Fresh Pork Quality Assessment by NIRS and NMR: Predicting Eating Quality and Elucidating Relationships with Key Chemical Components
Simple Summary
Abstract
1. Introduction
- NIRS and NMR parameters could be used to predict the IMF, collagen characteristics, pH, and sensory attributes of pork;
- Chemical measurements could be used to predict the eating quality of pork;
- Chemical measurements of SM could be predicted by LTL measurements.
- Study the difference in the IMF, collagen characteristics, pH, and sensory properties between pork LTL and SM;
- Build prediction models for the IMF, collagen characteristics, pH, and sensory attributes of pork using NIRS and NMR parameters;
- Investigate the effects of chemical measurements on pork eating quality;
- Investigate whether chemical measurements of the two pork muscles were correlated.
2. Materials and Methods
2.1. Sample Preparation
2.2. pH Measurement
2.3. Near-Infrared Spectroscopy (NIRS)
2.4. Nuclear Magnetic Resonance (NMR) Relaxometry
2.5. Chemical Assays
2.6. Sensory Evaluation
2.6.1. Consumers
2.6.2. Sensory Sessions
2.6.3. Questionnaires
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prieto, N.; Pawluczyk, O.; Dugan, M.E.R.; Aalhus, J.L. A Review of the Principles and Applications of Near-Infrared Spectroscopy to Characterize Meat, Fat, and Meat Products. Appl. Spectrosc. 2017, 71, 1403–1426. [Google Scholar] [CrossRef] [PubMed]
- Furtado, E.J.G.; Bridi, A.M.; Barbin, D.F.; Barata, C.C.P.; Peres, L.M.; Barbon, A.P.A.d.C.; Andreo, N.; Giangareli, B.d.L.; Terto, D.K.; Batista, J.P. Prediction of PH and Color in Pork Meat Using VIS-NIR near-Infrared Spectroscopy (NIRS). Food Sci. Technol. 2018, 39, 88–92. [Google Scholar] [CrossRef]
- González-Mohino, A.; Antequera, T.; Ventanas, S.; Caballero, D.; Mir-Bel, J.; Pérez-Palacios, T. Near-Infrared Spectroscopy-Based Analysis to Study Sensory Parameters on Pork Loins as Affected by Cooking Methods and Conditions. J. Sci. Food Agric. 2018, 98, 4227–4236. [Google Scholar] [CrossRef]
- Bertram, H.C.; Andersen, H.J. NMR and the Water-Holding Issue of Pork. J. Anim. Breed. Genet. 2007, 124, 35–42. [Google Scholar] [CrossRef]
