Ecological Characteristics of Large-Bodied Sharks in the East Sea of Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
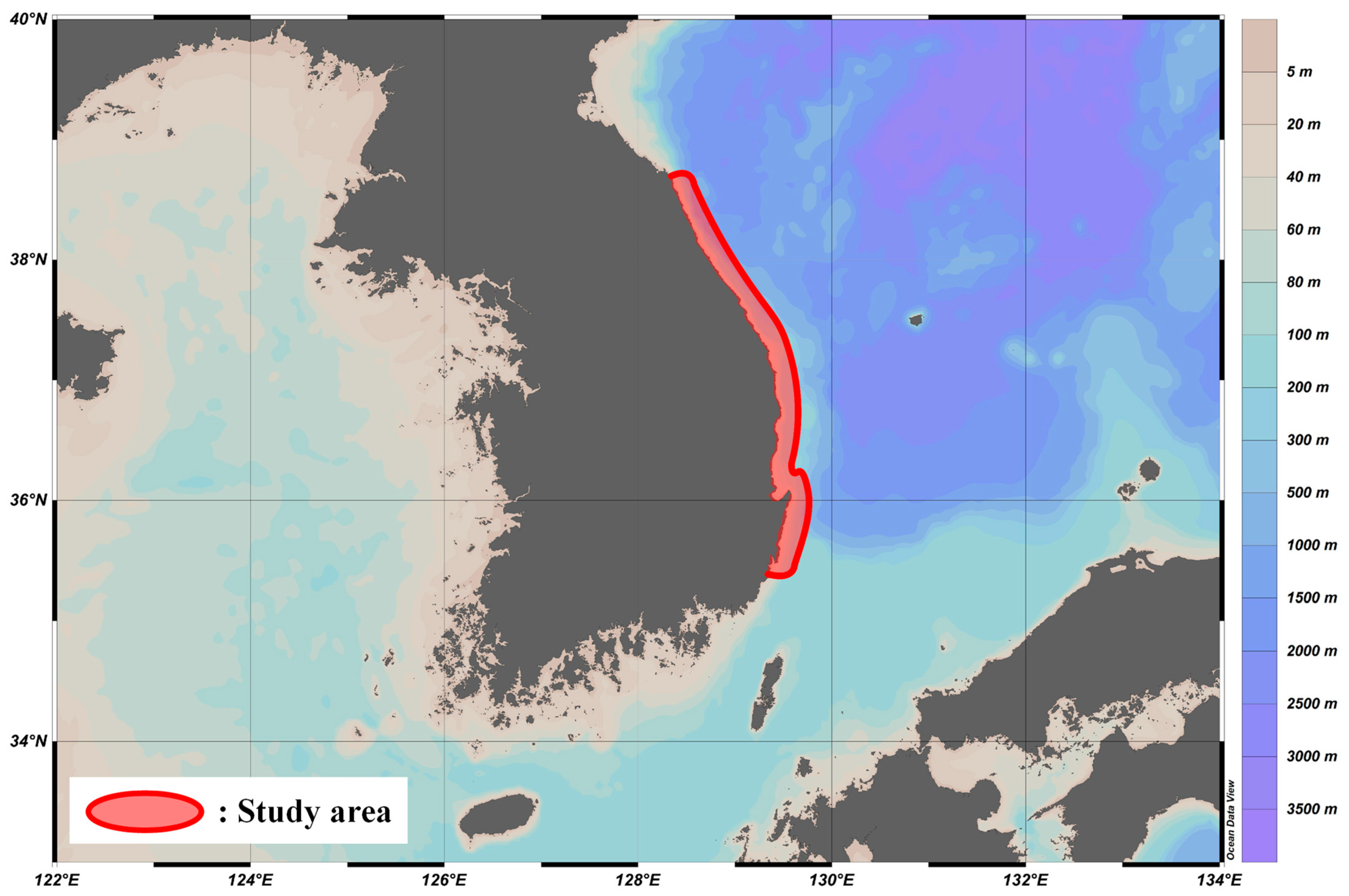
2.1. Data Collection and Sampling
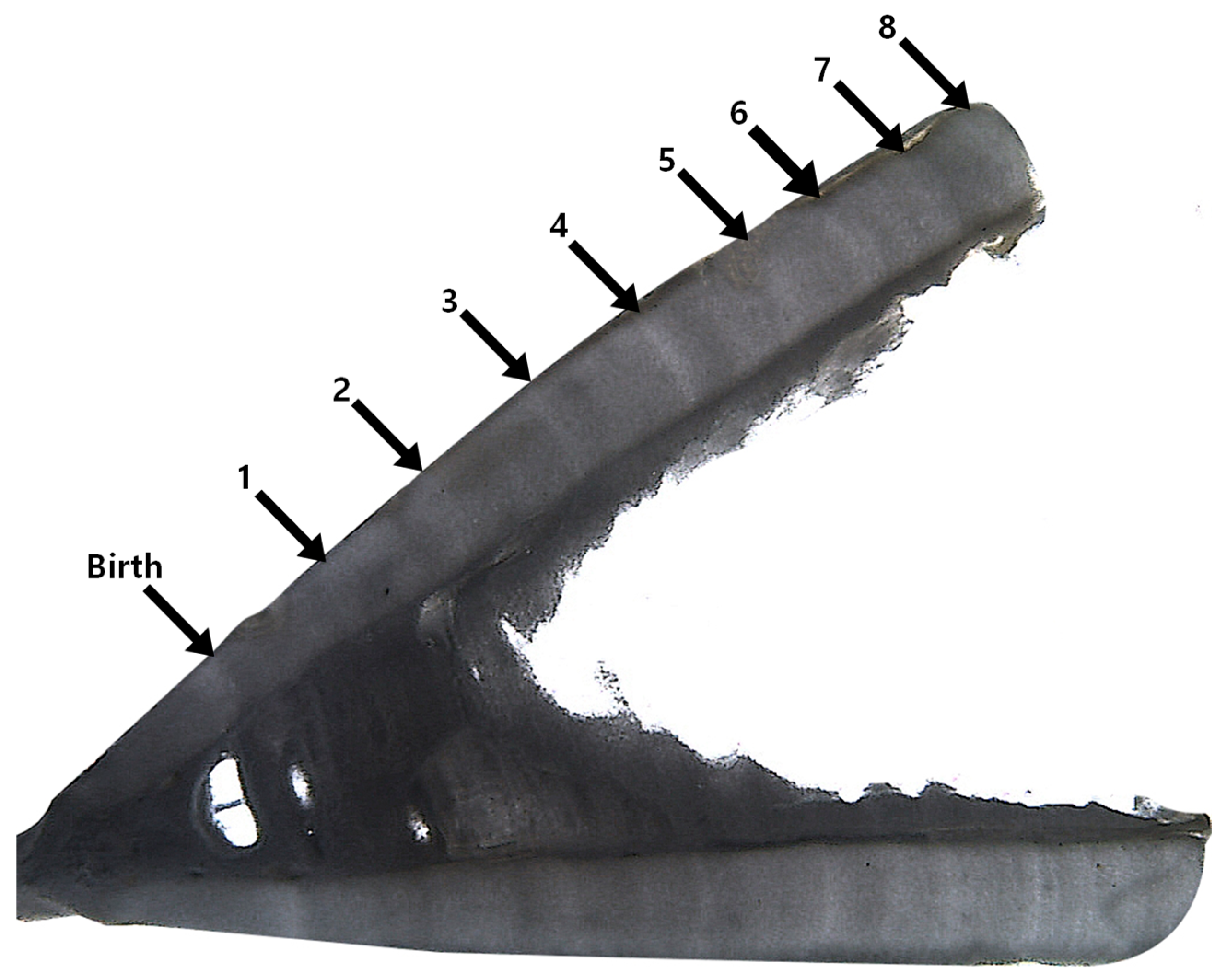
2.2. Age and Growth Analysis
2.3. Morphological Diet Analysis
2.4. Metabarcoding Analysis
2.5. Stable Isotope Analysis
3. Results
3.1. Monthly Occurrence
3.2. Age and Growth
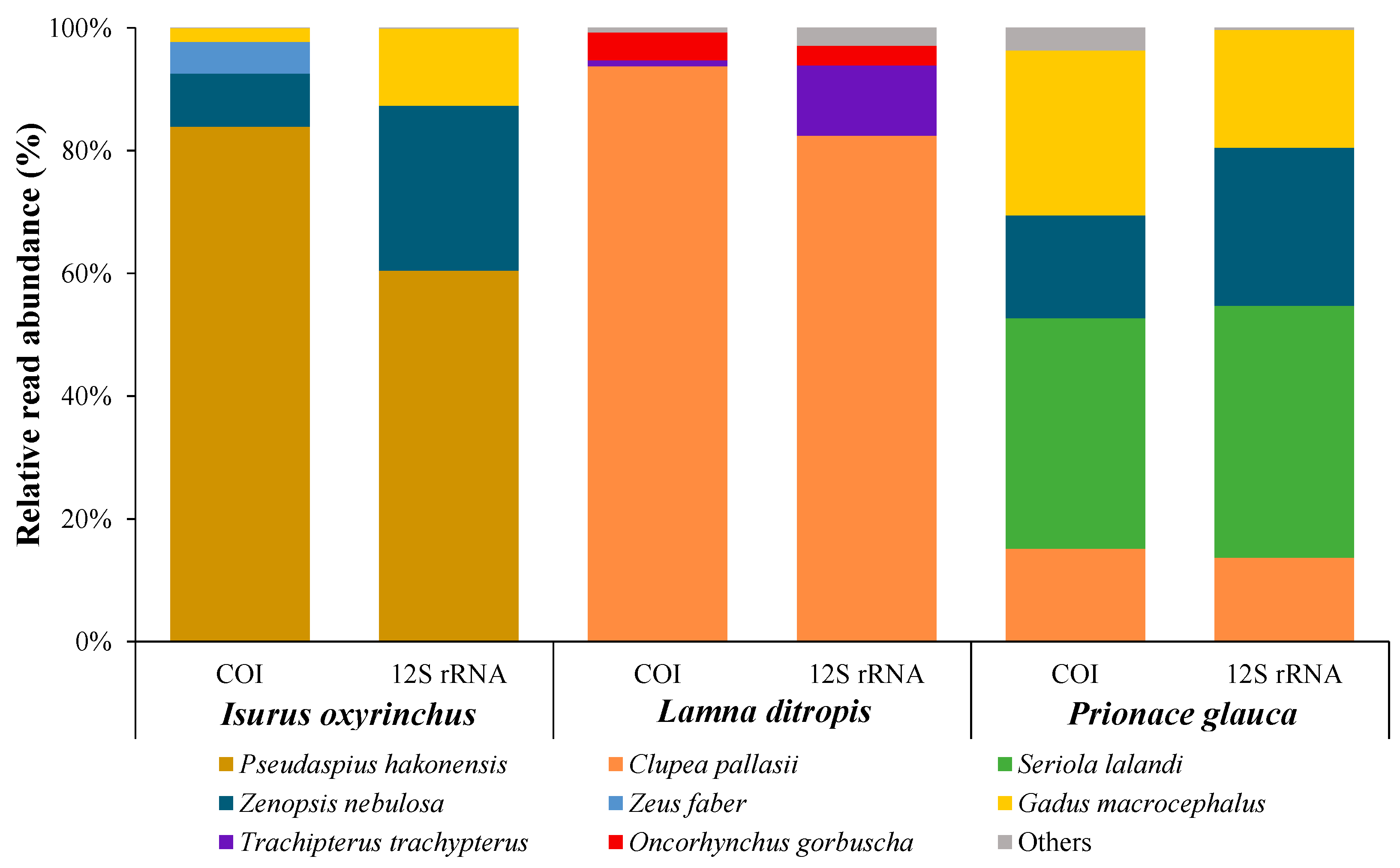
3.3. Diet Analysis
4. Discussion
4.1. Occurrence Patterns
4.2. Age and Growth Characteristics
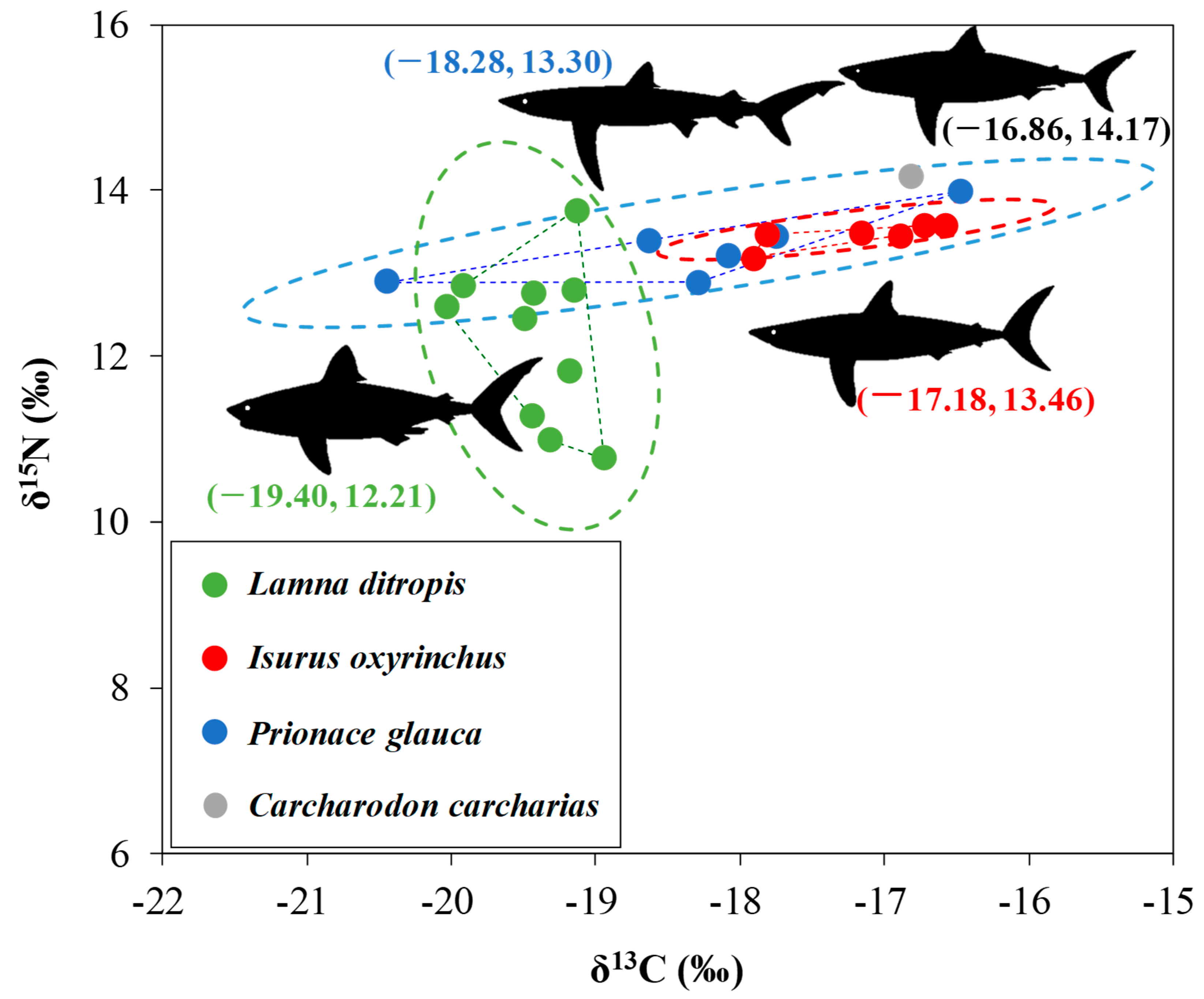
4.3. Feeding Ecology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Date | Gear | Species | Location | Total Length (cm) | Body Weight (kg) | Sex |
|---|---|---|---|---|---|---|---|
| 1 | 7 February 2024 | Set net | BS | Uljin | 126.6 | 7.0 | M |
| 2 | 21 March 2024 | Set net | SS | Uljin | 200.0 | 96.6 | F |
| 3 | 24 March 2024 | NA | SS | Goseong | NA | NA | NA |
| 4 | 25 March 2024 | Set net | SS | Goseong | 170.1 | 43.4 | M |
| 5 | 25 March 2024 | Set net | SS | Goseong | 182.5 | 62.5 | M |
| 6 | 25 March 2024 | Set net | SS | Goseong | 169.0 | 43.8 | M |
| 7 | 22 April 2024 | Set net | SM | Sokcho | 295.0 | 160.0 | NA |
| 8 | 26 April 2024 | Set net | SM | Donghae | 247.0 | 92.0 | F |
| 9 | 7 May 2024 | Set net | SM | Uljin | 253.0 | 113.7 | F |
| 10 | 25 May 2024 | Set net | WS | Uljin | 205.0 | 76.2 | F |
| 11 | 31 May 2024 | Set net | SS | Sokcho | 243.0 | 150.0 | F |
| 12 | 4 June 2024 | Gill net | BS | Ulsan | NA | NA | NA |
| 13 | 11 June 2024 | Set net | BS | Gangneung | 295.5 | 122.3 | F |
| 14 | 24 June 2024 | Gill net | SS | Samcheok | 257.0 | 186.6 | F |
| 15 | 26 June 2024 | Set net | SS | Uljin | 262.0 | 168.4 | F |
| 16 | 28 June 2024 | Trawl | SM | Uljin | NA | NA | NA |
| 17 | 2 July 2024 | Set net | BS | Uljin | 201.0 | 35.4 | M |
| 18 | 8 July 2024 | Set net | SS | Uljin | 223.0 | 124.3 | F |
| 19 | 10 July 2024 | Set net | BS | Gangneung | 261.0 | 96.0 | M |
