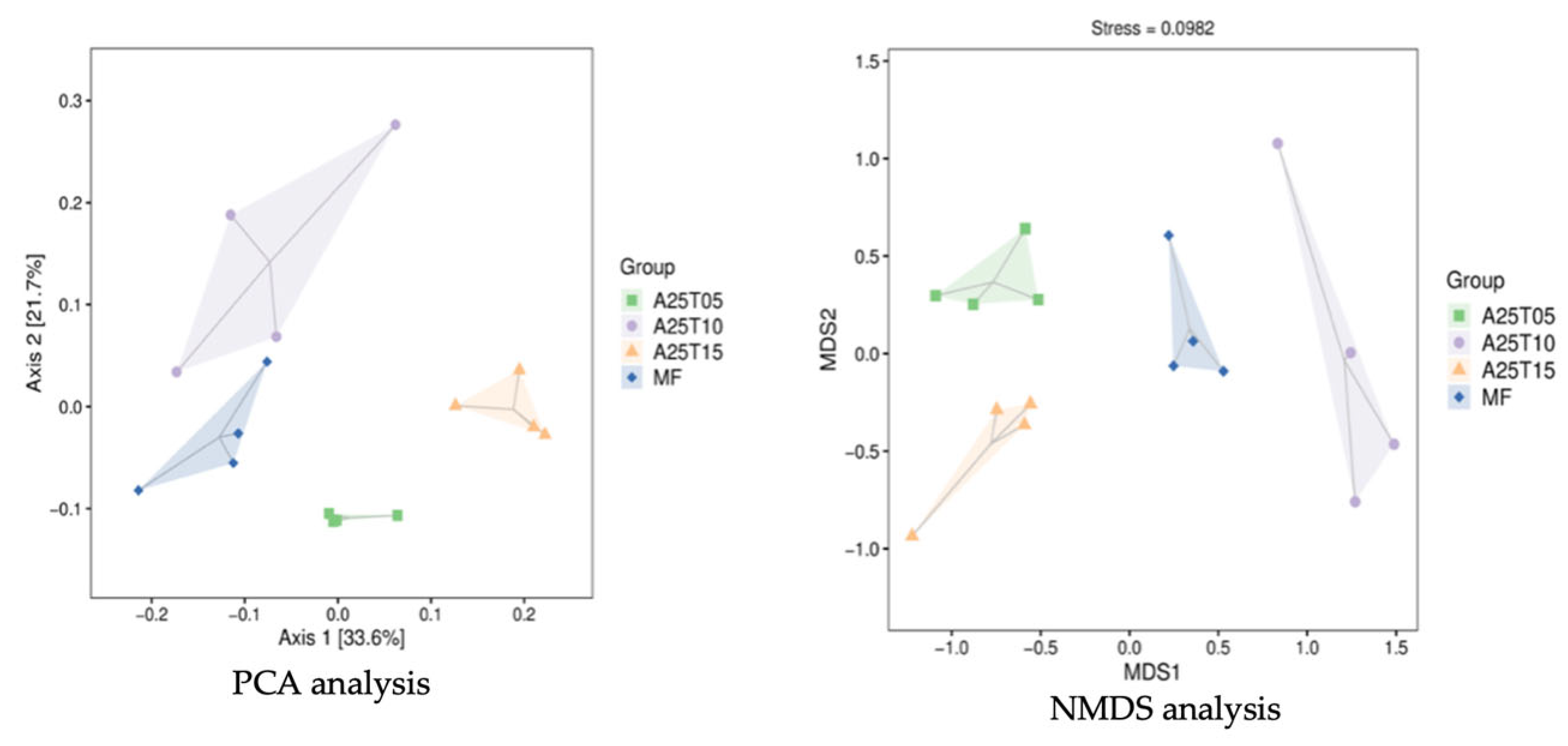
3.8.2. Analysis of β Diversity of Intestinal Flora
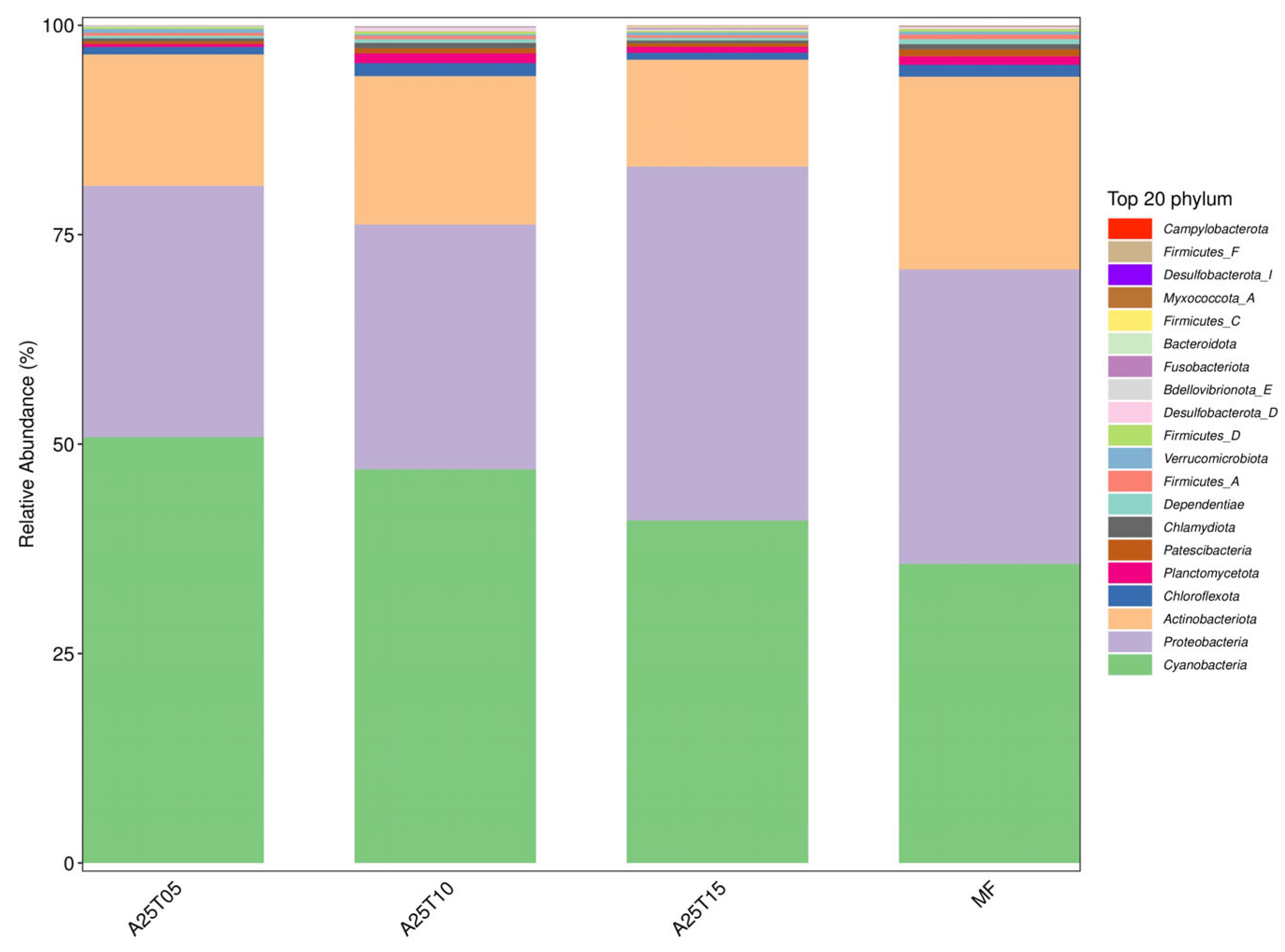
3.8.3. Analysis of Intestinal Flora Composition and Relative Abundance
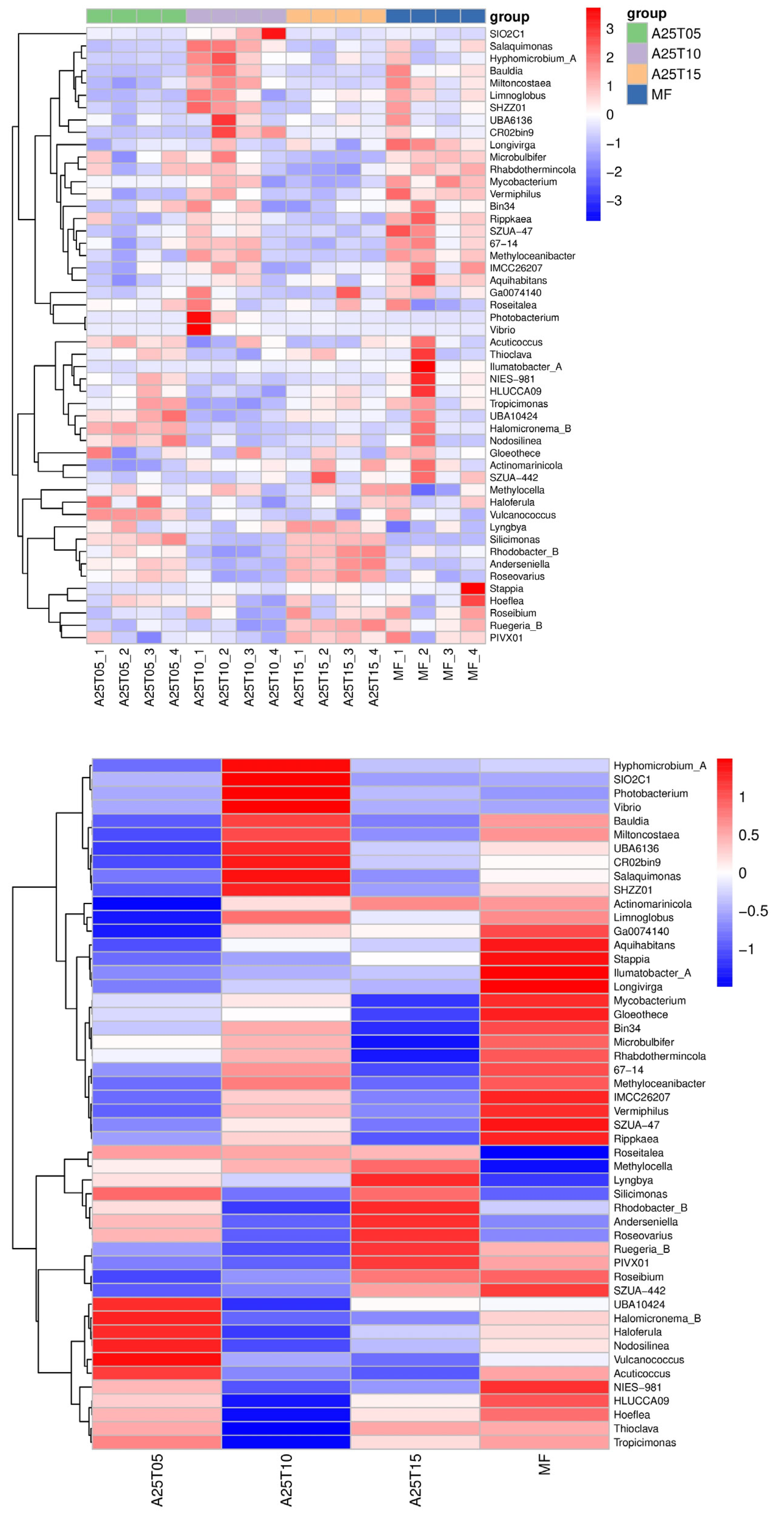
3.8.4. Analysis of Species Differences in Intestinal Flora
4. Discussion
4.1. Effects of Taurine Supplementation on Growth Performance and Feed Utilization of T. ovatus
4.2. Effects of Taurine Supplementation on Serum Biochemical Indexes of T. ovatus
4.3. Effects of Taurine Supplementation on Hepatic Antioxidant Indices of T. ovatus
4.4. Effects of Taurine Supplementation on the Expression of Growth and Quality-Related Genes in the Muscle of T. ovatus
4.5. Effects of Taurine Supplementation on the Intestinal Health and Alleviation of Inflammatory Response of T. ovatus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Pu, C.; Pei, Z.; Zhang, W.; Wei, Z.; Chen, H.; Huang, Y. Retrospect of fishmeal substitution in largemouth bass (Micropterus salmoides): A review. Fish Physiol. Biochem. 2025, 51, 21. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Do, T.V.; Tran, H.D. Dietary replacement of fish meal by defatted and fermented soybean meals with taurine supplementation for pompano fish: Effects on growth performance, nutrient digestibility, and biological parameters in a long-term feeding period. J. Anim. 2020, 98, skaa367. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, Q.; Zhang, X.; Zhu, T.; Guo, C.; Yang, Z.; Luo, J.; Yuan, Y.; Hu, X.; Jiao, L.; et al. Effect of dietary replacement of fish meal with low-gossypol cottonseed protein concentrate on growth performance and expressions of genes related to protein metabolism for swimming crab (Portunus trituberculatus). Aquaculture 2022, 549, 737820. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Replacement of fish meal with fermented plant proteins in the aquafeed industry: A systematic review and meta-analysis. Aquaculture 2023, 15, 62–88. [Google Scholar] [CrossRef]
- Hussain, S.M.; Bano, A.A.; Ali, S.; Rizwan, M.; Adrees, M.; Zahoor, A.F.; Sarker, P.K.; Hussain, M.; Arsalan, M.Z.-H.; Yong, J.W.H.; et al. Naeem, Substitution of fishmeal: Highlights of potential plant protein sources for aquaculture sustainability. Heliyon 2024, 10, e26573. [Google Scholar] [CrossRef]
- Kotzamanis, Y.; Tsironi, T.; Brezas, A.; Grigorakis, K.; Ilia, V.; Vatsos, I.; Romano, N.; Van Eys, J.; Kumar, V. High taurine supplementation in plant protein-based diets improves growth and organoleptic characteristics of European seabass (Dicentrarchus labrax). Sci. Rep. 2020, 10, 12294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liao, S.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W.; Huang, X.; Luo, M.; Lin, H.; Zhou, C. Dietary Effects of Fermented Cottonseed Meal Substituting Fishmeal on the Growth, Biochemical Indexes, Antioxidant Capacity, and Muscle Quality of Juvenile Golden Pompano (Trachinotus ovatus). Aquac. Nutr. 2024, 2024, 9972395. [Google Scholar] [CrossRef]
- Liu, X. Effects of Exogenous Taurine and Cysteine on Growth, Metabolism, Antioxidant and Immune Functions of Juvenile Golden Pompano (Trachinotus ovatus). Master’s Thesis, Dalian Ocean University, Dalian, China, 2023. [Google Scholar]
