Orthopaedic Injuries in 272 Dressage Horses: A Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Orthopaedic Examinations
2.3. Diagnostic Anaesthesia
2.4. Diagnostic Imaging
2.5. Injury Classification
2.5.1. Forelimb and Hindlimb Pain
2.5.2. Injury Type
- Osteoarthritis based on definitive radiological abnormalities [21] or the presence of osteophytes, periarticular new bone formation or modelling detected using ultrasonography (sacroiliac and lumbosacral joints);
- Soft tissue based on ultrasonographic abnormalities of ligaments, tendons, or muscles;
- Bone when osseous tissue was considered the primary source of pain;
- Developmental disease, including osteochondrosis and compression of incompletely ossified bones, and periarticular modelling (for example, osteophytes or entheseophytes) in horses less than four years of age.
2.5.3. Foot Pain
2.5.4. Cervicothoracolumbosacral Region
2.6. Treatment and Outcome
2.7. Data Analysis
3. Results
3.1. Horse Signalment and Work Level
3.2. Orthopaedic Examination
3.2.1. Reason for Initial Examination and Lameness Grades
3.2.2. Distribution of Lame Limbs
3.2.3. Neurological Dysfunction
3.2.4. Ridden Exercise
3.3. Anatomical Site of Injury
3.3.1. Foot Pain
3.3.2. Fetlock, Metacarpal and Metatarsal Region Injuries
3.3.3. Spinal Injuries
3.3.4. Neurological Dysfunction
3.4. Osteoarthritis, Soft Tissue Injury, Bone Injury and Developmental Disease
3.5. Follow-Up Outcome
3.5.1. Anatomical Site of Injury and Follow-Up Outcome
3.5.2. Type of Injury and Follow-Up Outcome
3.5.3. Neurological Dysfunction and Follow-Up Outcome
4. Discussion
4.1. Results Related to Hypotheses
4.1.1. Multifocal and Multilimbed Lameness
Management and Prevention of Multifocal and Multilimbed Injuries
4.1.2. Breed Differences
4.1.3. Paddock Turnout
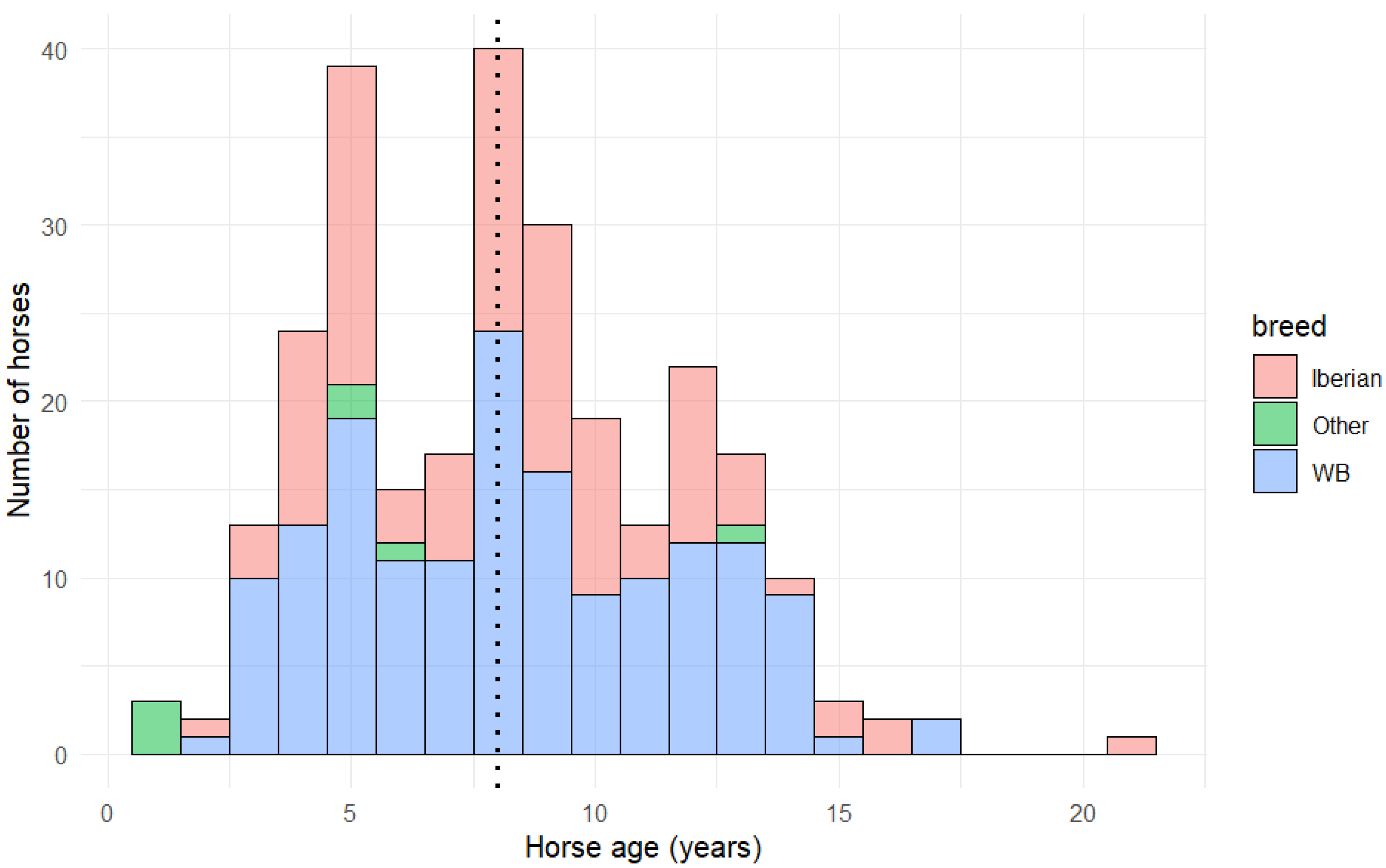
4.2. Horse Age
4.3. Distribution of Sources of Pain and Prevalence
4.3.1. Foot Pain
4.3.2. Suspensory Ligament Injury
4.3.3. Spinal Pain
4.3.4. Neurological Dysfunction
4.4. Prevention of Injury
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaneene, J.B.; Saffell, M.; Fedewa, D.J.; Gallagher, K.; Chaddock, H.M. The Michigan Equine Monitoring System. I. Design, Implementation and Population Estimates. Prev. Vet. Med. 1997, 29, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.C.; Dyson, S.J.; Tranquille, C.; Adams, V. Association of Type of Sport and Performance Level with Anatomical Site of Orthopaedic Injury Diagnosis. Equine Vet. J. 2006, 36 (Suppl. S36), 411–416. [Google Scholar] [CrossRef]
- Murray, R.C.; Walters, J.M.; Snart, H.; Dyson, S.J.; Parkin, T.D.H. Identification of Risk Factors for Lameness in Dressage Horses. Vet. J. 2010, 184, 27–36. [Google Scholar] [CrossRef]
- Murray, R.; Walters, J.; Snart, S.; Dyson, S.; Parkin, T. How do features of dressage arenas influence training surface properties which are potentially associated with lameness? Vet. J. 2010, 186, 172–179. [Google Scholar] [CrossRef]
- Tans, E.; Nauwelaerts, S.; Clayton, H.M. Dressage Training Affects Temporal Variables in Transitions between Trot and Halt. Comp. Exerc. Physiol. 2009, 6, 89–97. [Google Scholar] [CrossRef][Green Version]