| 20 | 14 July 2024 | Set net | SM | Goseong | 100.0 | 7.5 | NA |
| 21 | 17 July 2024 | Gill net | SS | Uljin | 183.0 | 87.3 | M |
| 22 | 19 July 2024 | Set net | SM | Samcheok | 126.0 | NA | NA |
| 23 | 19 July 2024 | Set net | SM | Samcheok | 120.0 | NA | NA |
| 24 | 19 July 2024 | Set net | SM | Samcheok | 112.0 | NA | NA |
| 25 | 20 July 2024 | Set net | SS | Donghae | NA | NA | NA |
| 26 | 20 July 2024 | Set net | BS | Donghae | NA | NA | NA |
| 27 | 22 July 2024 | Set net | SM | Uljin | 254.0 | 70.4 | F |
| 28 | 22 July 2024 | Set net | SM | Samcheok | 121.0 | NA | NA |
| 29 | 25 July 2024 | Set net | CS | Uljin | 77.9 | 2.4 | F |
| 30 | 25 July 2024 | Set net | SM | Yeongdeok | NA | NA | NA |
| 31 | 26 July 2024 | Set net | SS | Yeongdeok | 232.4 | 112.2 | M |
| 32 | 30 July 2024 | Set net | SS | Gangneung | 228.0 | 142.4 | F |
| 33 | 5 August 2024 | Set net | BS | Uljin | 262.0 | 78.4 | M |
| 34 | 7 August 2024 | Set net | BS | Uljin | 317.2 | 128.9 | M |
| 35 | 17 August 2024 | Fishing | SM | Samcheok | 320.0 | NA | NA |
| 36 | 27 August 2024 | Gill net | BS | Uljin | 230.0 | 55.8 | M |
| 37 | 10 September 2024 | Set net | SH | Uljin | 179.0 | 21.8 | F |
| 38 | 11 September 2024 | Set net | SM | Uljin | 100.0 | 9.0 | F |
| 39 | 13 September 2024 | Fishing | SM | Samcheok | NA | NA | NA |
| 40 | 14 October 2024 | Set net | SM | Uljin | 302.0 | 226.9 | F |
| 41 | 16 October 2024 | Set net | SM | Uljin | 296.0 | 184.4 | F |
| 42 | 18 October 2024 | Set net | SM | Uljin | 300.0 | 229.5 | F |
| 43 | 31 October 2024 | Gill net | SM | Uljin | NA | NA | NA |
| 44 | 12 November 2024 | Gill net | SS | Donghae | 242.0 | 114.5 | M |
References
- Motivarash, Y.B.; Fofandi, D.C.; Dabhi, R.M.; Makrani, R.A.; Tanna, P.D. Importance of sharks in ocean ecosystem. J. Entomol. Zool. Stud. 2020, 8, 611–613. [Google Scholar]
