Energy Processes During Rigor Mortis in the Adductor Muscle of the Lion’s Paw Scallop (Nodipecten subnodosus): Effects of Seasonality and Storage Temperature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Organisms
2.2. Storage Experiment
2.3. ATP, ADP, AMP
2.4. AEC
2.5. Glycogen
2.6. Arginine Phosphate
2.7. Arginine
2.8. Statistical Analysis
3. Results and Discussions
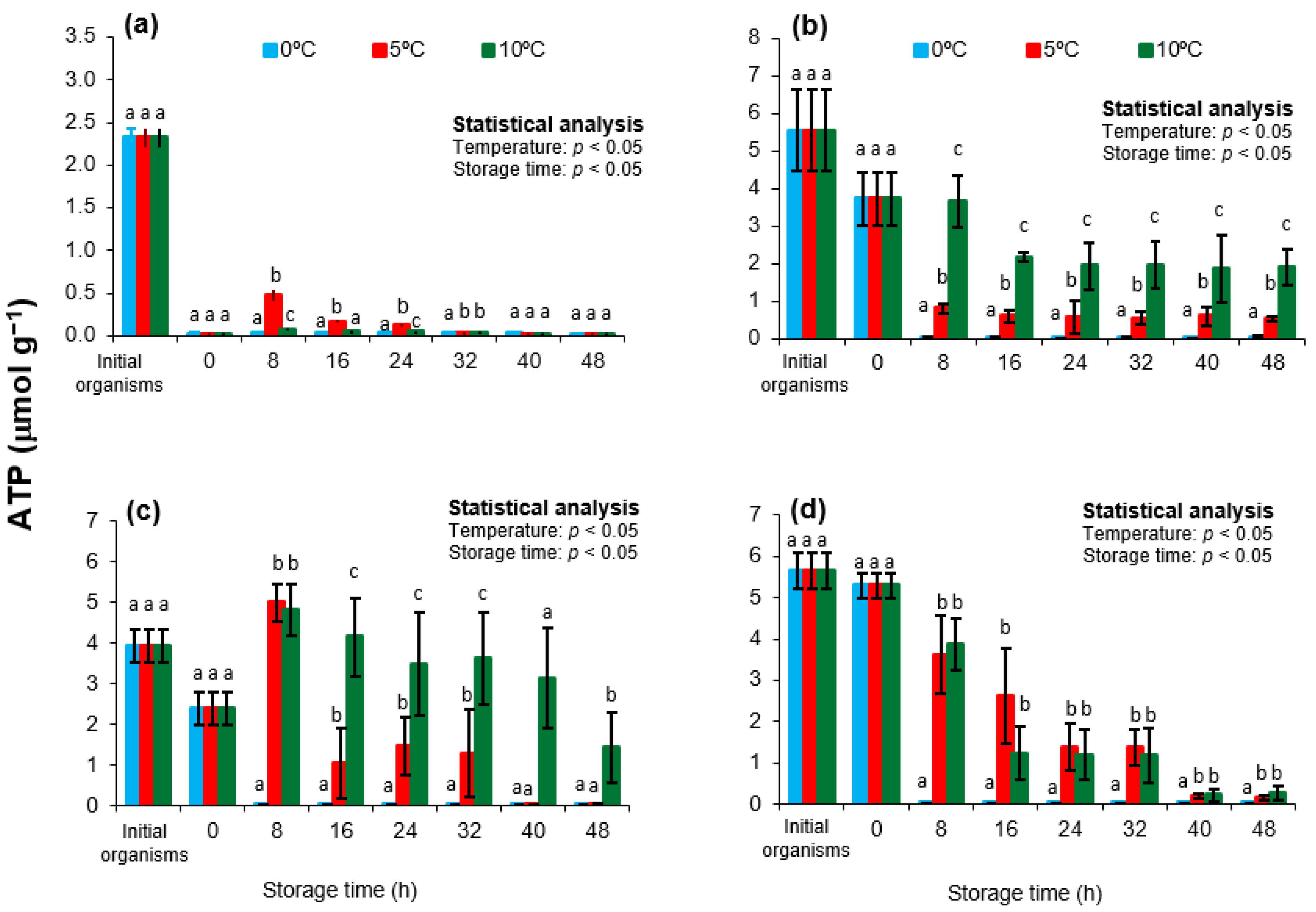
3.1. ATP, ADP and AMP
3.2. Adenylate Energy Charge (AEC)
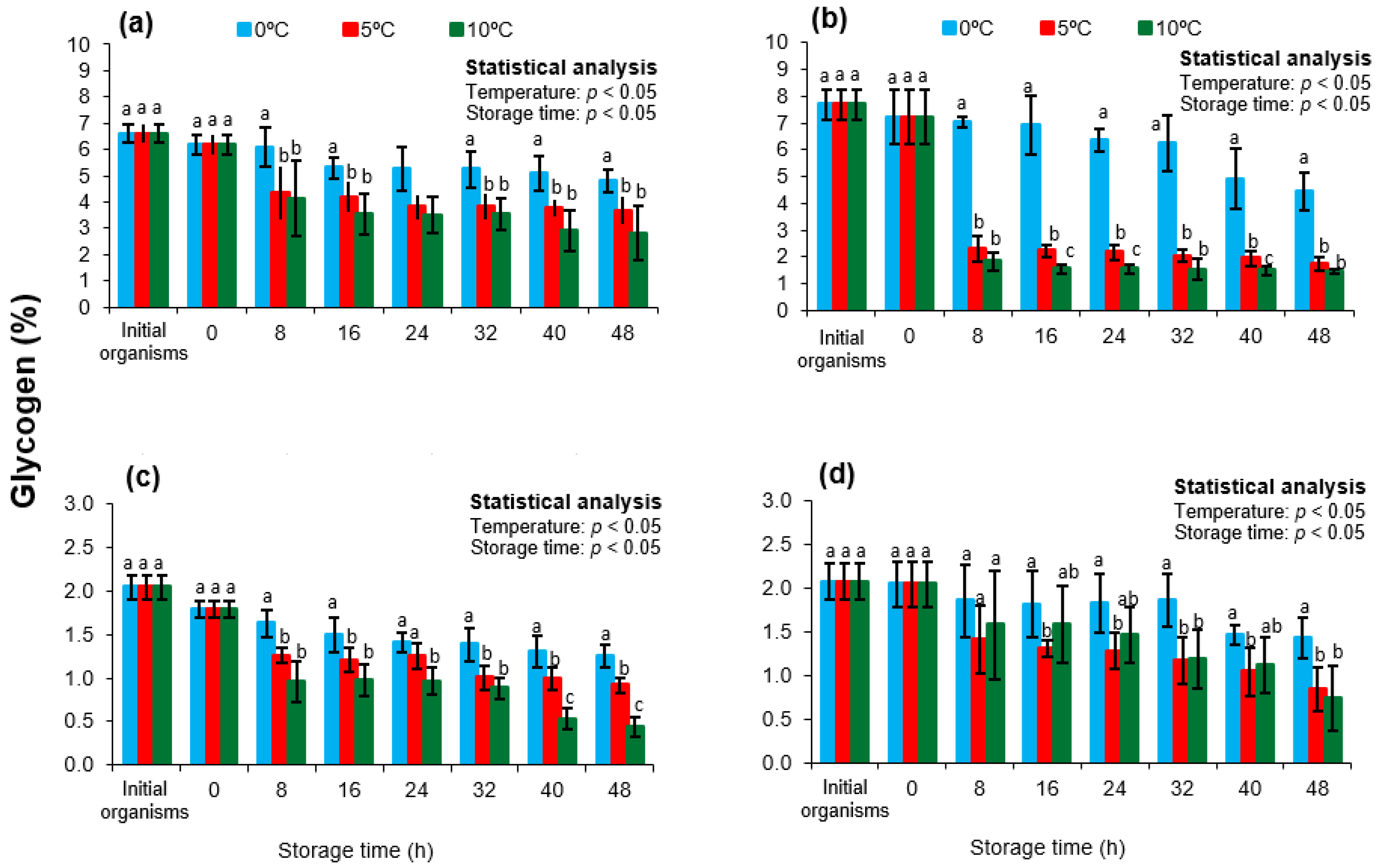
3.3. Glycogen
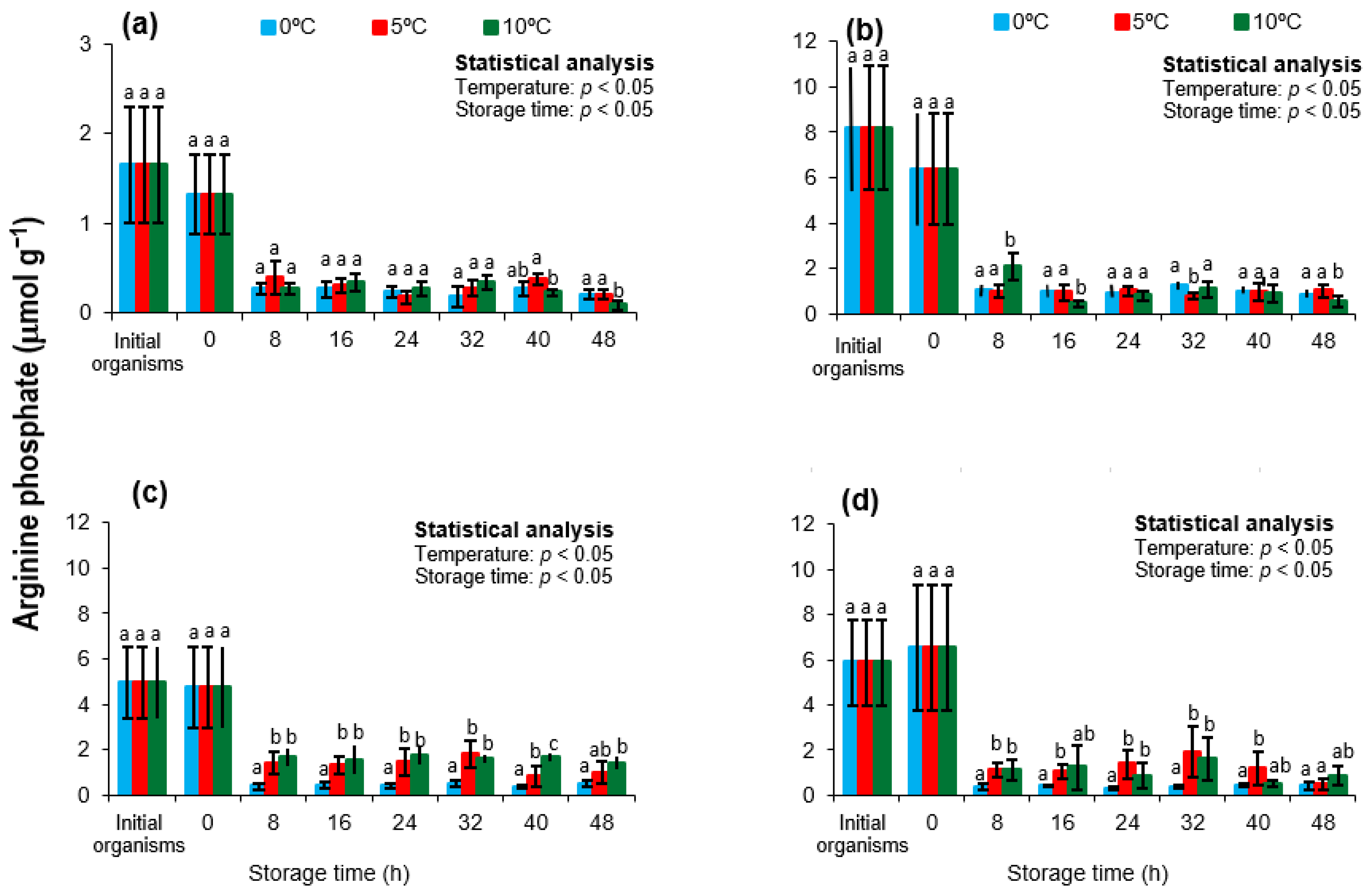
3.4. Arginine Phosphate
3.5. Arginine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nava-Gómez, G.E.; Cordero-Esquivel, B.; Díaz, F.; Bricelj, M.; García-Esquivel, Z. Survival, growth and biochemical composition of larvae of the the lion’s paw scallop, Nodipecten subnodosus, in batch-and flow-through culture. Aquaculture 2022, 555, 738181. [Google Scholar] [CrossRef]
- Massó-Rojas, J.A.E.; Morales-Bojórquez, E.; Arellano-Martínez, M.; Ceballos-Vázquez, B.P.; Talavera-Maya, J.; Ceseña-Espinoza, N. Almeja mano de león Nodipecten subnodosus. In Sustentabilidad y pesca Responsable en México: Evaluación y Manejo; SAGARPA e Instituto Nacional de Pesca: Mexico City, Mexico, 2014; pp. 17–48. [Google Scholar]
- Koch, V.; Rengstorf, A.; Taylor, M.; Mazón-Suástegui, J.M.; Sinsel, F.; Wolff, M. Comparative growth and mortality of cultured Lion’s Paw scallops (Nodipecten subnodosus) from Gulf of California and Pacific populations and their reciprocal transplants. Aquac. Res. 2015, 46, 185–201. [Google Scholar] [CrossRef]