- Clayton, H.M.; Hobbs, S.J.; Rhodin, M.; Hernlund, E.; Peterson, M.; Bos, R.; Bragança, F.S. Vertical Movement of Head, Withers, and Pelvis of High-Level Dressage Horses Trotting in Hand vs. Being Ridden. Animals 2025, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Holmström, M.; Fredricson, I.; Drevemo, S. Biokinematic Effects of Collection on the Trotting Gaits in the Elite Dressage Horse. Equine Vet. J. 1995, 27, 281–287. [Google Scholar] [CrossRef]
- Kold, S.; Dyson, S. Lameness in the dressage horse. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 1112–1123. [Google Scholar]
- Buthe, A. Dressage. In Essential Facts of Equine Physical Therapy, Rehabilitation and Sports Medicine, 1st ed.; Marques, J.P., Ed.; VBS GmbH: Babenhausen, Germany, 2024; pp. 759–762. [Google Scholar]
- Denoix, J.-M. The neck and trunk. In Biomechanics and Physical Training of the Horse, 1st ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2013; pp. 39–50. ISBN 978042910445. [Google Scholar]
- Rhodin, M.; Gómez Álvarez, C.B.; Byström, A.; Johnston, C.; van Weeren, P.R.; Roepstorff, L.; Weishaupt, M.A. The Effect of Different Head and Neck Positions on the Caudal Back and Hindlimb Kinematics in the Elite Dressage Horse at Trot. Equine Vet. J. 2009, 41, 274–279. [Google Scholar] [CrossRef]
- Clayton, H.M.; Hobbs, S.J. A Review of Biomechanical Gait Classification with Reference to Collected Trot, Passage and Piaffe in Dressage Horses. Animals 2019, 9, 763. [Google Scholar] [CrossRef]
- Hobbs, S.J.; Serra Braganca, F.M.; Rhodin, M.; Hernlund, E.; Peterson, M.; Clayton, H.M. Evaluating Overall Performance in High-Level Dressage Horse–Rider Combinations by Comparing Measurements from Inertial Sensors with General Impression Scores Awarded by Judges. Animals 2023, 13, 2496. [Google Scholar] [CrossRef]
- Walker, V.A.; Tranquille, C.A.; Newton, J.R.; Dyson, S.J.; Brandham, J.; Northrop, A.J.; Murray, R.C. Comparison of Limb Kinematics between Collected and Lengthened (Medium/Extended) Trot in Two Groups of Dressage Horses on Two Different Surfaces. Equine Vet. J. 2017, 49, 673–680. [Google Scholar] [CrossRef]
- Féderation Equestre Internationale. Dressage Handbook Guidelines for Judging; Féderation Equestre Internationale: Laussane, Switzerland, 2007. [Google Scholar]
- Barrey, E.; Desliens, F.; Poirel, D.; Biau, S.; Lemaire, S.; Rivero, J.L.; Langlois, B. Early Evaluation of Dressage Ability in Different Breeds. Equine Vet. J. 2002, 34 (Suppl. S34), 319–324. [Google Scholar] [CrossRef]
- Naccacha, F.; Metzger, J.; Distl, O. Genetic risk factors for osteochondrosis in various horse breeds. Equine Vet. J. 2018, 50, 556–563. [Google Scholar] [CrossRef]
- Ramos, S.; Pinto, A.; Crespo, J.; Marques, J.P.; Bettencourt, E.; Gama, L.; Monteiro, S. Osteochondrosis (osteochondritis dissecans) in Lusitano Horses: Prevalence and characteristics. J. Equine Vet. Sci. 2022, 117, 104063. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Lobo, M.; Perdomo-González, D.I.; Azor, P.J.; Valera, M. Orthopedic Diseases in the Pura Raza Española Horse: The Prevalence and Genetic Parameters of Angular Hoof Deviations. Animals 2023, 13, 3471. [Google Scholar] [CrossRef]
- Boado, A.; Pollard, D.; Dyson, S. A Retrospective Study of the Evolution of Orthopaedic Injuries in 70 Dressage Horses. Animals 2025, 15, 1740. [Google Scholar] [CrossRef]