- Bradai, M.N.; Saidi, B.; Enajjar, S. Overview on Mediterranean shark’s fisheries: Impact on the biodiversity. In Marine Ecology-Biotic and Abiotic Interactions; Türkoğlu, M., Önal, U., Ismen, A., Eds.; InTech: London, UK, 2018; pp. 211–230. [Google Scholar]
- Hewindati, Y.T.; Novita Sari, D. Effect of life history on Alopias pelagicus overexploitation vulnerability: A literature review. Leuser J. Environ. Stud. 2023, 1, 39–46. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Simpfendorfer, C.A.; Davidson, L.N.K.; Fordham, S.V.; Bräutigam, A.; Sant, G.; Welch, D.J. Challenges and priorities in shark and ray conservation. Curr. Biol. 2017, 27, R565–R572. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787.e8. [Google Scholar] [CrossRef]
- Niella, Y.; Butcher, P.; Holmes, B.; Barnett, A.; Harcourt, R. Forecasting intraspecific changes in distribution of a wide-ranging marine predator under climate change. Oecologia 2022, 198, 111–124. [Google Scholar] [CrossRef]
- Clarke, S.; Yokawa, K.; Matsunaga, H.; Nakano, H. Analysis of North Pacific Shark Data from Japanese Commercial Longline and Research/Training Vessel Records; Western and Central Pacific Fisheries Commission: Pohnpei, Micronesia, 2011. [Google Scholar]
- Ohshimo, S.; Fujinami, Y.; Shiozaki, K.; Kai, M.; Semba, Y.; Katsumata, N.; Ochi, D.; Matsunaga, H.; Minami, H.; Kiyota, M.; et al. Distribution, body length, and abundance of blue shark and shortfin mako offshore of northeastern Japan, as determined from observed pelagic longline data, 2000–2014. Fish. Oceanogr. 2016, 25, 259–276. [Google Scholar] [CrossRef]
- Tsai, W.-P.; Sun, C.-L.; Liu, K.-M.; Wang, S.-B.; Lo, N.C.H. CPUE standardization and catch estimate of blue shark by Taiwanese large-scale tuna longline fishery in the North Pacific Ocean. J. Mar. Sci. Technol. 2015, 23, 21. [Google Scholar] [CrossRef]
- Simpfendorfer, C.A.; Heupel, M.R.; White, W.T.; Dulvy, N.K. The importance of research and public opinion to conservation management of sharks and rays: A synthesis. Mar. Freshw. Res. 2011, 62, 518–527. [Google Scholar] [CrossRef]