- Wang, Z.; Liao, S.; Wang, J.; Wang, Y.; Huang, Z.; Yu, W.; Huang, X.-L.; Lin, H.-Z.; Luo, M.-L.; Cheng, Z.-Y.; et al. Effects of Fermented Cottonseed Meal Substitution for Fish Meal on Intestinal Enzymatic Activity, Inflammatory and Physical-Barrier-Related Gene Expression, and Intestinal Microflora of Juvenile Golden Pompano (Trachinotus ovatus). Fishes 2023, 8, 466. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, R.; He, X.; Cui, X.; Liao, Z.; Liu, Y.; Wei, H.; Zhuang, Z.; Chen, M.; Niu, J. A Novel Protein Sourced from Chinese Medicine Residue for Golden Pompano Feed: Endothelium Corneum Gigeriae Galli Residue (ECGGR). Aquac. Nutr. 2024, 2024, 1845188. [Google Scholar] [CrossRef]
- Macusi, E.D.; Cayacay, M.A.; Borazon, E.Q.; Sales, A.C.; Habib, A.; Fadli, N.; Santos, M.D. Protein Fishmeal Replacement in Aquaculture: A Systematic Review and Implications on Growth and Adoption Viability. Sustainability 2023, 15, 12500. [Google Scholar] [CrossRef]
- Daneshvar, N.; Shirini, F.; Langarudi, M.S.N.; Karimi-Chayjani, R. Taurine as a green bio-organic catalyst for the preparation of bio-active barbituric and thiobarbituric acid derivatives in water media. Bioorg. Chem. 2018, 77, 68–73. [Google Scholar] [CrossRef]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.; Spandidos, D.; Christodoulou, I.; Kyriakopoulos, A.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.C.; Li, M.H. Effect of supplemental taurine on juvenile channel catfish Ictalurus punctatus growth performance. Aquac. Nutr. 2018, 24, 310–314. [Google Scholar] [CrossRef]
- Gibson Gaylord, T.; Barrows, F.T.; Teague, A.M.; Johansen, K.A.; Overturf, K.E.; Shepherd, B. Supplementation of taurine and methionine to all-plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 269, 514–524. [Google Scholar] [CrossRef]
- Kim, S.; Takeuchi, T.; Yokoyama, M.; Murata, Y.; Kaneniwa, M.; Sakakura, Y. Effect of dietary taurine levels on growth and feeding behavior of juvenile Japanese flounder Paralichthys olivaceus. Aquaculture 2005, 250, 765–774. [Google Scholar] [CrossRef]
- Feidantsis, K.; Kaitetzidou, E.; Mavrogiannis, N.; Michaelidis, B.; Kotzamanis, Y.; Antonopoulou, E. Effect of taurine-enriched diets on the Hsp expression, MAPK activation and the antioxidant defence of the European sea bass (Dicentrarchus labrax). Aquac. Nutr. 2014, 20, 431–442. [Google Scholar] [CrossRef]
- Cao, X.; Li, Z.; Hu, Y. Effects of dietary taurine supplementation in low fish meal feed on growth, digestibility, intestinal enzyme activities of rice field eel (Monopterus albus). South China Fish. Sci. 2021, 17, 64–70. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, P.; Hu, H.; Li, Y.; Dai, J.; Zhang, Y.; Ai, Q.; Xu, W.; Zhang, W.; Mai, K. The tolerance and safety assessment of taurine as additive in a marine carnivorous fish, Scophthalmus maximus L. Aquac. Nutr. 2018, 24, 461–471. [Google Scholar] [CrossRef]
- Sun, S.-S.; Yan, L.-C.; Feng, L.; Jiang, W.-D.; Liu, Y.; Tang, L.; Wu, P.; Zhou, X.-Q. Taurine prevented the decline of fillet quality and muscle antioxidant capacity in on-growing grass carp (Ctenopharyngodon idella) fed non-fishmeal diet. Aquaculture 2024, 588, 740921. [Google Scholar] [CrossRef]