- Purce, D.N.; Donovan, D.A.; Maeda-Martínez, A.N.; Koch, V. Scope for growth of cultivated Pacific and Gulf of California populations of lion’s paw scallop Nodipecten subnodosus, and their reciprocal transplants. Lat. Am. J. Aquac. Res. 2020, 48, 538–551. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, E.I.; Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Varela-Romero, A.; Márquez-Ríos, E.; Muhlia-Almazán, A.T.; Castillo-Yáñez, F.J. Effect of seasonality and storage temperature on rigor mortis in the adductor muscle of lion’s paw scallop Nodipecten subnodosus. Aquaculture 2013, 388, 35–41. [Google Scholar] [CrossRef]
- Massó-Rojas, J.A.; Morales-Bojórquez, E.; Talavera-Mayer, J.; Fajardo-León, M.; Hernández-Valenzuela, R. Almeja mano de león. In Sustentabilidad y Pesca Responsable en México. Evaluación y Manejo. 1999–2000; Cisneros-Mata, M.A., Beléndez-Moreno, L.F.J., Zárate-Becerra, E., Gaspar-Dillanes, M.T., López-González, L., Saucedo-Ruiz, L., Tovar-Ávila, J., Eds.; Instituto Nacional de la Pesca: Mexico City, Mexico, 2001; pp. 349–366. [Google Scholar]
- Maldonado-Amparo, R.; Ramírez, J.L.; Avila, S.; Ibarra, A.M. Triploid lion-paw scallop (Nodipecten subnodosus Sowerby); growth, gametogenesis, and gametic cell frequencies when grown at a high food availability site. Aquaculture 2004, 235, 185–205. [Google Scholar] [CrossRef]