- Mullins, L.L.; Drymon, J.M.; Moore, M.; Skarke, A.; Moore, A.; Rodgers, J.C. Defining distribution and habitat use of west--central Florida’s coastal sharks through a research and education program. Ecol. Evol. 2021, 11, 16055–16069. [Google Scholar] [CrossRef]
- Park, J.; Yim, J.; Nam, J.; Suh, Y.-C. Comparative analysis of changes in fish catches by species and sea surface temperature change in coastal waters of the Republic of Korea. J. Coast. Res. 2024, 116, 270–273. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kim, H.C.; Ryu, J.-H.; Ahn, J.-S. Preliminary study on spatio-temporal variations of five giant and 17 large fish species around the Korean peninsula from 2011 to 2016. Fish. Aquat. Sci. 2022, 25, 298–310. [Google Scholar] [CrossRef]
- Huh, S.-H.; Park, J.M.; Park, S.C.; Kim, J.H.; Baeck, G.W. Feeding habits of 6 shark species in the Southern Sea of Korea. Korean J. Fish. Aquat. Sci. 2010, 43, 254–261. [Google Scholar] [CrossRef]
- Choi, Y. Distribution of the white shark, Carcharodon carcharias and other sharks around the Korean waters. Korean J. Ichthyol. 2009, 21, 44–51. [Google Scholar]
- Lee, W.-J.; Kim, J.-K. First reliable record of Echinorhinus cookei (Chondrichthyes: Elasmobranchii) collected from Busan, Korea. Korean J. Fish. Aquat. Sci. 2018, 51, 595–599. [Google Scholar] [CrossRef]
- Choi, Y.; Im, M.Y. Two unrecorded species of sharks in Korean waters. Korean J. Fish. Aquat. Sci. 2020, 53, 965–967. [Google Scholar] [CrossRef]
- Kim, J.-G.; Choi, Y. Taxonomic system of sharks (Chondrichthyes: Elasmobranchii) in Korean waters. Korean J. Ichthyol. 2024, 36, 84–93. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.-G.; Im, Y.; Kim, H. Pictorial Guidebook to Sharks of Korean Coastal Waters; National Institute of Fisheries Science: Busan, Republic of Korea, 2022. [Google Scholar]