- Dyson, S.; Thomson, K.; Quiney, L.; Bondi, A.; Ellis, A.D. Can Veterinarians Reliably Apply a Whole Horse Ridden Ethogram to Differentiate Nonlame and Lame Horses Based on Live Horse Assessment of Behaviour? Equine Vet. Educ. 2020, 32, 112–120. [Google Scholar] [CrossRef]
- Ross, M.W. Palpation. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 43–63. ISBN 978-1-4160-6069-7. [Google Scholar]
- Hahn, C.; Masty, J. Overview of neuroanatomy. In Equine Neurology, 2nd ed.; Furr, M., Reed, S., Eds.; Wiley-Blackwell: Oxford, UK, 2015; pp. 3–20. [Google Scholar]
- Bentz, B. How to Evaluate Clinically Indistinct Gait Deficits to Differentiate Musculoskeletal and Neurological. In Proceedings of the 55th Convention of American Association of Equine Practitioners, Las Vegas, NV, USA, 5–9 December 2009; pp. 50–56. [Google Scholar]
- Bassage, L.H.; Ross, M.W. Diagnostic Analgesia. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 100–135. [Google Scholar]
- Butler, J.A.; Colles, C.M.; Dyson, S.J.; Kold, S.E.; Poulos, P.W. Clinical Radiology of the Horse, 4th ed.; Wiley-Blackwell: Oxford, UK, 2016; ISBN 9781118912263. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Eribaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Perneger, T.V. Adjusting for Multiple Testing in Studies Is Less Important than Other Concerns. BMJ Br. Med. J. 1999, 318, 1288. [Google Scholar] [CrossRef] [PubMed]
- Barstow, A.; Dyson, S. Clinical Features and Diagnosis of Sacroiliac Joint Region Pain in 296 Horses: 2004-2014. Equine Vet. Educ. 2015, 27, 637–647. [Google Scholar] [CrossRef]
- Gruyaert, M.; Pollard, D.; Dyson, S. Relative Heights of the Withers and the Tubera Sacrale and Angulation of the Lumbar and Pelvic Regions in Horses with Hindlimb Proximal Suspensory Desmopathy, Sacroiliac Joint Region Pain and Other Orthopaedic Injuries. Equine Vet. Educ. 2023, 35, e429–e437. [Google Scholar] [CrossRef]
- Parkes, R.S.; Richard Newton, J.; Dyson, S.J. An Investigation of Risk Factors for Foot-Related Lameness in a United Kingdom Referral Population of Horses. Vet. J. 2013, 196, 218–225. [Google Scholar] [CrossRef]
- Gruyaert, M.; Pollard, D.; Dyson, S.J. An Investigation into the Occurrence of, and Risk Factors for, Concurrent Suspensory Ligament Injuries in Horses with Hindlimb Proximal Suspensory Desmopathy. Equine Vet. Educ. 2020, 32, 173–182. [Google Scholar] [CrossRef]
- Owen, K.R.; Dyson, S.J.; Parkin, T.D.H.; Singer, E.R.; Kristoffersen, M.; Mair, T.S. Retrospective Study of Palmar/Plantar Annular Ligament Injury in 71 Horses: 2001–2006. Equine Vet. J. 2008, 40, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Marneris, D.; Dyson, S.J. Clinical Features, Diagnostic Imaging Findings and Concurrent Injuries in 71 Sports Horses with Suspensory Branch Injuries. Equine Vet. Educ. 2014, 26, 312–332. [Google Scholar] [CrossRef]
- Dyson, S.; Nagy, A.; Murray, R. Clinical and Diagnostic Imaging Findings in Horses with Subchondral Bone Trauma of the Sagittal Groove of the Proximal Phalanx. Vet. Radiol. Ultrasound 2011, 52, 596–604. [Google Scholar] [CrossRef]
