What if Horses Were Humans? Comparing Rein Tension and Bit Pressures to Human Pressure Pain Thresholds
Abstract
Simple Summary
Abstract
1. Introduction
2. Pressure Pain Detection Threshold
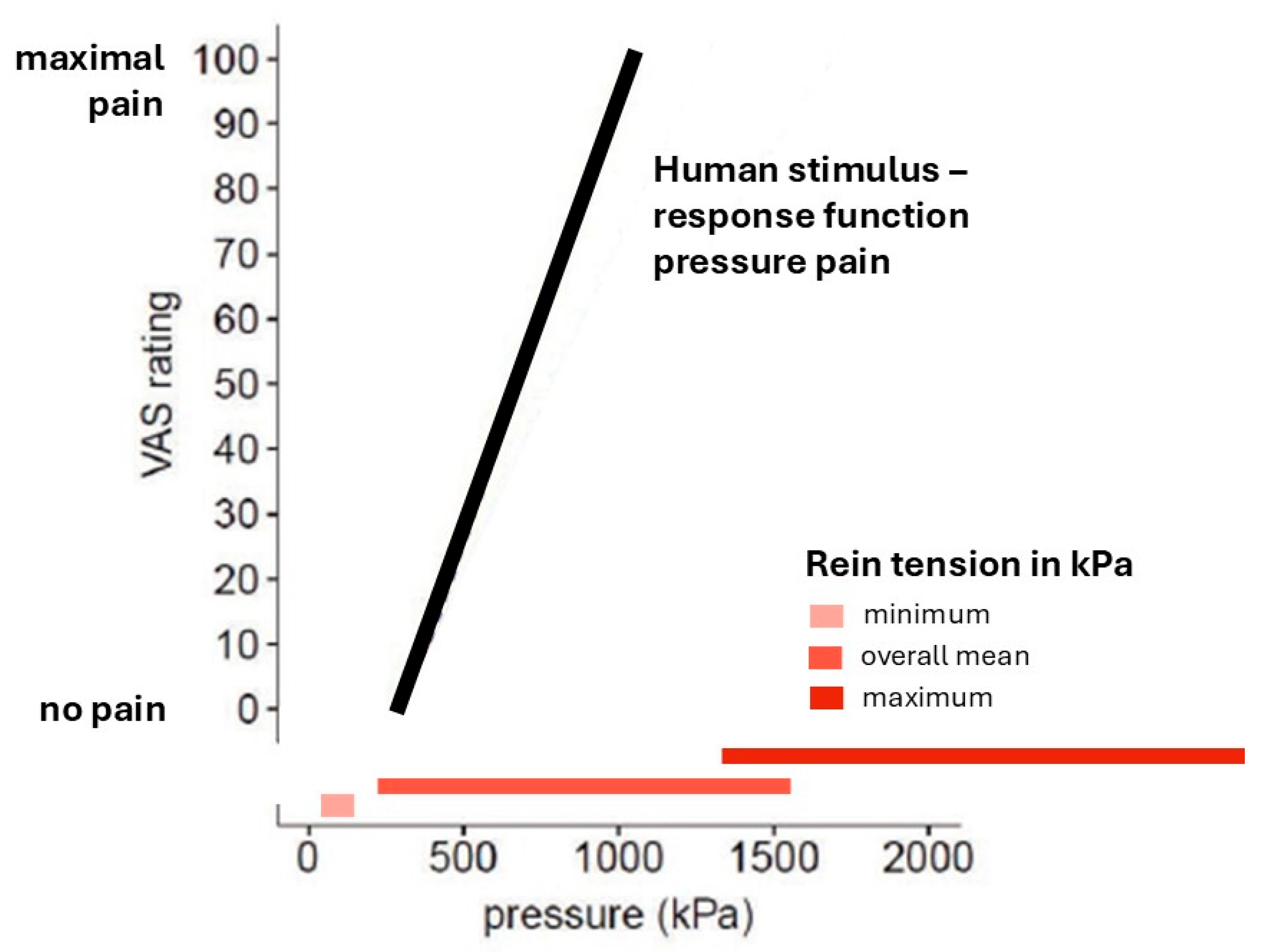
3. Stimulus Response Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPDT | Pressure pain detection threshold |
| kPa | Kilopascal |
| kg/cm2 | kilograms per square centimeter |
| N | Newton |
| QST | Quantitative sensory testing |
| SRF | Stimulus response function |
| NWR | Nociceptive withdrawal reflex |
References
- Wilkins, C.; McGreevy, P.; Henshall, C.; Mellor, D.; Tuomola, K.; Johannesen, C.P.; Lykins, A. Welfare in Dressage: The Visual and Scientific Evidence. Full 50-Minute Briefing our Research Team Delivered to the FEI Veterinary Committee on 9 April 2025. YouTube. 2025. Available online: https://www.youtube.com/watch?v=W6OgVEC4i28 (accessed on 12 October 2025).