- Kock, A.A.; Photopoulou, T.; Durbach, I.; Mauff, K.; Meÿer, M.; Kotze, D.; Griffiths, C.L.; O’Riain, M.J. Summer at the beach: Spatio-temporal patterns of white shark occurrence along the inshore areas of False Bay, South Africa. Mov. Ecol. 2018, 6, 7. [Google Scholar] [CrossRef]
- De Wysiecki, A.M.; Sánchez-Carnero, N.; Irigoyen, A.J.; Milessi, A.C.; Colonello, J.H.; Bovcon, N.D.; Cortés, F.; Barbini, S.A.; Cedrola, P.V.; Coller, N.M.; et al. Using temporally explicit habitat suitability models to infer the migratory pattern of a large mobile shark. Can. J. Fish. Aquat. Sci. 2020, 77, 1529–1539. [Google Scholar] [CrossRef]
- Link, J.S. Ecological considerations in fisheries management: When does it matter? Fisheries 2002, 27, 10–17. [Google Scholar] [CrossRef]
- Flinn, S.A.; Midway, S.R. Trends in growth modeling in fisheries science. Fishes 2021, 6, 1. [Google Scholar] [CrossRef]
- Skomal, G.B.; Natanson, L.J. Age and growth of the blue shark (Prionace glauca) in the North Atlantic Ocean. Fish. Bull.-Natl. Ocean. Atmos. Adm. 2003, 101, 627–639. [Google Scholar]
- Bishop, S.D.H.; Francis, M.P.; Duffy, C.; Montgomery, J.C. Age, growth, maturity, longevity and natural mortality of the shortfin mako shark (Isurus oxyrinchus) in New Zealand waters. Mar. Freshw. Res. 2006, 57, 143–154. [Google Scholar] [CrossRef]
- Goldman, K.J.; Musick, J.A. Growth and maturity of salmon sharks (Lamna ditropis) in the eastern and western North Pacific, and comments on back-calculation methods. Fish. Bull. 2006, 104, 278. [Google Scholar]
- Goldman, K.J.; Cailliet, G.M.; Andrews, A.H.; Natanson, L.J. Assessing the age and growth of chondrichthyan fishes. Biol. Sharks Their Relat. 2012, 13, 423. [Google Scholar]
- Huxley, J.S. Problems of Relative Growth; Dover Publishing: New York, NY, USA, 1932. [Google Scholar]
- von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Walford, L.A. A new graphic method of describing the growth of animals. Biol. Bull. 1946, 90, 141–147. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 10 September 2025).