- Lykkjen, S.; Roed, K.H.; Dolvik, N.I. Osteochondrosis and Osteochondral Fragments in Standardbred Trotters: Prevalence and Relationships. Equine Vet. J. 2012, 44, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Byam-Cook, K.L.; Singer, E.R. Is There a Relationship between Clinical Presentation, Diagnostic and Radiographic Findings and Outcome in Horses with Osteoarthritis of the Small Tarsal Joints? Equine Vet. J. 2009, 41, 118–123. [Google Scholar] [CrossRef]
- Smyth, E.A.; Newman, P.; Waddington, G.; Weissensteiner, J.R.; Drew, M.K. Injury Prevention Strategies Specific to Pre-Elite Athletes Competing in Olympic and Professional Sports—A Systematic Review. J. Sci. Med. Sport. 2019, 22, 887–901. [Google Scholar] [CrossRef]
- Dyson, S. Can Lameness Be Graded Reliably? Equine Vet. J. 2011, 43, 379–382. [Google Scholar] [CrossRef]
- Şahin, H.; Akalın, Y.; Çevik, N.; Avci, Ö.; Sağlıcak, H.; Öztürk, A. Dose-Independent Adverse Effects of Corticosteroid Injections on Rotator Cuff Healing in a Rat Model. Acta Orthop. Traumatol. Turc. 2025, 59, 100–104. [Google Scholar] [CrossRef]
- Buchner, H.H.F.; Savelberg, H.H.C.M.; Schamhardt, H.C.; Barneveld, A. Head and trunk movement adaptations in horses with experimentally induced fore or hind limb lameness. Equine Vet. J. 1996, 28, 71–76. [Google Scholar] [CrossRef]
- Weishaupt, M.A. Adaptation strategies of horses with lameness. Vet. Clin. N. Am. Equine Pract. 2007, 24, 79–100. [Google Scholar] [CrossRef]
- Plaas, A.; Sandy, J.D.; Liu, H.; Diaz, M.A.; Schenkman, D.; Magnus, R.P.; Bolam-Bretl, C.; Kopesky, P.W.; Wang, V.M.; Galante, J.O. Biochemical Identification and Immunolocalizaton of Aggrecan, ADAMTS5 and Inter-Alpha-Trypsin-Inhibitor in Equine Degenerative Suspensory Ligament Desmitis. J. Orthop. Res. 2011, 29, 900–906. [Google Scholar] [CrossRef]
- Momen, M.; Brounts, S.H.; Binversie, E.E.; Sample, S.J.; Rosa, G.J.M.; Davis, B.W.; Muir, P. Selection Signature Analyses and Genome-Wide Association Reveal Genomic Hotspot Regions That Reflect Differences between Breeds of Horse with Contrasting Risk of Degenerative Suspensory Ligament Desmitis. G3 Genes Genomes Genet. 2022, 12, jkac179. [Google Scholar] [CrossRef] [PubMed]
- Mero, J.L.; Scarlett, J.M. Diagnostic Criteria for Degenerative Suspensory Ligament Desmitis in Peruvian Paso Horses. J. Equine Vet. Sci. 2005, 25, 224–228. [Google Scholar] [CrossRef]
- Holmström, M.; Magnusson, L.-E.; Philipsson, J. Variation in Conformation of Swedish Warmblood Horses and Conformational Characteristics of Elite Sport Horses. Equine Vet. J. 1990, 22, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Van Weeren, P.R.; Crevier-Denoix, N. Equine Conformation: Clues to Performance and Soundness? Equine Vet. J. 2006, 38, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.W.; McIlwraith, C.W. Conformation and Lameness. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 15–32. ISBN 978-1-4160-6069-7. [Google Scholar]
- Holroyd, K.; Dixon, J.J.; Mair, T.; Bolas, N.; Bolt, D.M.; David, F.; Weller, R. Variation in Foot Conformation in Lame Horses with Different Foot Lesions. Vet. J. 2013, 195, 361–365. [Google Scholar] [CrossRef]
- Ripollés-Lobo, M.; Perdomo-González, D.I.; Azor, P.J.; Valera, M. Evaluation of Potential Effects and Genetic Parameters in Conformational Limb Defects in Pura Raza Española Horses. Ital. J. Anim. Sci. 2023, 22, 407–417. [Google Scholar] [CrossRef]