- Daskalova, A. Farmed fish welfare: Stress, post-mortem muscle metabolism, and stress-related meat quality changes. Int. Aquat. Res. 2019, 11, 113–124. [Google Scholar] [CrossRef]
- Sultana, K.; Jayathilakan, K.; Sajeevkumar, V.A. Chemistry of Animal Tissues. In Advances in Food Chemistry; Chauhan, O.P., Ed.; Springer: Singapore, 2022; pp. 385–437. [Google Scholar]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO Fisheries Technical Paper No. 348; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995; p. 195. [Google Scholar]
- Sikorski, Z.E.; Kolakowska, A.; Burt, R. Postharvest biochemical and microbial changes. In Seafood: Resources, Nutritional Composition and Preservation; Sikorski, Z.E., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 55–75. [Google Scholar]
- Kumar, S.; Gorea, R.K. Correlation of postmortem changes with time since death. In Textbook of Forensic Science; Shrivastava, P., Lorente, J.A., Srivastava, A., Badiye, A., Kapoor, N., Eds.; Springer: Singapore, 2023; pp. 197–243. [Google Scholar]
- Hwang, G.C.; Ushio, H.; Watabe, S.; Iwamoto, M.; Hashimoto, K. The effect of thermal acclimation on rigor mortis progress of carp stored at different temperatures. Nippon Suisan Gakkaishi 1991, 57, 541–548. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Cheftel, H. Introducción a la Bioquímica y Tecnología de los Alimentos Vol. I; Editorial Acribia, S.A.: Zaragoza, Spain, 1976; pp. 65–97. [Google Scholar]