- Bertram, H.C.; Straadt, I.K.; Jensen, J.A.; Dall Aaslyng, M. Relationship between Water Mobility and Distribution and Sensory Attributes in Pork Slaughtered at an Age between 90 and 180 Days. Meat Sci. 2007, 77, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.C.; Andersen, H.J.; Karlsson, A.H. Comparative Study of Low-Field NMR Relaxation Measurements and Two Traditional Methods in the Determination of Water Holding Capacity of Pork. Meat Sci. 2001, 57, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Elbourne, A.; Truong, V.K.; Cozzolino, D. Shining Light into Meat—A Review on the Recent Advances in in Vivo and Carcass Applications of near Infrared Spectroscopy. Int. J. Food Sci. Technol. 2020, 55, 935–941. [Google Scholar] [CrossRef]
- Wheeler, T.L.; Shackelford, S.D.; Koohmaraie, M. Variation in Proteolysis, Sarcomere Length, Collagen Content, and Tenderness among Major Pork Muscles. J. Anim. Sci. 2000, 78, 958–965. [Google Scholar] [CrossRef]
- Fortin, A.; Robertson, W.M.; Tong, A.K.W. The Eating Quality of Canadian Pork and Its Relationship with Intramuscular Fat. Meat Sci. 2005, 69, 297–305. [Google Scholar] [CrossRef]
- Rincker, P.J.; Killefer, J.; Ellis, M.; Brewer, M.S.; McKeith, F.K. Intramuscular Fat Content Has Little Influence on the Eating Quality of Fresh Pork Loin Chops. J. Anim. Sci. 2008, 86, 730–737. [Google Scholar] [CrossRef]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Meta-Analysis of the Relationship between Collagen Characteristics and Meat Tenderness. Meat Sci. 2022, 185, 108717. [Google Scholar] [CrossRef]
- Arkfeld, E.K.; Wilson, K.B.; Overholt, M.F.; Harsh, B.N.; Lowell, J.E.; Hogan, E.K.; Klehm, B.J.; Bohrer, B.M.; Mohrhauser, D.A.; King, D.A.; et al. Pork Loin Quality Is Not Indicative of Fresh Belly or Fresh and Cured Ham Quality. J. Anim. Sci. 2016, 94, 5155–5167. [Google Scholar] [CrossRef] [PubMed]
- Huff-Lonergan, E.; Baas, T.J.; Malek, M.; Dekkers, J.C.M.; Prusa, K.; Rothschild, M.F. Correlations among Selected Pork Quality Traits. J. Anim. Sci. 2002, 80, 617–627. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Brun, A.; Gispert, M. Intramuscular Fat Content in Different Muscles, Locations, Weights and Genotype-Sexes and Its Prediction in Live Pigs with Computed Tomography. Animal 2019, 13, 666–674. [Google Scholar] [CrossRef]
- McCarney, E.R.; Dykstra, R.; Dykstra, C.G.; FitzPatrick, A. Automated Eating Quality Measurements on Lamb Carcases in a Processing Plant Using Unilateral NMR. Appl. Magn. Reson. 2023, 54, 1377–1389. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 2004, 29, 688–691. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Nakashima, Y.; Shiba, N. Nondestructive Measurement of Intramuscular Fat Content of Fresh Beef Meat by a Hand-Held Magnetic Resonance Sensor. Int. J. Food Prop. 2021, 24, 1722–1736. [Google Scholar] [CrossRef]