- Gómez, M.; Molina, A.; Sánchez-Guerro, M.; Valera, A. Prediction of adult conformation traits from shape characteristics of Pura Razo Española foals. Livestock Sci. 2021, 253, 104701. [Google Scholar] [CrossRef]
- Sánchez, M.J.; Azor, P.J.; Molina, A.; Parkin, T.; Rivero, J.L.; Valera, M. Prevalence, risk factors and genetic parameters of cresty neck in Pura Raza Español horses. Equine Vet. J. 2017, 49, 196–200. [Google Scholar] [CrossRef]
- Hall, C.; Kay, R. Living the good life? A systematic review of behavioural signs of affective state in the domestic horse (Equus caballus) and factors relating to quality of life. Part I: Fulfilment of species-specific needs. Anim. Welf. 2024, 33, e40. [Google Scholar] [CrossRef]
- Brenner, J.S.; Watson, A. Overuse Injuries, Overtraining, and Burnout in Young Athletes. Pediatrics 2024, 153, e2023065129. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Nibeyro, S.D.; White, N.A.; Werpy, N.M. Outcome of Medical Treatment for Horses with Foot Pain: 56 Cases. Equine Vet. J. 2010, 42, 680–685. [Google Scholar] [CrossRef]
- Biggi, M.; Dyson, S. High-Field Magnetic Resonance Imaging Investigation of Distal Border Fragments of the Navicular Bone in Horses with Foot Pain. Equine Vet. J. 2011, 43, 302–308. [Google Scholar] [CrossRef]
- Dyson, S.; Murray, R. Verification of Scintigraphic Imaging for Injury Diagnosis in 264 Horses with Foot Pain. Equine Vet. J. 2007, 39, 350–355. [Google Scholar] [CrossRef]
- Gutierrez-Nibeyro, S.; Werpy, N.; Gold, S.; Olguin, S.; Schaeffer, D. Standing MRI lesions of the distal interphalangeal joint and podotrochlear apparatus occur with a high frequency in Warmblood horses. Vet. Radiol. Ultrasound 2020, 61, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where Tendons and Ligaments Meet Bone: Attachment Sites (‘entheses’) in Relation to Exercise and/or Mechanical Load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.M. Functional Anatomy of Tendons and Ligaments in the Distal Limbs (Manus and Pes). Vet. Clin. N. Am. Equine Pract. 1994, 10, 273–322. [Google Scholar] [CrossRef]
- Minshall, G.; Wright, I. Arthroscopic diagnosis and treatment of intra-articular insertional injuries of the suspensory ligament branches in 18 horses. Equine Vet. J. 2006, 38, 10–14. [Google Scholar] [CrossRef]
- Penell, J.C.; Egenvall, A.; Bonnett, B.N.; Olson, P.; Pringle, J. Specific Causes of Morbidity among Swedish Horses Insured for Veterinary Care between 1997 and 2000. Vet. Rec. 2005, 157, 470–477. [Google Scholar] [CrossRef]
- Dyson, S. Proximal Suspensory Desmitis in the Hindlimb—42 Cases. Br. Vet. J. 1994, 150, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Murray, R. Management of hindlimb proximal suspensory desmopathy by neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy: 155 horses (2003–2008). Equine Vet. J. 2012, 44, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Routh, J.; Strang, C.; Gilligan, S.; Dyson, S. An Investigation of the Association between Hindlimb Conformation and Suspensory Desmopathy in Sports Horses. Equine Vet. Educ. 2020, 32, 183–192. [Google Scholar] [CrossRef]