- Mai, H.; Li, Y.; Song, Z.; Zeng, Y.; Lin, P.; Sun, Z.; Mai, K.; Tan, B.; Ye, C. Effect of taurine on growth and immune response of Pacific white shrimp (Litopenaeus vannamei) cultured at different temperatures. Aquaculture 2025, 594, 741393. [Google Scholar] [CrossRef]
- Güroy, D.; Karadal, O.; Güroy, B.; Emre, Y.; Emre, N.; Eraslan, D.; Yalım, F.B.; Mantoğlu, S.; Demir, A. Dietary Taurine Improves The Growth Performance, Health Status and Liver Histopathology of Meagre (Argyrosomus regius) Fed A Reduced Fish Meal Diet. Ann. Anim. Sci. 2024, 24, 851–866. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Cheng, K.; Ma, X.; Wu, G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part II: Effects of supplementation with methionine or taurine on growth, feed utilization, and health. Amino Acids 2021, 53, 49–62. [Google Scholar] [CrossRef]
- Shi, M.; Yao, X.; Qu, K.; Liu, Y.; Tan, B.; Xie, S. Effects of taurine supplementation in low fishmeal diet on growth, immunity and intestinal health of Litopenaeus vannamei. Aquac. Rep. 2023, 32, 101713. [Google Scholar] [CrossRef]
- Liu, X.; Mai, K.; Liu, F.; Ai, Q. Interactive efect of dietary taurine and protein sources on fed in-take, growth performance and body composition of turbot(Scophthalmus maximus). Period. Ocean Univ. China 2018, 5, 25–31. [Google Scholar]
- Wei, L.; Peng, Z.; Yan, L.; Gao, X.; Zhai, H.; Wang, W.; Ren, T.; Han, Y. Effects of Taurine Supplementation in Low Fishmeal Diet on Intestinal Structure, Immunity and Antioxidant Capacity of Sebastes schlegeli. Feed. Ind. 2022, 43, 35–40. [Google Scholar] [CrossRef]
- Fu, S.; Qian, K.; Liu, H.; Song, F.; Ye, J. Effects of fish meal replacement with low-gossypol cottonseed meal on the intestinal barrier of juvenile golden pompano (Trachinotus ovatus). Aquac. Res. 2022, 53, 285–299. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, C.; Xu, F.; Luo, X.-B.; Huang, X.-L.; Huang, Z.; Yu, W.; Xun, P.; Wu, Y.; Lin, H. Effects of partial replacement of fish meal with porcine meat meal on growth performance, antioxidant status, intestinal morphology, gut microflora and immune response of juvenile golden pompano (Trachinotus ovatus). Aquaculture 2022, 561, 738646. [Google Scholar] [CrossRef]
- Shen, J.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Effects of replacement of fishmeal with cottonseed protein concentrate on the growth, intestinal microflora, haematological and antioxidant indices of juvenile golden pompano (Trachinotus ovatus). Aquac. Nutr. 2020, 26, 1119–1130. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, C.; Wang, M.; Li, L.; Lin, R.; Han, D.; Zhu, X.; Zhang, L. Effects of hypoxic stress on liver metabolism, oxidative stress, and immunity in yellow catfish (Pelteobagrus fulvidraco) at different water temperatures. Aquaculture 2025, 598, 742088. [Google Scholar] [CrossRef]
- Cai, W.; Li, X.; Cai, M.; Tang, Z.; Zhu, B.; Yang, M.; Hu, Y.; Dai, J. Effects of replacing fishmeal with soybean meal on growth performance, liver antioxidant capacity and intestinal health in juvenile Asian red-tailed catfish (Hemibagrus wyckioides). Aquac. Rep. 2025, 40, 102646. [Google Scholar] [CrossRef]