- Pooke, D.; McCarney, E. NMR Measurement of Intra-Muscular Fat; Meat & Livestock Australia (MLA): Sydney, Australia, 2019. [Google Scholar]
- Bertram, H.C.; Karlsson, A.H.; Rasmussen, M.; Pedersen, O.D.; Dønstrup, S.; Andersen, H.J. Origin of Multiexponential T2 Relaxation in Muscle Myowater. J. Agric. Food Chem. 2001, 49, 3092–3100. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Collagen Characteristics Affect the Texture of Pork Longissimus and Biceps Femoris. Transl. Anim. Sci. 2022, 6, txac129. [Google Scholar] [CrossRef]
- Kolar, K. Colorimetric Determination of Hydroxyproline as Measure of Collagen Content in Meat and Meat Products: NMKL Collaborative Study. J. Assoc. Off. Anal. Chem. 1990, 73, 54–57. [Google Scholar] [CrossRef]
- Channon, H.A.; D’Souza, D.N.; Dunshea, F.R. Developing a Cuts-Based System to Improve Consumer Acceptability of Pork: Impact of Gender, Ageing Period, Endpoint Temperature and Cooking Method. Meat Sci. 2016, 121, 216–227. [Google Scholar] [CrossRef]
- Prieto, N.; Roehe, R.; Lavín, P.; Batten, G.; Andrés, S. Application of near Infrared Reflectance Spectroscopy to Predict Meat and Meat Products Quality: A Review. Meat Sci. 2009, 83, 175–186. [Google Scholar] [CrossRef]
- Anderson, S. Determination of Fat, Moisture, and Protein in Meat and Meat Products by Using the FOSS FoodScan near-Infrared Spectrophotometer with FOSS Artificial Neural Network Calibration Model and Associated Database: Collaborative Study. J. AOAC Int. 2007, 90, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Savenije, B.; Geesink, G.H.; van der Palen, J.G.P.; Hemke, G. Prediction of Pork Quality Using Visible/near-Infrared Reflectance Spectroscopy. Meat Sci. 2006, 73, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Brøndum, J.; Munck, L.; Henckel, P.; Karlsson, A.; Tornberg, E.; Engelsen, S.B. Prediction of Water-Holding Capacity and Composition of Porcine Meat by Comparative Spectroscopy. Meat Sci. 2000, 55, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Walker, N.P.; Mills, W.E. Prediction of Pork Quality Characteristics Using Visible and near–Infrared Spectroscopy. Trans. ASAE 2002, 45, 1519. [Google Scholar] [CrossRef]