- Leclercq, A.; Byström, A.; Söderlind, M.; Persson, E.; Rhodin, M.; Engell, M.T.; Hernlund, E. Evaluation of feedback methods for improved detection of hindlimb lameness in horses among riding instructors and trainers. Front. Vet. Sci. 2022, 9, 992954. [Google Scholar] [CrossRef]
- Butler, J.A.; Colles, C.M.; Dyson, S.J.; Kold, S.E.; Poulos, P.W. The vertebral column. In Clinical Radiology of the Horse, 4th ed.; Wiley-Blackwell: Oxford, UK, 2016; pp. 531–608. [Google Scholar]
- Espinosa-Mur, P.; Phillips, K.L.; Galuppo, L.D.; DeRouen, A.; Benoit, P.; Anderson, E.; Shaw, K.; Puchalski, S.; Peters, D.; Kass, P.H.; et al. Radiological Prevalence of Osteoarthritis of the Cervical Region in 104 Performing Warmblood Jumpers. Equine Vet. J. 2021, 53, 972–978. [Google Scholar] [CrossRef]
- Dyson, S.; Quiney, L.; Phillips, K.; Zheng, S.; Aleman, M. Radiological Abnormalities of the Cervicothoracic Vertebrae in Warmblood Horses with Primary Neck-Related Clinical Signs versus Controls. Vet. Radiol. Ultrasound 2024, 65, 755–768. [Google Scholar] [CrossRef]
- Dyson, S.; Phillips, K.; Zheng, S.; Aleman, M. Congenital Variants of the Ventral Laminae of the Sixth and Seventh Cervical Vertebrae Are Not Associated with Clinical Signs or Other Radiological Abnormalities of the Cervicothoracic Region in Warmblood Horses. Equine Vet. J. 2024, 57, 419–430. [Google Scholar] [CrossRef]
- Dyson, S.; Zheng, S.; Aleman, M. Primary Phenotypic Features Associated with Caudal Neck Pathology in Warmblood Horses. J. Vet. Intern. Med. 2024, 38, 2380–2390. [Google Scholar] [CrossRef]
- Sleutjens, J.; Voorhout, G.; Van Der Kolk, J.H.; Wijnberg, I.D.; Back, W. The Effect of Ex Vivo Flexion and Extension on Intervertebral Foramina Dimensions in the Equine Cervical Spine. Equine Vet. J. 2010, 42, 425–430. [Google Scholar] [CrossRef]
- MacKechnie-Guire, R.; Pfau, T. Differential Rotational Movement of the Thoracolumbosacral Spine in High-Level Dressage Horses Ridden in a Straight Line, in Sitting Trot and Seated Canter Compared to in-Hand Trot. Animals 2021, 11, 888. [Google Scholar] [CrossRef]
- García-López, J.M. Neck, Back, and Pelvic Pain in Sport Horses. Vet. Clin. N. Am. Equine Pract. 2018, 34, 235–251. [Google Scholar] [CrossRef]
- Nagy, A.; Dyson, S.; Barr, A. Ultrasonographic Findings in the Lumbosacral Joint of 43 Horses with No Clinical Signs of Back Pain or Hindlimb Lameness. Vet. Radiol. Ultrasound 2010, 51, 533–539. [Google Scholar] [CrossRef]
- Girodroux, M.; Dyson, S.; Murray, R. Osteoarthritis of the Thoracolumbar Synovial Intervertebral Articulations: Clinical and Radiographic Features in 77 Horses with Poor Performance and Back Pain. Equine Vet. J. 2009, 41, 130–138. [Google Scholar] [CrossRef]
- Stubbs, N. Epaxial musculature, motor control, and its relationship with back pain in the horse: Objective clinical physical therapy, pathological and imaging studies. In Proceedings of the 57th Annual Convention of the American Association of Equine Practitioners, San Antonio, TX, USA, 18–22 November 2012; pp. 153–157. [Google Scholar]
- Zimmerman, M.; Dyson, S.; Murray, R. Close, Impinging and Overriding Spinous Processes in the Thoracolubar Spine: The Relationship between Radiological and Scintigraphic Findings and Clinical Signs. Equine Vet. J. 2012, 44, 178–184. [Google Scholar] [CrossRef]