- Cuckson, P. Bits Work Because They Hurt. In Horse Sport; Jennifer Anstey, Horse Media Group Online: Nobleton, ON, Canada, 2025. [Google Scholar]
- Uldahl, M.; Bundgaard, L.; Dahl, J.; Clayton, H.M. Pre-Competition Oral Findings in Danish Sport Horses and Ponies Competing at High Level. Animals 2022, 12, 616. [Google Scholar] [CrossRef]
- Tuomola, K.; Mäki-Kihniä, N.; Kujala-Wirth, M.; Mykkänen, A.; Valros, A. Oral Lesions in the Bit Area in Finnish Trotters After a Race: Lesion Evaluation, Scoring, and Occurrence. Front. Vet. Sci. 2019, 6, 206. [Google Scholar] [CrossRef] [PubMed]
- Tuomola, K.; Mäki-Kihniä, N.; Valros, A.; Mykkänen, A.; Kujala-Wirth, M. Bit-Related Lesions in Event Horses After a Cross-Country Test. Front. Vet. Sci. 2021, 8, 651160. [Google Scholar] [CrossRef] [PubMed]
- Tuomola, K.; Mäki-Kihniä, N.; Valros, A.; Mykkänen, A.; Kujala-Wirth, M. Risk factors for bit-related lesions in Finnish trotting horses. Equine Vet. J. 2021, 53, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Tell, A.; Egenvall, A.; Lundström, T.; Wattle, O. The prevalence of oral ulceration in Swedish horses when ridden with bit and bridle and when unridden. Vet. J. 2008, 178, 405–410. [Google Scholar] [CrossRef]
- Björnsdóttir, S.; Frey, R.; Kristjansson, T.; Lundström, T. Bit-related lesions in Icelandic competition horses. Acta Vet. Scand. 2014, 56, 40. [Google Scholar] [CrossRef]
- Mata, F.; Johnson, C.; Bishop, C. A cross-sectional epidemiological study of prevalence and severity of bit-induced oral trauma in polo ponies and race horses. J. Appl. Anim. Welf. Sci. 2015, 18, 259–268. [Google Scholar] [CrossRef]
- Mellor, D.J. Mouth Pain in Horses: Physiological Foundations, Behavioural Indices, Welfare Implications, and a Suggested Solution. Animals 2020, 10, 572. [Google Scholar] [CrossRef]
- Torcivia, C.; McDonnell, S. In-Person Caretaker Visits Disrupt Ongoing Discomfort Behavior in Hospitalized Equine Orthopedic Surgical Patients. Animals 2020, 10, 210. [Google Scholar] [CrossRef]
- Short, C.E. Fundamentals of pain perception in animals. Appl. Anim. Behav. Sci. 1998, 59, 125–133. [Google Scholar] [CrossRef]
- Kavaliers, M. Evolutionary and comparative aspects of nociception. Brain Res. Bull. 1988, 21, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U. Comparative Physiology of Nociception and Pain. Physiology 2018, 33, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.S.; Lewin, G.R. Nociceptors: A phylogenetic view. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2009, 195, 1089–1106. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Tong, L.; Stewart, M.; Johnson, I.; Appleyard, R.; Wilson, B.; James, O.; Johnson, C.; McGreevy, P. A Comparative Neuro-Histological Assessment of Gluteal Skin Thickness and Cutaneous Nociceptor Distribution in Horses and Humans. Animals 2020, 10, 2094. [Google Scholar] [CrossRef]
- Nothnagel, H.; Puta, C.; Lehmann, T.; Baumbach, P.; Menard, M.B.; Gabriel, B.; Gabriel, H.H.W.; Weiss, T.; Musial, F. How stable are quantitative sensory testing measurements over time? Report on 10-week reliability and agreement of results in healthy volunteers. J. Pain Res. 2017, 10, 2067–2078. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.D. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 2006, 10, 77–88. [Google Scholar] [CrossRef]
- Magerl, W.; Krumova, E.K.; Baron, R.; Tölle, T.; Treede, R.D.; Maier, C. Reference data for quantitative sensory testing (QST): Refined stratification for age and a novel method for statistical comparison of group data. Pain 2010, 151, 598–605. [Google Scholar] [CrossRef]
- Spohn, D.; Musial, F.; Rolke, R. Naturopathic reflex therapies for the treatment of chronic pain—Part 2: Quantitative sensory testing as a translational tool. Forsch. Komplementärmedizin 2013, 20, 225–230. [Google Scholar] [CrossRef]
- Pfau, D.B.; Krumova, E.K.; Treede, R.D.; Baron, R.; Toelle, T.; Birklein, F.; Eich, W.; Geber, C.; Gerhardt, A.; Weiss, T.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain 2014, 155, 1002–1015. [Google Scholar] [CrossRef]
- Haggard, P.; de Boer, L. Oral somatosensory awareness. Neurosci. Biobehav. Rev. 2014, 47, 469–484. [Google Scholar] [CrossRef]
- Cook, W.R. Damage by the bit to the equine interdental space and second lower premolar. Equine Vet. Educ. 2011, 23, 355–360. [Google Scholar] [CrossRef]