- Hoving-Bolink, A.H.; Vedder, H.W.; Merks, J.W.M.; de Klein, W.J.H.; Reimert, H.G.M.; Frankhuizen, R.; van den Broek, W.H.A.M.; Lambooij, e.E. Perspective of NIRS Measurements Early Post Mortem for Prediction of Pork Quality. Meat Sci. 2005, 69, 417–423. [Google Scholar] [CrossRef]
- AUS-MEAT. AUS-MEAT List of Approved Equipment Suppliers; AUS-MEAT: Murarrie, Australia, 2023. [Google Scholar]
- Brown, R.J.S.; Capozzi, F.; Cavani, C.; Cremonini, M.A.; Petracci, M.; Placucci, G. Relationships between 1H NMR Relaxation Data and Some Technological Parameters of Meat: A Chemometric Approach. J. Magn. Reson. 2000, 147, 89–94. [Google Scholar] [CrossRef]
- Hullberg, A.; Bertram, H.C. Relationships between Sensory Perception and Water Distribution Determined by Low-Field NMR T2 Relaxation in Processed Pork—Impact of Tumbling and RN− Allele. Meat Sci. 2005, 69, 709–720. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of Water-Holding Capacity of Meat: The Role of Postmortem Biochemical and Structural Changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Li, R.; Yang, H.; Wang, H.; Wang, H.; Tan, M. Influence of Multiple Freeze-Thaw Cycles on Quality Characteristics of Beef Semimembranous Muscle: With Emphasis on Water Status and Distribution by LF-NMR and MRI. Meat Sci. 2019, 147, 44–52. [Google Scholar] [CrossRef]
- Bertram, H.C.; Aaslyng, M.D.; Andersen, H.J. Elucidation of the Relationship between Cooking Temperature, Water Distribution and Sensory Attributes of Pork—A Combined NMR and Sensory Study. Meat Sci. 2005, 70, 75–81. [Google Scholar] [CrossRef]
- Fjelkner-Modig, S.; Tornberg, E. Water Distribution in Porcine M. Longissimus Dorsi in Relation to Sensory Properties. Meat Sci. 1986, 17, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A Structural Approach to Understanding the Interactions between Colour, Water-Holding Capacity and Tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.S.; Wheeler, T.L.; Shackelford, S.D.; Koohmaraie, M. Variation in Palatability and Biochemical Traits within and among Eleven Beef Muscles. J. Anim. Sci. 2004, 82, 534–550. [Google Scholar] [CrossRef]
- Chambaz, A.; Scheeder, M.R.L.; Kreuzer, M.; Dufey, P.-A. Meat Quality of Angus, Simmental, Charolais and Limousin Steers Compared at the Same Intramuscular Fat Content. Meat Sci. 2003, 63, 491–500. [Google Scholar] [CrossRef]