- Suryadevara, M.; Mishra, G.V.; Parihar, P.; Javvaji, C.K.; Sood, A.; Reddy, H.; Sudheesh Reddy, N.; Shelar, S.S.; Parihar, P.; Shelar, S.S. Role of End Plate Changes and Paraspinal Muscle Pathology in Lower Back Pain: A Narrative Review. Cureus 2024, 16, e61319. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, N.C.; Riggs, C.M.; Hodges, P.W.; Jeffcott, L.B.; Hodgson, D.R.; Clayton, H.M.; Mc Gowan, C.M. Osseous Spinal Pathology and Epaxial Muscle Ultrasonography in Thoroughbred Racehorses. Equine Vet. J. 2010, 42, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Townsend, H.G.G.; Leach, D.H.; Doige, C.E.; Kirkaldy-Willis, W.H. Relationship between Spinal Biomechanics and Pathological Changes in the Equine Thoracolumbar Spine. Equine Vet. J. 1986, 18, 107–112. [Google Scholar] [CrossRef]
- Stubbs, N.C.; Kaiser, L.J.; Hauptman, J.; Clayton, H.M. Dynamic Mobilisation Exercises Increase Cross Sectional Area of Musculus Multifidus. Equine Vet. J. 2011, 43, 522–529. [Google Scholar] [CrossRef]
- Stubbs, N.C.; Hodges, P.W.; Jeffcott, L.B.; Cowin, G.; Hodgson, D.R.; McGowan, C.M. Functional Anatomy of the Caudal Thoracolumbar and Lumbosacral Spine in the Horse. Equine Vet. J. 2006, 38, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Boado, A.; Nagy, A.; Dyson, S. Ultrasonographic Features Associated with the Lumbosacral or Lumbar 5–6 Symphyses in 64 Horses with Lumbosacral-Sacroiliac Joint Region Pain (2012–2018). Equine Vet. Educ. 2020, 32, 136–143. [Google Scholar] [CrossRef]
- Denoix, J.-M.; Jacquet, S. Diagnosis and Treatment of Lumbosacral and Sacroiliac Pain in Horses. In Proceedings of the 10th International Congress of World Equine Veterinary Association, Moscow, Russia, 28 January–1 February 2008; pp. 290–297. [Google Scholar]
- Tallaj, A.; Coudry, V.; Denoix, J.M. Transrectal Ultrasonographic Examination of the Sacroiliac Joints of the Horse: Technique and Normal Images. Equine Vet. Educ. 2019, 31, 666–671. [Google Scholar] [CrossRef]
- Tallaj, A.; Coudry, V.; Denoix, J.-M. Transrectal ultrasonographic examination of the sacroiliac joints of the horse: Abnormal findings and lesions. Equine Vet. Educ. 2020, 32, 33–38. [Google Scholar] [CrossRef]
- Denoix, J.-M.; Pailloux, J.-P. Physical Therapy and Massage for the Horse; Manson: London, UK, 1996; pp. 142–187. ISBN 1874545146/9781874545149/9782224018672/2224018673. [Google Scholar]
- Dyson, S. An Approach to the Sport Horse with Potential Thoracolumbar, Lumbosacral or Sacroiliac Joint Region Pain. In Proceedings of the American Association of Equine Practitioners, Focus Meeting on Lameness and Imaging, Fort Collins, CO, USA, 31 July 2007; pp. 142–148. [Google Scholar]
- McGowan, C.M.; Golland, L.C.; Evans, D.L.; Hodgson, D.R.; Rose, R.J. Effects of Prolonged Training, Overtraining and Detraining on Skeletal Muscle Metabolites and Enzymes. Equine Vet. J. 2002, 34 (Suppl. S34), 257–263. [Google Scholar] [CrossRef]
- Aicale, R.; Tarantino, D.; Maffulli, N. Overuse Injuries in Sport: A Comprehensive Overview. J. Orthop. Surg. Res. 2018, 13, 309. [Google Scholar] [CrossRef] [PubMed]
| Osteoarthritis |
|
| Subchondral Bone Injury |
|