- Manfredi, J.; Clayton, H.M.; Rosenstein, D. Radiographic study of bit position within the horse’s oral cavity. Equine Comp. Exerc. Physiol. 2005, 2, 195–201. [Google Scholar] [CrossRef]
- Benoist, C.C.; Cross, G.H. A Photographic Methodology for Analyzing Bit Position Under Rein Tension. J. Equine Vet. Sci. 2018, 67, 102–111. [Google Scholar] [CrossRef]
- Mantyh, P.W. The neurobiology of skeletal pain. Eur. J. Neurosci. 2014, 39, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Heleski, C.R.; McGreevy, P.D.; Kaiser, L.J.; Lavagnino, M.; Tans, E.; Bello, N.; Clayton, H.M. Effects on behaviour and rein tension on horses ridden with or without martingales and rein inserts. Vet. J. 2009, 181, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Herbsleb, M.; Bär, K.J.; Weiss, T. Dissociation of Endogenous Pain Inhibition Due to Conditioned Pain Modulation and Placebo in Male Athletes Versus Nonathletes. Front. Psychol. 2020, 11, 553530. [Google Scholar] [CrossRef] [PubMed]
- Mühlemann, S.; Leandri, M.; Risberg, Å.I.; Spadavecchia, C. Comparison of Threshold and Tolerance Nociceptive Withdrawal Reflexes in Horses. Animals 2021, 11, 3380. [Google Scholar] [CrossRef]
- Sherrington, C. The Integrative Action of the Nervous System; Oxford University Press: Oxford, UK, 1906. [Google Scholar]
- Casey, C.K. Chasing Pain: The Search for a Neurobiological Mechanism; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Haussler, K.K. Pressure Algometry for the Detection of Mechanical Nociceptive Thresholds in Horses. Animals 2020, 10, 2195. [Google Scholar] [CrossRef]
- Love, E.J.; Murrell, J.; Whay, H.R. Thermal and mechanical nociceptive threshold testing in horses: A review. Vet. Anaesth. Analg. 2011, 38, 3–14. [Google Scholar] [CrossRef]
- Doherty, O.; Conway, R.; McGreevy, P. Using an Equine Cadaver Head to Investigate Associations Between Sub-Noseband Space, Noseband Tension, and Sub-Noseband Pressure at Three Locations. Animals 2025, 15, 2141. [Google Scholar] [CrossRef]
- Crago, F.; Shea, G.; James, O.; Schemann, K.; McGreevy, P.D. An opportunistic pilot study of radiographs of equine nasal bones at the usual site of nosebands. J. Vet. Behav. 2019, 29, 70–76. [Google Scholar] [CrossRef]
- Gigliuto, C.; De Gregori, M.; Malafoglia, V.; Raffaeli, W.; Compagnone, C.; Visai, L.; Petrini, P.; Avanzini, M.A.; Muscoli, C.; Viganò, J.; et al. Pain assessment in animal models: Do we need further studies? J. Pain Res. 2014, 7, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Meyer, R.A.; Campbell, J.N. Peripheral mechanisms of somatic pain. Anesthesiology 1988, 68, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Torcivia, C.; McDonnell, S. Equine Discomfort Ethogram. Animals 2021, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Pascuzzo, R.; Leach, M.C.; Dai, F.; Lebelt, D.; Vantini, S.; Minero, M. Can grimace scales estimate the pain status in horses and mice? A statistical approach to identify a classifier. PLoS ONE 2018, 13, e0200339. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Forkman, B.; Lindegaard, C.; Andersen, P.H. An equine pain face. Vet. Anaesth. Analg. 2015, 42, 103–114. [Google Scholar] [CrossRef]

| Rein Tension | ||
|---|---|---|
| Intensity | kPa | kg/cm2 |
| Minimum | 91.2–107.87 | 0.93–1.1 |
| Overall mean | 225.55–1520.03 | 2.3–15.5 |
| Maximum | 1314.09–4285.51 | 13.4–43.7 |
| Human Pressure Pain Detection Thresholds (PPDTs) | ||
|---|---|---|
| Location | kPa | kg/cm2 |
| Face | 232.4 | 2.37 |
| Hand | 445.3 | 4.54 |
| Foot | 535.5 | 5.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musial, F.; Weiss, T. What if Horses Were Humans? Comparing Rein Tension and Bit Pressures to Human Pressure Pain Thresholds. Animals 2025, 15, 2989. https://doi.org/10.3390/ani15202989
Musial F, Weiss T. What if Horses Were Humans? Comparing Rein Tension and Bit Pressures to Human Pressure Pain Thresholds. Animals. 2025; 15(20):2989. https://doi.org/10.3390/ani15202989
Chicago/Turabian StyleMusial, Frauke, and Thomas Weiss. 2025. "What if Horses Were Humans? Comparing Rein Tension and Bit Pressures to Human Pressure Pain Thresholds" Animals 15, no. 20: 2989. https://doi.org/10.3390/ani15202989
APA StyleMusial, F., & Weiss, T. (2025). What if Horses Were Humans? Comparing Rein Tension and Bit Pressures to Human Pressure Pain Thresholds. Animals, 15(20), 2989. https://doi.org/10.3390/ani15202989