- Pradhan, L.K.; Sahoo, P.K.; Sarangi, P.; Chauhan, N.R.; Das, S.K. Suppression of Chronic Unpredictable Stress-Persuaded Increased Monoamine Oxidase Activity by Taurine Promotes Significant Neuroprotection in Zebrafish Brain. Neurochem. Res. 2023, 48, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Y.; Ai, Q.; Mao, P.; Tian, Q.; Zhong, L.; Xiao, T.; Chu, W. Effect of dietary taurine supplementation on growth performance, digestive enzyme activities and antioxidant status of juvenile black carp (Mylopharyngodon piceus) fed with low fish meal diet. Aquac. Res. 2018, 49, 3187–3195. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N. Stimulatory effect of dietary taurine on growth performance, digestive enzymes activity, antioxidant capacity, and tolerance of common carp, Cyprinus carpio L., fry to salinity stress. Fish Physiol. Biochem. 2018, 44, 639–649. [Google Scholar] [CrossRef]
- Liu, M.; Gao, J.; Guo, H.; Zhu, K.; Liu, S.; Zhang, N.; Zhu, T.; Zhang, D. Influence of aquaculture environments on the muscle quality of Golden Pompano (Trachinotus ovatus) in the Beibu Gulf: A multifaceted analysis of nutritional, textural, and flavor profiles. LWT 2024, 212, 116957. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, M.; Jiang, H.; Li, M. Dietary Supplementation of Crystalline Amino Acid Improves Growth Performance and Health of Yellow Catfish That Reduced by Plant Proteins Replacement of Fishmeal. Aquac. Nutr. 2022, 2022, 7145090. [Google Scholar] [CrossRef]
- Lunger, A.N.; McLean, E.; Gaylord, T.G.; Kuhn, D.; Craig, S.R. Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 271, 401–410. [Google Scholar] [CrossRef]
- Richard, N.; Colen, R.; Aragão, C. Supplementing taurine to plant-based diets improves lipid digestive capacity and amino acid retention of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 2017, 468, 94–101. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Zhang, B.; Yan, L.; Wang, P.; Zhao, C.; Lin, H.; Qiu, L.; Zhou, C. Effects of High Dietary Carbohydrate Levels on Growth Performance, Enzyme Activities, Expression of Genes Related to Liver Glucose Metabolism, and the Intestinal Microbiota of Lateolabrax maculatus Juveniles. Fishes 2023, 8, 431. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Rotman, F.; Stuart, K.; Drawbridge, M. Effects of taurine supplementation in live feeds on larval rearing performance of California yellowtail Seriola lalandi and white seabass Atractoscion nobilis. Aquac. Res. 2017, 48, 1232–1239. [Google Scholar] [CrossRef]
- Velasquez, A.; Pohlenz, C.; Barrows, F.T.; Gaylord, T.G.; Gatlin, D.M. Assessment of taurine bioavailability in pelleted and extruded diets with red drum Sciaenops ocellatus. Aquaculture 2015, 449, 2–7. [Google Scholar] [CrossRef]
- Matsunari, H.; Takeuchi, T.; Takahashi, M.; Mushiake, K. Effect of dietary taurine supplementation on growth performance of yellowtail juveniles Seriola quinqueradiata. Fish. Sci. 2005, 71, 1131–1135. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, H.; Wang, R.; Huang, Z.; Zhou, C.; Yu, W.; Xun, P.; Tan, L.; Wang, Y.; Wang, J. Effect of dietary vitamin B 6 supplementation on growth and intestinal microflora of juvenile golden pompano (Trachinotus ovatus). Aquac. Res. 2019, 50, 2359–2370. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effects of dietary leucine levels on intestinal antioxidant status and immune response for juvenile golden pompano (Trachinotus ovatus) involved in Nrf2 and NF-κB signaling pathway. Fish Shellfish Immunol. 2020, 107, 336–345. [Google Scholar] [CrossRef]