- Le, T.T.; Nguyen, H.T.; Pham, M.A. Rigor mortis development and effects of filleting conditions on the quality of Tra catfish (Pangasius hypophthalmus) fillets. J. Food Sci. Technol. 2020, 57, 1320–1330. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Tilapia Culture, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020; 348p. [Google Scholar]
- Vasconcellos, B.M.; Guimarães Ribeiro, V.; Campos, N.D.N.; da Silva Romão Mota, L.G.; Moreira, M.F. A comprehensive review of arginine kinase proteins: What we need to know? Biochem. Biophys. Rep. 2024, 8, 101837. [Google Scholar] [CrossRef]
- Tremblay, I.; Guderley, H.E. Scallops show that muscle metabolic capacities reflect locomotor style and morphology. Physiol. Biochem. Zool. 2014, 87, 231–244. [Google Scholar] [CrossRef]
- Racotta, I.S.; Ramírez, J.L.; Ibarra, A.M.; Rodríguez-Jaramillo, M.C.; Palacios, E. Growth and gametogenesis in the lions-paw-scallop Nodipecten (Lyropecten) subnodosus. Aquaculture 2003, 217, 335–349. [Google Scholar] [CrossRef]
- Wongso, S.; Ushio, H.; Ohshima, T.; Yamanaka, H. Changes in content of octopine, acidic opines, related amino acids and phosphoarginine in the adductor muscle of three species of scallop during storage. J. Food Biochem. 1998, 22, 65–81. [Google Scholar] [CrossRef]
- Angel-Dapa, M.A.; Arellano-Martínez, M.; Ceballos-Vázquez, B.P.; Acosta-Salmón, H.; Saucedo, P.E. Comparative analysis of the reproductive strategy of lion’s paw scallop Nodipecten subnodosus in Baja California Sur, Mexico. Lat. Am. J. Aquat. Res. 2015, 43, 616–620. [Google Scholar] [CrossRef]