- Kim, I.S.; Choi, Y.; Lee, C.L.; Lee, Y.J.; Kim, B.J.; Kim, J.H. Illustrated Book of Korean Fishes; Kyo-Hak Publ: Seoul, Republic of Korea, 2005; 615p. [Google Scholar]
- Kim, J.K.; Ryu, J.H.; Kwun, H.J.; Ji, H.S.; Park, J.H.; Myoung, S.H.; Song, Y.S.; Lee, S.J.; Yu, H.J.; Bae, S.E.; et al. Distribution Map of Sea Fishes in the Korean Peninsula; Mapledesign Publ: Busan, Republic of Korea, 2019; 541p. [Google Scholar]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Food habits of albacore, bluefin tuna, and bonito in California waters. Fish. Bull. 1971, 152, 1–105. [Google Scholar]
- Tournayre, O.; Leuchtmann, M.; Filippi--Codaccioni, O.; Trillat, M.; Piry, S.; Pontier, D.; Charbonnel, N.; Galan, M. In silico and empirical evaluation of twelve metabarcoding primer sets for insectivorous diet analyses. Ecol. Evol. 2020, 10, 6310–6332. [Google Scholar] [CrossRef]
- Tabassum, N.; Lee, J.-H.; Lee, S.-R.; Kim, J.-U.; Park, H.; Kim, H.-W.; Kim, J.-H. Molecular diet analysis of Adélie penguins (Pygoscelis adeliae) in the Ross Sea using fecal DNA. Biology 2022, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Santos, C.C.; Domingo, A.; Carlson, J.; Natanson, L.J.; Travassos, P.; Macías, D.; Cortés, E.; Miller, P.; Hazin, F.; Mas, F.; et al. Movements, habitat use, and diving behavior of shortfin mako in the Atlantic Ocean. Front. Mar. Sci. 2021, 8, 686343. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Broderick, A.C.; Coker, J.W.; Coyne, M.S.; Dodd, M.; Frick, M.G.; Godfrey, M.H.; Godley, B.J.; Griffin, D.B.; Hartog, K.; et al. Evaluating the landscape of fear between apex predatory sharks and mobile sea turtles across a large dynamic seascape. Ecology 2015, 96, 2117–2126. [Google Scholar] [CrossRef]
- Byrne, M.E.; Vaudo, J.J.; Harvey, G.C.M.; Johnston, M.W.; Wetherbee, B.M.; Shivji, M. Behavioral response of a mobile marine predator to environmental variables differs across ecoregions. Ecography 2019, 42, 1569–1578. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Calò, A.; Di Franco, A.; Milisenda, G.; Aglieri, G.; Cattano, C.; Milazzo, M.; Guidetti, P. Small-scale fisheries catch more threatened elasmobranchs inside partially protected areas than in unprotected areas. Nat. Commun. 2022, 13, 4381. [Google Scholar] [CrossRef]
- Sims, D.W.; Humphries, N.E.; Bradford, R.W.; Bruce, B.D. Lévy flight and Brownian search patterns of a free--ranging predator reflect different prey field characteristics. J. Anim. Ecol. 2012, 81, 432–442. [Google Scholar] [CrossRef]
- Nasby-Lucas, N.; Dewar, H.; Sosa-Nishizaki, O.; Wilson, C.; Hyde, J.R.; Vetter, R.D.; Wraith, J.; Block, B.A.; Kinney, M.J.; Sippel, T.; et al. Movements of electronically tagged shortfin mako sharks (Isurus oxyrinchus) in the eastern North Pacific Ocean. Anim. Biotelemetry 2019, 7, 12. [Google Scholar] [CrossRef]
- Smoothey, A.F.; Lee, K.A.; Peddemors, V.M. Long-term patterns of abundance, residency and movements of bull sharks (Carcharhinus leucas) in Sydney Harbour, Australia. Sci. Rep. 2019, 9, 18864. [Google Scholar] [CrossRef]
- Balchin, G.P.; Schuller, A.; Di Stefano, I.; Robertson, M.; Pollard, K.; Hughes, W.O.H. Seasonality, long-term trends and co-occurrence of sharks in a top predator assemblage. PLoS ONE 2025, 20, e0318011. [Google Scholar] [CrossRef]
- Carreón-Zapiain, M.T.; Favela-Lara, S.; González-Pérez, J.O.; Tavares, R.; Leija-Tristán, A.; Mercado-Hernández, R.; Compeán-Jiménez, G.A. Size, age, and spatial–temporal distribution of shortfin mako in the Mexican Pacific Ocean. Mar. Coast. Fish. 2018, 10, 402–410. [Google Scholar] [CrossRef]