- Liu, R.; Fan, Q.; Quan, D.; Zhao, Z.; He, H.; Hu, W.; Wu, Q.; Wang, F.; Zhang, C.; Shao, W. Effects of replacing fish meal with soybean meal in feed and adding taurine on the growth and some physiological indexes of Culter alburnus. Freshw. Fish. 2015, 45. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Khaoian, P.; Fukada, H.; Suzuki, N.; Masumoto, T. Feeding fermented soybean meal diet supplemented with taurine to yellowtail Seriola quinqueradiata affects growth performance and lipid digestion. Aquac. Res. 2015, 46, 1101–1110. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, H.; Zhu, K.; Guo, L.; Liu, B.-S.; Zhang, N.; Liu, B.; Yang, J.; Jiang, S.; Zhang, D. Dietary taurine intake affects growth and taurine synthesis regulation in golden pompano, Trachinotus ovatus (Linnaeus 1758). Aquaculture 2021, 530, 735918. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, C.; Lin, H.; Wang, J.; Wang, Y.; Yu, W. Effect of dietary carbohydrate on glycometabolism of juvenile golden pompano (Trachinotus ovatus) related to growth performance, hepatic histopathology, transcriptome profiles and identification of differentially expressed genes. Aquac. Rep. 2022, 27, 101362. [Google Scholar] [CrossRef]
- Ma, Q. Dietary Taurine Intake Affects Growth and Taurine Synthesis Regulation in Trachinotus ovatus. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar]
- Shi, Y.; Hu, Y.; Wang, Z.; Zhou, J.; Zhang, J.; Zhong, H.; Fu, G.; Zhong, L. The Protective Effect of Taurine on Oxidized Fish-Oil-Induced Liver Oxidative Stress and Intestinal Barrier-Function Impairment in Juvenile Ictalurus punctatus. Antioxidants 2021, 10, 1690. [Google Scholar] [CrossRef]
- Bañuelos-Vargas, I.; López, L.M.; Pérez-Jiménez, A.; Peres, H. Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on hepatic intermediary metabolism and antioxidant status of totoaba juveniles (Totoaba macdonaldi). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 170, 18–25. [Google Scholar] [CrossRef]
- Liu, J.; Guo, H.; Zhu, K.; Liu, B.; Zhang, N.; Zhang, D.C. Effects of exogenous taurine supplementation on the growth, antioxidant capacity, intestine immunity, and resistance against Streptococcus agalactiae in juvenile golden pompano (Trachinotus ovatus) fed with a low-fishmeal diet. Front. Immunol. 2022, 13, 1036821. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, W.-S.; Luo, W.; Zhang, X.-Q.; Zhu, W.-L.; Wang, J.; Liao, L.-Y.; Li, G.-H. Polymorphisms and their association with growth traits in the growth hormone gene of yellow catfish, Pelteobagrus fulvidraco. Aquaculture 2017, 469, 117–123. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Johansson, V.; Benedet, S.; Einarsdottir, I.E.; Hildahl, J.; Agustsson, T.; Jönsson, E. Growth Hormone Endocrinology of Salmonids: Regulatory Mechanisms and Mode of Action. Fish Physiol. Biochem. 2002, 27, 227–242. [Google Scholar] [CrossRef]
- Ben-Zaken, S.; Meckel, Y.; Nemet, D.; Eliakim, A. Can IGF-I polymorphism affect power and endurance athletic performance? Growth Horm. IGF Res. 2013, 23, 175–178. [Google Scholar] [CrossRef]