- Listrat, A.; Gagaoua, M.; Normand, J.; Gruffat, D.; Andueza, D.; Mairesse, G.; Mourot, B.; Chesneau, G.; Gobert, C.; Picard, B. Contribution of Connective Tissue Components, Muscle Fibres and Marbling to Beef Tenderness Variability in Longissimus Thoracis, Rectus Abdominis, Semimembranosus and Semitendinosus Muscles. J. Sci. Food Agric. 2020, 100, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.L.; Shackelford, S.D.; Koohmaraie, M. Technical Note: Sampling Methodology for Relating Sarcomere Length, Collagen Concentration, and the Extent of Postmortem Proteolysis to Beef and Pork Longissimus Tenderness. J. Anim. Sci. 2002, 80, 982–987. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.M.C.; Micol, D.; Hocquette, J.-F.; Moloney, A.P.; Nuernberg, K.; Bauchart, D.; Boudjellal, A.; Scollan, N.D.; Richardson, R.I.; et al. Sensory Quality of Meat from Eight Different Types of Cattle in Relation with Their Biochemical Characteristics. J. Integr. Agric. 2016, 15, 1550–1563. [Google Scholar] [CrossRef]
- Schönfeldt, H.C.; Strydom, P.E. Effect of Age and Cut on Tenderness of South African Beef. Meat Sci. 2011, 87, 206–218. [Google Scholar] [CrossRef]
- Listrat, A.; Gagaoua, M.; Andueza, D.; Gruffat, D.; Normand, J.; Mairesse, G.; Picard, B.; Hocquette, J.-F. What Are the Drivers of Beef Sensory Quality Using Metadata of Intramuscular Connective Tissue, Fatty Acids and Muscle Fiber Characteristics? Livest. Sci. 2020, 240, 104209. [Google Scholar] [CrossRef]
- Purslow, P.P. Contribution of Collagen and Connective Tissue to Cooked Meat Toughness; Some Paradigms Reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef]
- Bidner, B.S.; Ellis, M.; Brewer, M.S.; Campion, D.; Wilson, E.R.; Mckeith, F.K. Effect of Ultimate PH on the Quality Characteristics of Pork. J. Muscle Foods 2004, 15, 139–154. [Google Scholar] [CrossRef]
- Silva, J.A.; Patarata, L.; Martins, C. Influence of Ultimate PH on Bovine Meat Tenderness during Ageing. Meat Sci. 1999, 52, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Lomiwes, D.; Farouk, M.M.; Wu, G.; Young, O.A. The Development of Meat Tenderness Is Likely to Be Compartmentalised by Ultimate PH. Meat Sci. 2014, 96, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of Inosine Monophosphate and Its Degradation Products during Aging of Pork of Different Qualities in Relation to Basic Taste and Retronasal Flavor Perception of the Meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef]