- Ribot-Carballal, M.C.; Galván-Magaña, F.; Quiñónez-Velázquez, C. Age and growth of the shortfin mako shark, Isurus oxyrinchus, from the western coast of Baja California Sur, Mexico. Fish. Res. 2005, 76, 14–21. [Google Scholar] [CrossRef]
- Carlson, J.; Passerotti, M.; McCandless, C. Age, growth and maturity of blue shark (Prionace glauca) in the northwest Atlantic Ocean. Collect. Vol. Sci. Pap. ICCAT 2023, 80, 269–283. [Google Scholar]
- Cerna, F.; Licandeo, R. Age and growth of the shortfin mako (Isurus oxyrinchus) in the south-eastern Pacific off Chile. Mar. Freshw. Res. 2009, 60, 394–403. [Google Scholar] [CrossRef]
- Joung, S.-J.; Lyu, G.-T.; Hsu, H.-H.; Liu, K.-M.; Wang, S.-B. Age and growth estimates of the blue shark Prionace glauca in the central South Pacific Ocean. Mar. Freshw. Res. 2018, 69, 1346–1354. [Google Scholar] [CrossRef]
- Semba, Y.; Aoki, I.; Yokawa, K. Size at maturity and reproductive traits of shortfin mako, Isurus oxyrinchus, in the western and central North Pacific. Mar. Freshw. Res. 2011, 62, 20–29. [Google Scholar] [CrossRef]
- Carrera-Fernández, M.; Galván-Magaña, F.; Ceballos-Vázquez, B.P. Reproductive biology of the blue shark Prionace glauca (Chondrichthyes: Carcharhinidae) off Baja California Sur, México. aqua 2010, 16, 101–110. [Google Scholar]
- Montealegre-Quijano, S.; Cardoso, A.T.C.; Silva, R.Z.; Kinas, P.G.; Vooren, C.M. Sexual development, size at maturity, size at maternity and fecundity of the blue shark Prionace glauca (Linnaeus, 1758) in the Southwest Atlantic. Fish. Res. 2014, 160, 18–32. [Google Scholar] [CrossRef]
- Bethea, D.; Buckel, J.; Carlson, J. Foraging ecology of the early life stages of four sympatric shark species. Mar. Ecol. Prog. Ser. 2004, 268, 245–264. [Google Scholar] [CrossRef]
- Alonso, H.; Granadeiro, J.P.; Waap, S.; Xavier, J.; Symondson, W.O.C.; Ramos, J.A.; Catry, P. An holistic ecological analysis of the diet of Cory’s shearwaters using prey morphological characters and DNA barcoding. Mol. Ecol. 2014, 23, 3719–3733. [Google Scholar] [CrossRef] [PubMed]
- Collie, J.S.; Spencer, P.D. Modeling predator–prey dynamics in a fluctuating environment. Can. J. Fish. Aquat. Sci. 1994, 51, 2665–2672. [Google Scholar] [CrossRef]
- Arnés-Urgellés, C.; Salinas-de-León, P.; Rastoin-Laplane, E.; Vaca-Pita, L.; Suárez-Moncada, J.; Páez-Rosas, D. The effects of climatic variability on the feeding ecology of the scalloped hammerhead shark (Sphyrna lewini) in the tropical Eastern Pacific. Front. Mar. Sci. 2021, 8, 625748. [Google Scholar] [CrossRef]
- Bird, C.S.; Veríssimo, A.; Magozzi, S.; Abrantes, K.G.; Aguilar, A.; Al-Reasi, H.; Barnett, A.; Bethea, D.M.; Biais, G.; Borrell, A.; et al. A global perspective on the trophic geography of sharks. Nat. Ecol. Evol. 2018, 2, 299–305. [Google Scholar] [CrossRef]
- Zhang, C.I.; Kim, S.; Gunderson, D.; Marasco, R.; Lee, J.B.; Park, H.W.; Lee, J.H. An ecosystem-based fisheries assessment approach for Korean fisheries. Fish. Res. 2009, 100, 26–41. [Google Scholar] [CrossRef]
- Adams, K.R.; Gibbs, L.; Knott, N.A.; Broad, A.; Hing, M.; Taylor, M.D.; Davis, A.R. Coexisting with sharks: A novel, socially acceptable and non-lethal shark mitigation approach. Sci. Rep. 2020, 10, 17497. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.F.; Banks, S.C.; Crewe, T.L.; Campbell, H.A. The rise of animal biotelemetry and genetics research data integration. Ecol. Evol. 2023, 13, e9885. [Google Scholar] [CrossRef] [PubMed]