| Large (≥50% of the total cross-sectional [CSA] area) core lesion of a tendon or ligament (non-synovial environment) |
|
| Small (>25% < 50% of CSA) core or diffuse lesion of a tendon or ligament |
|
| Tenosynovitis |
|
| Anatomical Area/Injury | Number | Percentage |
|---|---|---|
| Distal interphalangeal (DIP) joint osteoarthritis | 83 | 76.8 |
| Collateral desmitis of DIP joint | 18 | 16.7 |
| Ungular cartilages | 10 | 9.3 |
| Podotrochlear apparatus | 10 | 9.3 |
| Deep digital flexor tendon | 13 | 12.0 |
| Distal phalanx | 26 | 24.1 |
| Anatomical Structure | Number | Percentage |
|---|---|---|
| Forelimb suspensory (SL) ligament branch(es) | 16 | 5.9 |
| Forelimb proximal suspensory desmitis | 56 | 20.6 |
| Forelimb superficial digital flexor tendon | 15 | 5.2 |
| Forelimb accessory ligament of the deep digital flexor tendon | 5 | 1.8 |
| Hindlimb SL branch(es) | 16 | 5.9 |
| Hindlimb proximal suspensory desmopathy | 4 | 1.5 |
| Joint | Number of Horses | Percentage |
|---|---|---|
| DIP and PIP joints | 83 | 30.5 |
| MCP/MTP joints | 71 | 26.1 |
| Distal tarsal joints | 26 | 9.6 |
| Tarsocrural joint | 8 | 2.9 |
| Carpal joints | 8 | 2.9 |
| Femorotibial and femoropatellar joints | 21 | 7.7 |
| Caudal cervical articular process joints | 19 | 7.0 |
| Lumbosacroiliac joints | 36 | 13.2 |
| Anatomical Area | Injury | Maintained Performance | Decreased Performance | Retired | Chi-squared/Fisher’s Exact Test p-Value * |
|---|---|---|---|---|---|
| Foot | Yes (n = 93) | 40 (43.0%) | 39 (41.9%) | 14 (15.1%) | 0.696 |
| No (n = 145) | 61 (42.1%) | 67 (46.2%) | 17 (11.7%) | ||
| Fetlock | Yes (n = 68) | 27 (9.0%) | 29 (42.6%) | 12 (17.7%) | 0.406 |
| No (n = 170) | 74 (43.5%) | 77 (45.3%) | 19 (11.2%) | ||
| Metacarpal/metatarsal | Yes (n = 78) | 34 (43.6%) | 36 (46.2%) | 8 (10.3%) | 0.675 |
| No (n = 160) | 67 (41.9%) | 70 (43.8%) | 23 (14.4%) | ||
| Cranial cervical | Yes (n = 1) | 0 | 0 | 1 (100.0%) | - |
| No (n = 40) | 12 (30.0%) | 25 (62.5%) | 3 (7.5%) | ||
| Caudal cervical | Yes (n = 9) | 4 (44.4%) | 2 (22.2%) | 3 (33.3%) | 0.021 |
| No (n = 81) | 29 (35.8%) | 47 (58.0) | 5 (6.2%) | ||
| Thoracolumbar | Yes (n = 23) | 8 (38.1%) | 12 (57.1%) | 1 (4.8%) | 0.872 |
| No (n = 69) | 25 (36.2%) | 37 (53.6%) | 7 (10.1%) | ||
| Lumbosacroiliac | Yes (n = 23) | 7 (25.9%) | 13 (61.9%) | 1 (4.8%) | 0.872 |
| No (n = 67) | 24 (35.8%) | 36 (53.7%) | 7 (10.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boado, A.; Pollard, D.; Lopez-Sanroman, F.J.; Dyson, S. Orthopaedic Injuries in 272 Dressage Horses: A Retrospective Study. Animals 2025, 15, 2972. https://doi.org/10.3390/ani15202972
Boado A, Pollard D, Lopez-Sanroman FJ, Dyson S. Orthopaedic Injuries in 272 Dressage Horses: A Retrospective Study. Animals. 2025; 15(20):2972. https://doi.org/10.3390/ani15202972
Chicago/Turabian StyleBoado, Ana, Danica Pollard, Francisco Javier Lopez-Sanroman, and Sue Dyson. 2025. "Orthopaedic Injuries in 272 Dressage Horses: A Retrospective Study" Animals 15, no. 20: 2972. https://doi.org/10.3390/ani15202972
APA StyleBoado, A., Pollard, D., Lopez-Sanroman, F. J., & Dyson, S. (2025). Orthopaedic Injuries in 272 Dressage Horses: A Retrospective Study. Animals, 15(20), 2972. https://doi.org/10.3390/ani15202972