- Meng, Q.; Epler, M.J.; Lin, C.; Karinch, A.M.; Vary, T.C.; Pan, M. Insulin-like growth factor-2 activation of intestinal glutamine transport is mediated by mitogen-activated protein kinases. J. Gastrointest. Surg. 2004, 8, 40–47. [Google Scholar] [CrossRef]
- Nakamura, K.; Gonzales-Plasus, M.M.; Ushigusa-Ito, T.; Masuda, R.; Kabeya, N.; Kondo, H.; Hirono, I.; Satoh, S.; Haga, Y. Taurine synthesis via the cysteic acid pathway: Effect of dietary cysteic acid on growth, body taurine content, and gene expression of taurine-synthesizing enzymes, growth hormone, and insulin-like growth factor 1 in Japanese flounder Paralichthys olivaceus. Fish. Sci. 2021, 87, 353–363. [Google Scholar] [CrossRef]
- Wiriduge, H.A.S.W.; Zhang, Y.; Liu, J.; Yang, M.; Zhang, W.; Mai, K. Dietary taurine improves muscle growth and texture characteristics in juvenile turbot (Scophthalmus maximus). Aquac. Rep. 2020, 17, 100305. [Google Scholar] [CrossRef]
- Kotzamanis, Y.; Kumar, V.; Tsironi, T.; Grigorakis, K.; Ilia, V.; Vatsos, I.; Brezas, A.; Van Eys, J.; Gisbert, E. Taurine supplementation in high-soy diets affects fillet quality of European sea bass (Dicentrarchus labrax). Aquaculture 2020, 520, 734655. [Google Scholar] [CrossRef]
- Kolkovski, S.; Tandler, A.; Kissil, G.W.; Gertler, A. The effect of dietary exogenous digestive enzymes on ingestion, assimilation, growth and survival of gilthead seabream (Sparus aurata, Sparidae, Linnaeus) larvae. Fish Physiol. Biochem. 1993, 12, 203–209. [Google Scholar] [CrossRef]
- Luo, L.; Wen, H.; Wang, L.; Li, Q.; Long, Y.; Guo, J.; Yang, X. Effects of taurine on growth, quality, digestive and metabolic enzyme activities of grass carp (Ctenopharyngodon idellus). J. Anim. Nutr. 2006, 3, 166–171. [Google Scholar]
- Bolnick, D.I.; Snowberg, L.K.; Hirsch, P.E.; Lauber, C.L.; Knight, R.; Caporaso, J.G.; Svanbäck, R. Individuals’ diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch). Ecol. Lett. 2014, 17, 979–987. [Google Scholar] [CrossRef]
- Miyake, S.; Ngugi, D.K.; Stingl, U. Diet strongly influences the gut microbiota of surgeonfishes. Mol. Ecol. 2015, 24, 656–672. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Zhong, H.; Zhang, J.; Che, C.; Fu, G.; Hu, Y.; Mai, K. Taurine supplements in high-fat diets improve survival of juvenile Monopterus albus by reducing lipid deposition and intestinal damage. Aquaculture 2022, 547, 737431. [Google Scholar] [CrossRef]
- Li, S.; Luo, X.; Liao, Z.; Xu, H.; Liang, M.; Mai, K.; Zhang, Y. Additional supplementation of sulfur-containing amino acids in the diets improves the intestinal health of turbot fed high-lipid diets. Fish Shellfish Immunol. 2022, 130, 368–379. [Google Scholar] [CrossRef]
- Meng, X.; Li, W.; Nie, G. Progress in the study of factors influencing the intestinal flora of fish. J. Aquac. 2019, 1, 143–155. [Google Scholar]
- Xu, J.; Wu, P.; Jiang, W.-D.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Optimal dietary protein level improved growth, disease resistance, intestinal immune and physical barrier function of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 55, 64–87. [Google Scholar] [CrossRef]
- Aly, S.M.; Abdel-Galil Ahmed, Y.; Abdel-Aziz Ghareeb, A.; Mohamed, M.F. Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 2008, 25, 128–136. [Google Scholar] [CrossRef]