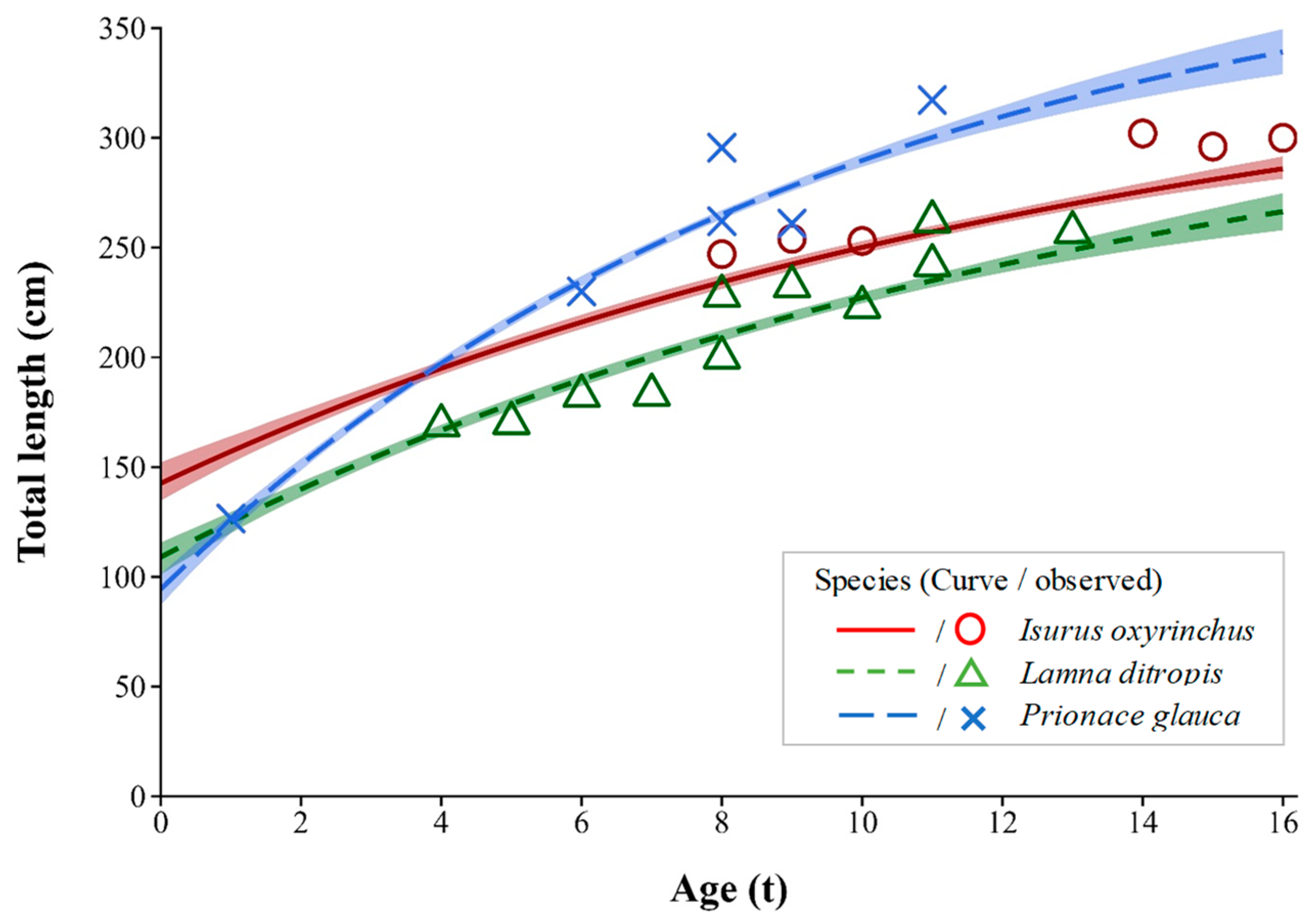
| Species | n | Estimates of Parameters | 95% Confidence Intervals | ||||
|---|---|---|---|---|---|---|---|
| L∞ | K | t0 | L∞ | K | t0 | ||
| Isurus oxyrinchus | 6 | 352.6 | 0.072 | −7.23 | 320.3–432.6 | 0.043–0.098 | −10.07–−5.57 |
| Lamna ditropis | 11 | 338.3 | 0.073 | −5.37 | 295.5–431.2 | 0.043–0.103 | −7.07–−4.07 |
| Prionace glauca | 6 | 395.8 | 0.104 | −2.61 | 365.5–439.2 | 0.083–0.127 | −3.16–−2.15 |
| Species | Isurus oxyrinchus | Lamna ditropis | Prionace glauca | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prey organism | %F | %N | %W | IRI | %IRI | %F | %N | %W | IRI | %IRI | %F | %N | %W | IRI | %IRI | |
| Pisces | 100.0 | 96.0 | 99.99 | 19,599.4 | 99.6 | 80.0 | 31.6 | 95.8 | 10,190.4 | 73.7 | 83.3 | 67.6 | 99.8 | 13,954.9 | 90.1 | |
| Aptocyclus ventricosus | 10.0 | 1.3 | 9.1 | |||||||||||||
| Clupea pallasii | 20.0 | 10.5 | 8.6 | 16.7 | 23.5 | 41.9 | ||||||||||
| Gadus macrocephalus | 50.0 | 14.7 | 21.4 | |||||||||||||
| Gymnocanthus herzensteini | 16.7 | 5.9 | 7.1 | |||||||||||||
| Hippoglossoides pinetorum | 20.0 | 4.0 | 3.8 | |||||||||||||
| Monacanthidae | 20.0 | 4.0 | 0.9 | |||||||||||||
| Pleuronectidae | 16.7 | 11.8 | 2.9 | |||||||||||||
| Salmonidae | 20.0 | 8.0 | 34.4 | |||||||||||||
| Scomber japonicus | 10.0 | 1.3 | 1.2 | |||||||||||||
| Seriola quinqueradiata | 16.7 | 5.9 | 10.3 | |||||||||||||
| Trachipterus ishikawae | 10.0 | 1.3 | 53.7 | |||||||||||||
| Zeidae | 20.0 | 4.0 | 40.3 | 16.7 | 2.9 | 16.1 | ||||||||||
| Unidentified Pisces | 60.0 | 76.0 | 20.6 | 60.0 | 17.1 | 23.2 | 16.7 | 2.9 | 0.2 | |||||||
| Cephalopoda | 20.0 | 4.0 | + | 80.1 | 0.4 | 50.0 | 68.4 | 4.2 | 3631.0 | 26.3 | 50.0 | 29.4 | 0.1 | 1477.4 | 9.5 | |
| Todarodes pacificus | 10.0 | 1.3 | 2.3 | |||||||||||||
| Watasenia scintillans | 16.7 | 2.9 | 0.1 | |||||||||||||
| Unidentified Cephalopoda | 20.0 | 4.0 | + | 50.0 | 67.1 | 1.9 | 50.0 | 26.5 | + | |||||||
| Anomura | 16.7 | 2.9 | 0.1 | 49.9 | 0.3 | |||||||||||
| Total | 100.0 | 100.0 | 19,679.5 | 100.0 | 100.0 | 100.0 | 13,821.4 | 100.0 | 100.0 | 100.0 | 15,482.2 | 100.0 | ||||
| Comparison | COI | 12S rRNA |
|---|---|---|
| Isurus oxyrinchus vs. Lamna ditropis | 0.0000 | 0.0001 |
| Isurus oxyrinchus vs. Prionace glauca | 0.1094 | 0.3808 |
| Lamna ditropis vs. Prionace glauca | 0.1565 | 0.1369 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, G.C.; Baek, J.-I.; Gal, J.-K.; Lee, S.-K.; Shim, J.-M.; Kim, M.-J. Ecological Characteristics of Large-Bodied Sharks in the East Sea of Korea. Animals 2025, 15, 2974. https://doi.org/10.3390/ani15202974
Seong GC, Baek J-I, Gal J-K, Lee S-K, Shim J-M, Kim M-J. Ecological Characteristics of Large-Bodied Sharks in the East Sea of Korea. Animals. 2025; 15(20):2974. https://doi.org/10.3390/ani15202974
Chicago/Turabian StyleSeong, Gi Chang, Jeong-Ik Baek, Jong-Ku Gal, Sun-Kil Lee, Jeong-Min Shim, and Maeng-Jin Kim. 2025. "Ecological Characteristics of Large-Bodied Sharks in the East Sea of Korea" Animals 15, no. 20: 2974. https://doi.org/10.3390/ani15202974
APA StyleSeong, G. C., Baek, J.-I., Gal, J.-K., Lee, S.-K., Shim, J.-M., & Kim, M.-J. (2025). Ecological Characteristics of Large-Bodied Sharks in the East Sea of Korea. Animals, 15(20), 2974. https://doi.org/10.3390/ani15202974