- Madeira, M.S.; Costa, P.; Alfaia, C.M.; Lopes, P.A.; Bessa, R.J.B.; Lemos, J.P.C.; Prates, J.A.M. The Increased Intramuscular Fat Promoted by Dietary Lysine Restriction in Lean but Not in Fatty Pig Genotypes Improves Pork Sensory Attributes. J. Anim. Sci. 2013, 91, 3177–3187. [Google Scholar] [CrossRef]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of Intramuscular Fat Content on the Quality of Pig Meat—1. Composition of the Lipid Fraction and Sensory Characteristics of m. Longissimus Lumborum. Meat Sci. 1999, 53, 59–65. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour Formation in Meat and Meat Products: A Review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Jensen, H.; Karlsson, A.H. The Gender Background of Texture Attributes of Pork Loin. Meat Sci. 2018, 136, 79–84. [Google Scholar] [CrossRef]
- Jeleníková, J.; Pipek, P.; Miyahara, M. The Effects of Breed, Sex, Intramuscular Fat and Ultimate PH on Pork Tenderness. Eur. Food Res. Technol. 2008, 227, 989–994. [Google Scholar] [CrossRef]
- Barton-Gade, P.; Bejerholm, A.C. Eating Quality in Pork. Pig Farming 1985, 33, 56–57. [Google Scholar]
- Melody, J.L.; Lonergan, S.M.; Rowe, L.J.; Huiatt, T.W.; Mayes, M.S.; Huff-Lonergan, E. Early Postmortem Biochemical Factors Influence Tenderness and Water-Holding Capacity of Three Porcine Muscles1. J. Anim. Sci. 2004, 82, 1195–1205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maiorano, G.; Filetti, F.; Manchisi, A.; Pastorelli, G.; Corino, C. Intramuscular Collagen Properties in Longissimus Dorsi and Semimembranosus Muscles of Heavy Pig. Atti Soc. Sci. Vet. 2000, 54, 407–408. [Google Scholar][Green Version]
- Voutila, L.; Mullen, A.M.; Ruusunen, M.; Troy, D.; Puolanne, E. Thermal Stability of Connective Tissue from Porcine Muscles. Meat Sci. 2007, 76, 474–480. [Google Scholar] [CrossRef]
- Li, X.; Ha, M.; Warner, R.D.; Hewitt, R.J.E.; D’Souza, D.N.; Dunshea, F.R. Genetic Lines Influenced the Texture, Collagen and Intramuscular Fat of Pork Longissimus and Semimembranosus. Meat Sci. 2024, 207, 109376. [Google Scholar] [CrossRef]
- Xu, S.; Falsafi, S.R. Juiciness of Meat, Meat Products, and Meat Analogues: Definition, Evaluation Methods, and Influencing Factors. Food Rev. Int. 2023, 40, 2344–2377. [Google Scholar] [CrossRef]
- Knecht, D.; Duziński, K.; Jankowska-Mąkosa, A. Pork Ham and Belly Quality Can Be Estimated from Loin Quality Measurements? Meat Sci. 2018, 145, 144–149. [Google Scholar] [CrossRef]