- Huang, J.; Ran, X.; Liu, X.; Hu, W.; Tang, R.; Zheng, J.; Chen, Y.; He, Y.; Lin, M. Effects of bile acids and taurine on growth, liver health and intestinal barrier of largemouth bass. J. Fish. 2024, 48, 099603. [Google Scholar] [CrossRef]



| Ingredients | Diets | |||
|---|---|---|---|---|
| A25T05 | A25T10 | A25T15 | MF | |
| Fishmeal | 18.75 | 18.75 | 18.75 | 25 |
| Soy protein concentrate | 12 | 12 | 12 | 12 |
| Corn protein powder | 10 | 10 | 10 | 10 |
| Soybean meal | 8 | 8 | 8 | 8 |
| Fermented cottonseed meal | 6.19 | 6.19 | 6.19 | 0 |
| Corn starch | 12.65 | 12.65 | 12.65 | 14.44 |
| Porcine blood cell protein powder | 8 | 8 | 8 | 8 |
| Beer yeast powder | 2 | 2 | 2 | 2 |
| Fish oil | 4.9 | 4.9 | 4.9 | 4.64 |
| Soybean oil | 4.9 | 4.9 | 4.9 | 4.64 |
| Vitamin and mineral premix 1 | 1 | 1 | 1 | 1 |
| Ca(H2PO4)2 | 0.5 | 0.5 | 0.5 | 0.5 |
| Choline chloride | 0.5 | 0.5 | 0.5 | 0.5 |
| Lecithin | 1 | 1 | 1 | 1 |
| Microcrystalline cellulose | 7.54 | 7.04 | 6.54 | 6.71 |
| Betaine | 0.5 | 0.5 | 0.5 | 0.5 |
| Lysine | 0.31 | 0.31 | 0.31 | 0.16 |
| Methionine | 0.15 | 0.15 | 0.15 | 0.09 |
| Glycine | 0.61 | 0.61 | 0.61 | 0.82 |
| Taurine | 0.5 | 1 | 1.5 | 0 |
| Total | 100 | 100 | 100 | 100 |
| Nutrient levels 2 | ||||
| Ash | 5.05 | 5.05 | 5.05 | 5.67 |
| Crude protein | 42.34 | 42.34 | 42.34 | 42.35 |
| Crude lipid | 12.47 | 12.47 | 12.47 | 12.48 |
| Gene | Sequence (5′-3′) | Reference |
|---|---|---|
| GH-qF | GCCAGTCAGGACGGAG | [7] |
| GH-qR | AGGAGGCGGGGCTACA | |
| GHR1-qF | GGTGGAGTTCATTGAGGTGGAT | [7] |
| GHR1-qR | TGGTGGCTGACAGGTTGG | |
| GHR2-qF | CACCACCTCTACCTCCTCTG | [7] |
| GHR2-qR | CCCTCTTCGGCGTTCATA | |
| IGF1-qF | GACGCTTACAGGAGGAGAA | [7] |
| IGF1-qR | GCTGCTGGATGTGTTCAC | |
| IGF2-qF | CTGTGACCTCAACCTGCT | [7] |
| IGF2-qR | CTCTGCCACTCCTCGTATT | |
| β-actin-qF | TACGAGCTGCCTGACGGACA | [7] |
| β-actin-qR | GGCTGTGATCTCCTTCTGCA | |
| myoG-qF | AACCAGAGGCTGCCCAAGG | [7] |
| myoG-qR | GCTGTCCCGTCTCAGTGTCC | |
| myf5-qF | AAGAACGAGAGTTTGGGCGA | [7] |
| myf5-qR | AGGACGTGGTATATGGGCCT | |
| MSTN-qF | GACGGGAACAGGCACATACG | [7] |
| MSTN-qR | GCAGCCACACGGTCAACACT | |
| CatL-qF | CCACTGGCACCTCTGCAAGA | [7] |
| CatL-qR | GCCCGTAGCACTGTTTGCCC | |
| CatB-qF | TCTGCCTGGGACTTCTGGACCA | [7] |
| CatB-qR | ACACTTGAGGACGCACTGAG | |
| Nrf2-qF | TTGCCTGGACACAACTGCTGTTAC | [7] |
| Nrf2-qR | TCTGTGACGGTGGCAGTGGAC | |
| Keap1-qF | CAGATAGACAGCGTGGTGAAGGC | [7] |
| Keap1-qR | GACAGTGAGACAGGTTGAAGAACTCC | |
| HO-1-qF | AGAAGATTCAGACAGCAGCAGAACAG | [7] |
| HO-1-qR | TCATACAGCGAGCACAGGAGGAG | |
| SOD-qF | CCTCATCCCCCTGCTTGGTA | [7] |
| SOD-qR | CCAGGGAGGGATGAGAGGTG | |
| GSH-Px-qF | GCTGAGAGGCTGGTGCAAGTG | [7] |
| GSH-Px-qR | TTCAAGCGTTACAGCAGGAGGTTC | |
| CAT-qF | GGATGGACAGCCTTCAAGTTCTCG | [7] |
| CAT-qR | TGGACCGTTACAACAGTGCAGATG | |
| il-1β-qF | CGGACTCGAACGTGGTCACATTC | [9] |
| il-1β-qR | AATATGGAAGGCAACCGTGCTCAG | |
| IL-8-qF | TGCATCACCACGGTGAAAAA | [9] |
| IL-8-qR | GCATCAGGGTCCAGACAAATC | |
| TNF-α-qF | CGCAATCGTAAAGAGTCCCA | [9] |
| TNF-α-qR | AAGTCACAGTCGGCGAAATG | |
| il-10-qF | CTCCAGACAGAAGACTCCAGCA | [9] |
| il-10-qR | GGAATCCCTCCACAAAACGAC | |
| NF-κB-qF | TGCGACAAAGTCCAGAAAGAT | [9] |
| NF-κB-qR | CTGAGGGTGGTAGGTGAAGGG | |
| ZO-1-qF | TTTGTGGCAGGAGTTCT | [9] |
| ZO-1-qR | TTCTTGTTGGGGATGAT | |
| Occludin-qF | TACGCCTACAAGACCCGCA | [9] |
| Occludin-qR | CACCGCTCTCTCTGATAAA | |