- Herault, F.; Vincent, A.; Dameron, O.; Le Roy, P.; Cherel, P.; Damon, M. The Longissimus and Semimembranosus Muscles Display Marked Differences in Their Gene Expression Profiles in Pig. PLoS ONE 2014, 9, e96491. [Google Scholar] [CrossRef]
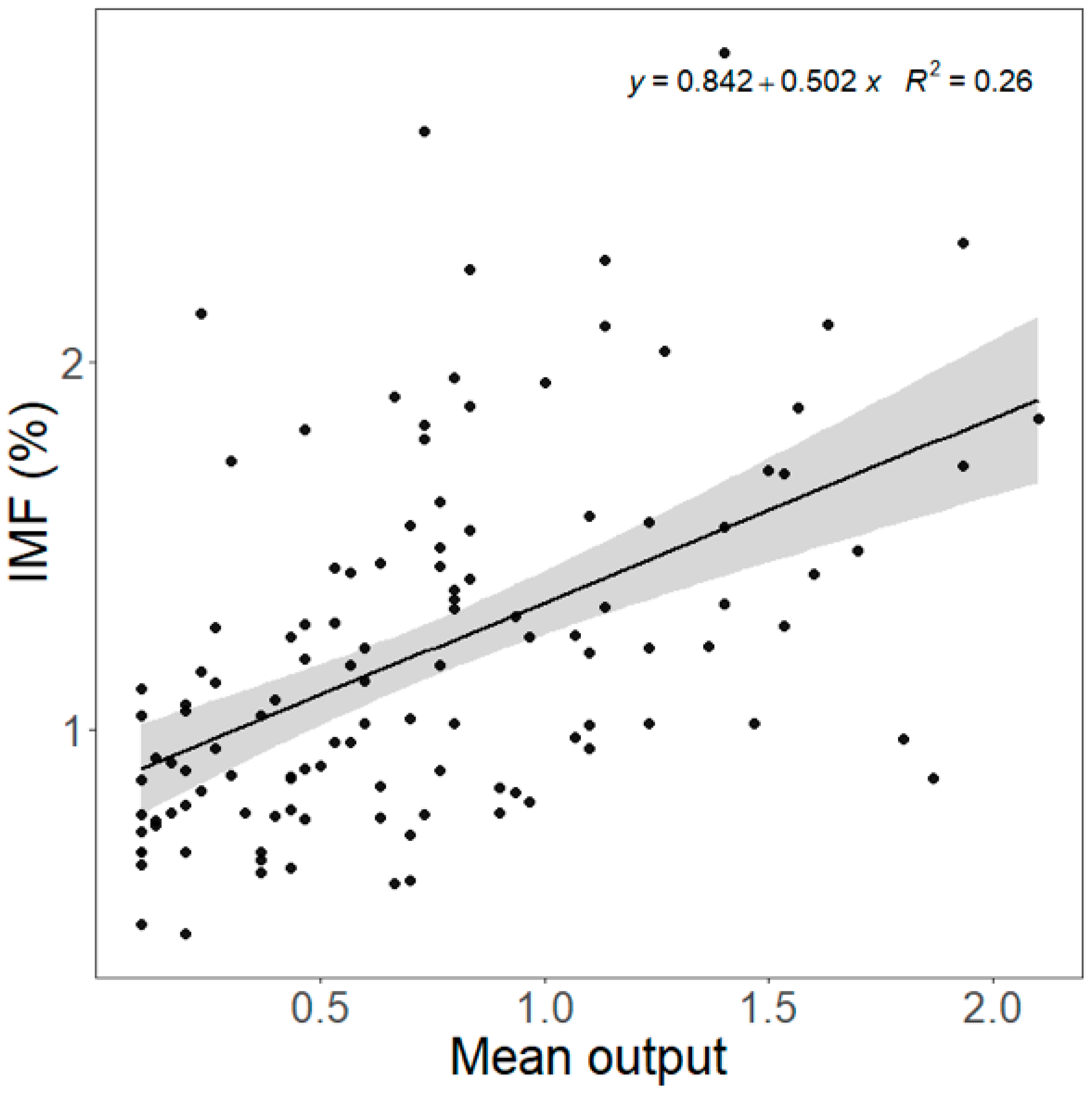
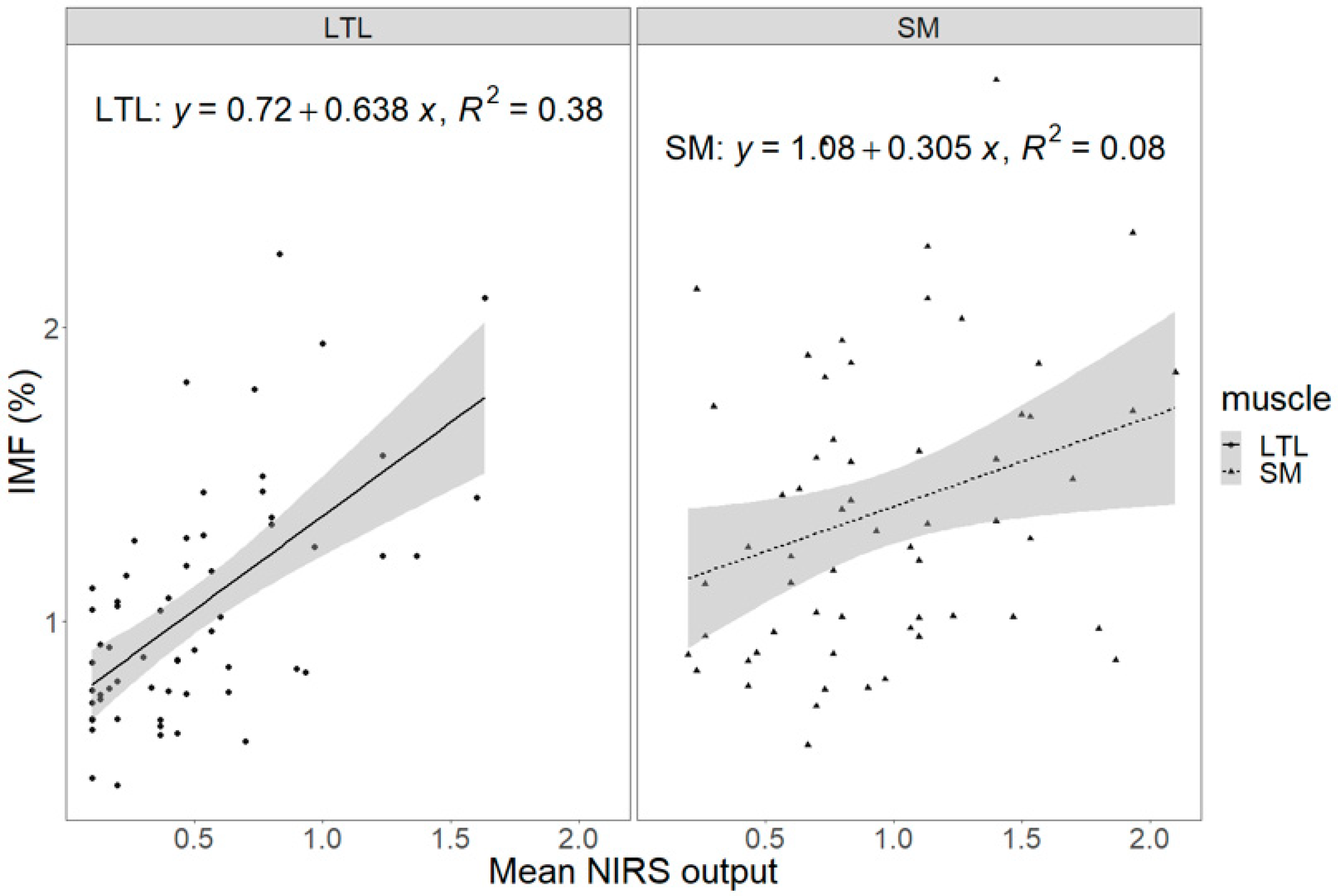

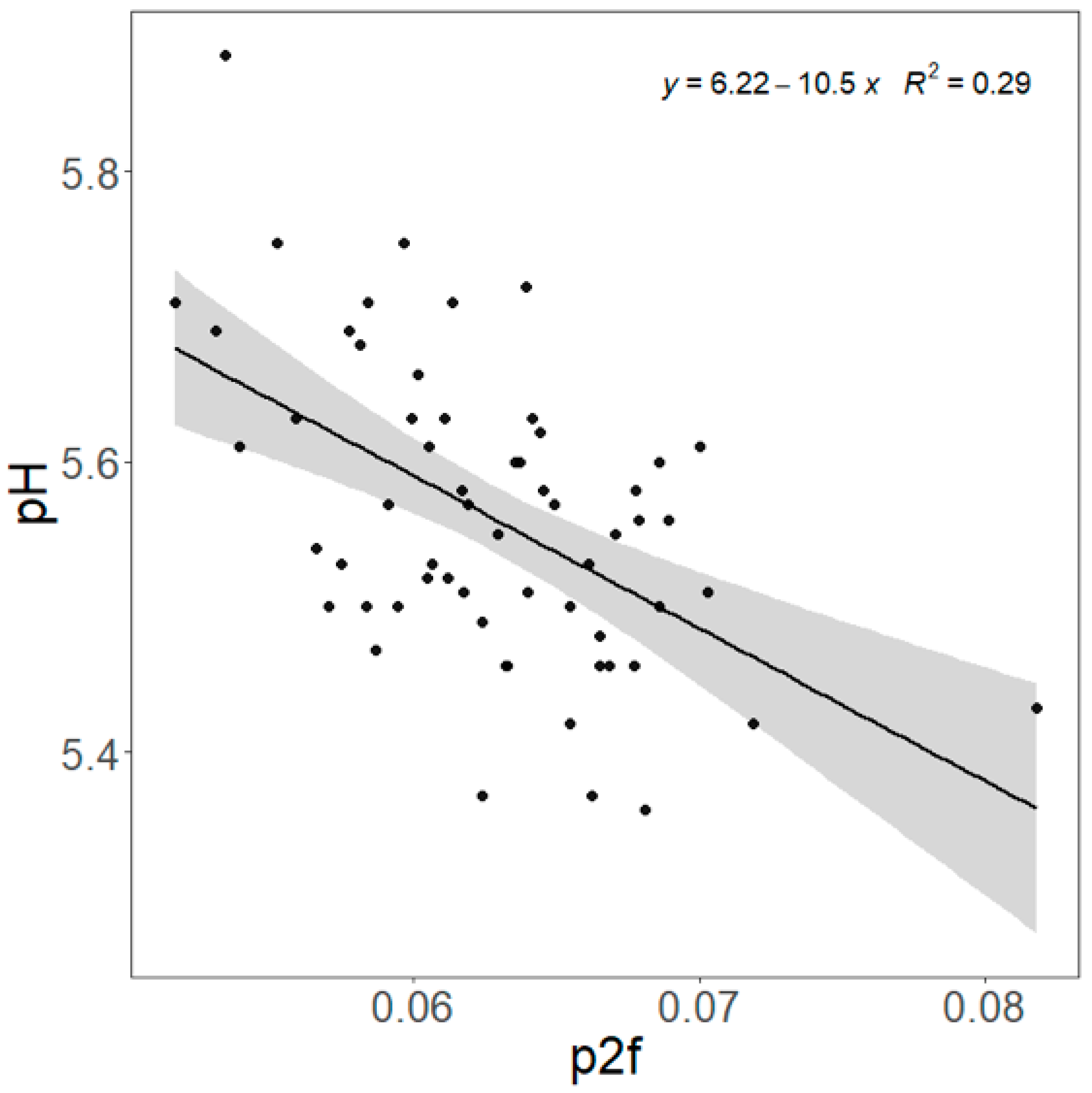
| LTL (n = 60) | SM (n = 60) | p-Value 1 | |
|---|---|---|---|
| pH | 5.56 ± 0.014 (5.33–5.88) | 5.65 ± 0.018 (5.46–5.99) | <0.001 |
| Collagen content (mg/g) | 4.56 ± 0.073 (3.51–5.57) | 4.73 ± 0.108 (3.21–8.51) | 0.20 |
| Collagen solubility (%) | 10.2 ± 0.258 (6.13–18.5) | 9.19 ± 0.239 (5.78–13.4) | 0.004 |
| IMF 2 (%) | 1.04 ± 0.051 (0.443–2.25) | 1.38 ± 0.065 (0.579–2.84) | <0.001 |
| LTL (n = 593) | SM (n = 595) | p-Value 1 | |
|---|---|---|---|
| Sensory Score (1–100) | |||
| Tenderness | 48.8 ± 2.26 | 51.7 ± 2.26 | 0.014 |
| Juiciness | 48.1 ± 2.42 | 54.1 ± 2.42 | <0.001 |
| Flavor | 52.0 ± 1.73 | 54.0 ± 1.73 | 0.063 |
| Overall liking | 52.0 ± 1.73 | 54.7 ± 1.73 | 0.010 |
| Consumer Probability | |||
| No off-flavor | 0.996 ± 0.004 | 0.992 ± 0.007 | 0.013 |
| Purchase intent 2 | 0.298 ± 0.031 | 0.351 ± 0.033 | 0.093 |
| Quality grading 3 | 0.284 ± 0.030 | 0.318 ± 0.032 | 0.27 |
| Tenderness | Juiciness | Liking of Flavor | Overall Liking | |||||
|---|---|---|---|---|---|---|---|---|
| Slope 1 | p-Value | Slope | p-Value | Slope | p-Value | Slope | p-Value | |
| pH | 14.1 ± 6.41 | 0.029 3 | 12.6 ± 5.49 | 0.023 | 11.7 ± 5.05 | 0.022 | 14.2 ± 5.53 | 0.011 |
| Collagen content (mg/g) | −3.86 ± 1.21 | 0.002 | −2.76 ± 1.05 | 0.010 | −2.60 ± 0.97 | 0.008 | −3.42 ± 1.05 | 0.001 |
| Collagen solubility (%) | −0.79 ± 0.45 | 0.083 | −0.83 ± 0.04 | 0.039 | −0.68 ± 0.37 | 0.065 | −0.69 ± 0.39 | 0.082 |
| IMF 2 (%) | 0.40 ±1.68 | 0.81 | 0.88 ± 1.48 | 0.55 | 2.91 ± 1.36 | 0.034 | 1.58 ± 1.46 | 0.28 |
| Slope 1 | R2 | p-Value | |
|---|---|---|---|
| pH | 0.397 ± 0.085 | 0.273 | <0.001 |
| Collagen content (mg/g) | 0.312 ± 0.079 | 0.214 | <0.001 |
| Collagen solubility (%) | 0.348 ± 0.134 | 0.104 | 0.012 |
| IMF (%) | 0.165 ± 0.101 | 0.044 | 0.11 |
| Muscle | Tenderness | Juiciness | Liking of Flavor | Overall Liking | ||||
|---|---|---|---|---|---|---|---|---|
| Slope 1 | p-Value | Slope | p-Value | Slope | p-Value | Slope | p-Value | |