| Claudin-3-qF | CTCCTCTGCTGCTCCTGTCC | [9] |
| Claudin-3-qR | CGTAGTCTTTCCTTTCTAACCCTG | |
| Claudin-15-qF | AAGGTATGAAATAGGAGAAGGGC | [9] |
| Claudin-15-qR | TGGTTTGATAAGGCAGAGGGTA |
| Item | Diets | |||
|---|---|---|---|---|
| A25T05 | A25T10 | A25T15 | MF | |
| WGR (%) | 245.48 ± 12.91 b | 304.11 ± 21.18 ab | 380.46 ± 17.99 a | 286.48 ± 89.39 ab |
| SGR (%) | 2.22 ± 0.07 | 2.49 ± 0.09 | 2.80 ± 0.07 | 2.38 ± 0.45 |
| FCR | 2.65 ± 0.22 | 2.30 ± 0.10 | 1.86 ± 0.05 | 2.57 ± 0.91 |
| FE | 1.3 ± 0.06 | 1.27 ± 0.03 | 1.1 ± 0.00 | 1.27 ± 0.12 |
| PER | 0.93 ± 0.03 | 1.1 ± 0.06 | 1.33 ± 0.03 | 1.03 ± 0.22 |
| VSI (%) | 1.01 ± 0.10 | 1.02 ± 0.31 | 1.22 ± 0.27 | 1.08 ± 0.39 |
| HSI (%) | 7.49 ± 0.93 | 7.61 ± 1.50 | 7.56 ± 1.04 | 7.96 ± 0.99 |
| CF (%) | 1.70 ± 0.07 | 1.76 ± 0.02 | 1.79 ± 0.08 | 1.76 ± 0.19 |
| Enzyme | Diets | |||
|---|---|---|---|---|
| A25T05 | A25T10 | A25T15 | MF | |
| ALT(U/L) | 2.52 ± 0.48 | 3.31 ± 0.49 | 2.83 ± 0.9 | 3.13 ± 0.84 |
| AST(U/L) | 35.38 ± 8.25 | 30.68 ± 4.45 | 27.81 ± 2.06 | 35.9 ± 2.8 |
| Enzyme | Diets | |||
|---|---|---|---|---|
| A25T05 | A25T10 | A25T15 | MF | |
| SOD (U/mg) | 17.48 ± 0.65 b | 18.8 ± 0.43 a | 8.1 ± 0.55 d | 11.57 ± 0.34 c |
| CAT/(U/mg) | 5.85 ± 0.20 d | 20.34 ± 0.38 a | 15.6 ± 0.74 b | 11.45 ± 0.23 c |
| GSH-Px/(U/mg) | 0.0345 ± 0.00125 a | 0.0234 ± 0.00085 c | 0.031 ± 0.0000 b | 0.0339 ± 0.00114 a |
| T-AOC/(U/mg) | 0.0796 ± 0.00201 c | 0.0614 ± 0.00129 d | 0.0862 ± 0.0016 b | 0.0922 ± 0.00169 a |
| MDA (nmol/mg) | 1.9 ± 0.05 d | 2.6 ± 0.03 c | 2.74 ± 0.09 b | 3.62 ± 0.05 a |
| Enzyme | Diets | |||
|---|---|---|---|---|
| A25T05 | A25T10 | A25T15 | MF | |
| Chymotrypsin (U/mg) | 0.0436 ± 0.00225 d | 0.0834 ± 0.0026 a | 0.0615 ± 0.00122 b | 0.0538 ± 0.00127 c |
| AMY(U/mg) | 0.0084 ± 0.0003 b | 0.0081 ± 0.00012 b | 0.0094 ± 0.00026 a | 0.0073 ± 0.0001 c |
| LPS (U/mg) | 0.0415 ± 0.00146 b | 0.028 ± 0.00015 d | 0.0383 ± 0.00153 c | 0.0445 ± 0.00093 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ye, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W.; Lin, H.; Ma, Z.; Zhou, C. Taurine Supplementation in Low-Fishmeal of Golden Pompano (Trachinotus ovatus) Diets: Improving Intestinal Health and Alleviation of Inflammatory Response. Animals 2025, 15, 3080. https://doi.org/10.3390/ani15213080
Wang Z, Ye H, Huang Z, Wang J, Wang Y, Yu W, Lin H, Ma Z, Zhou C. Taurine Supplementation in Low-Fishmeal of Golden Pompano (Trachinotus ovatus) Diets: Improving Intestinal Health and Alleviation of Inflammatory Response. Animals. 2025; 15(21):3080. https://doi.org/10.3390/ani15213080
Chicago/Turabian StyleWang, Zhanzhan, Hongkai Ye, Zhong Huang, Jun Wang, Yun Wang, Wei Yu, Heizhao Lin, Zhenhua Ma, and Chuanpeng Zhou. 2025. "Taurine Supplementation in Low-Fishmeal of Golden Pompano (Trachinotus ovatus) Diets: Improving Intestinal Health and Alleviation of Inflammatory Response" Animals 15, no. 21: 3080. https://doi.org/10.3390/ani15213080
APA StyleWang, Z., Ye, H., Huang, Z., Wang, J., Wang, Y., Yu, W., Lin, H., Ma, Z., & Zhou, C. (2025). Taurine Supplementation in Low-Fishmeal of Golden Pompano (Trachinotus ovatus) Diets: Improving Intestinal Health and Alleviation of Inflammatory Response. Animals, 15(21), 3080. https://doi.org/10.3390/ani15213080