| Both | −1.60 ± 1.76 | 0.36 | −0.36 ± 1.55 | 0.81 | 0.42 ± 1.44 | 0.77 | 0.07 ± 1.53 | 0.96 |
| LTL | 1.11 ± 3.07 | 0.72 | 1.00 ± 2.69 | 0.71 | −0.47 ± 2.64 | 0.86 | −0.35 ± 2.82 | 0.90 |
| SM | −2.47 ± 2.33 | 0.29 | −1.99 ± 1.98 | 0.32 | 0.38 ± 1.90 | 0.84 | −0.44 ± 2.17 | 0.84 |
| Tenderness | Juiciness | Liking of Flavor | Overall Liking | |||||
|---|---|---|---|---|---|---|---|---|
| Slope 1 | p-Value | Slope | p-Value | Slope | p-Value | Slope | p-Value | |
| p2f | −252 ± 217.0 | 0.25 | −245 ± 190.5 | 0.20 | −323 ± 183.5 | 0.083 | −363 ± 195.4 | 0.068 |
| p21 | 87.1 ± 31.23 | 0.007 3 | 41.1 ± 28.69 | 0.16 | 38.6 ± 28.20 | 0.18 | 54.8 ± 29.70 | 0.070 |
| p22 | −92.0 ± 33.25 | 0.008 | −40.2 ± 30.61 | 0.20 | −35.4 ± 30.13 | 0.25 | 52.7 ± 31.77 | 0.10 |
| T21 (ms) | 0.80 ± 0.78 | 0.31 | −0.12 ± 0.69 | 0.86 | 0.91 ± 0.67 | 0.18 | 1.16 ± 0.71 | 0.11 |
| T22 (ms) | −0.33 ± 0.17 | 0.056 | −0.26 ± 0.15 | 0.078 | −0.37 ± 0.14 | 0.010 | −0.21 ± 0.16 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Hastie, M.; Ha, M.; Warner, R.D.; Steel, C.C.; McGilchrist, P.; McCarney, E.; D’Souza, D.N.; Hewitt, R.J.E.; Pethick, D.W.; et al. Fresh Pork Quality Assessment by NIRS and NMR: Predicting Eating Quality and Elucidating Relationships with Key Chemical Components. Animals 2025, 15, 2973. https://doi.org/10.3390/ani15202973
Li X, Hastie M, Ha M, Warner RD, Steel CC, McGilchrist P, McCarney E, D’Souza DN, Hewitt RJE, Pethick DW, et al. Fresh Pork Quality Assessment by NIRS and NMR: Predicting Eating Quality and Elucidating Relationships with Key Chemical Components. Animals. 2025; 15(20):2973. https://doi.org/10.3390/ani15202973
Chicago/Turabian StyleLi, Xiying, Melindee Hastie, Minh Ha, Robyn D. Warner, Cameron C. Steel, Peter McGilchrist, Evan McCarney, Darryl N. D’Souza, Robert J. E. Hewitt, David W. Pethick, and et al. 2025. "Fresh Pork Quality Assessment by NIRS and NMR: Predicting Eating Quality and Elucidating Relationships with Key Chemical Components" Animals 15, no. 20: 2973. https://doi.org/10.3390/ani15202973
APA StyleLi, X., Hastie, M., Ha, M., Warner, R. D., Steel, C. C., McGilchrist, P., McCarney, E., D’Souza, D. N., Hewitt, R. J. E., Pethick, D. W., Corlett, M. T., Stewart, S. M., & Dunshea, F. R. (2025). Fresh Pork Quality Assessment by NIRS and NMR: Predicting Eating Quality and Elucidating Relationships with Key Chemical Components. Animals, 15(20), 2973. https://doi.org/10.3390/ani15202973