- Beltrán-Lugo, A.I.; Maeda-Martínez, A.N.; Pacheco-Aguilar, R.; Nolasco-Soria, H.G. Seasonal variations in chemical, physical, textural, and microstructural properties of adductor muscles of Pacific lions-paw scallop (Nodipecten subnodosus). Aquaculture 2006, 258, 619–632. [Google Scholar] [CrossRef]
- Massa, A.E.; Paredi, M.E.; Crupkin, M. Nucleotide catabolism in cold stored adductor muscle of scallop (Zygochlamys patagonica). J. Food Biochem. 2002, 26, 295–305. [Google Scholar] [CrossRef]
- Yin, Z.; Li, M.; Cai, Y.; Qi, L.; Yuan, C.; Tian, Y. Examination of the effects of dry storage stress on mitochondrial energy synthesis in the scallop Mizuhopecten yessoensis. Aquaculture 2023, 575, 739770. [Google Scholar] [CrossRef]
- Pistorius, K.; Vosloo, A.; Vosloo, D. The effects of emersion and re-immersion on gill recruitment, perfusion, and metabolic rates of south african abalone (Haliotis midae). J. Shellfish Res. 2025, 44, 11–19. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamanaka, H.; Yamakawa, H. Postmortem biochemical changes in the muscle of disk abalone during storage. Nippon Suisan Gakkaishi 1992, 58, 2081–2088. [Google Scholar] [CrossRef]
- Zhou, L.; Li, M.; Zhong, Z.; Chen, H.; Wang, X.; Wang, M.; Sun, Y.; Li, C. Biochemical and metabolic responses of the deep-sea mussel Bathymodiolus platifrons to cadmium and copper exposure. Aquat. Toxicol. 2021, 236, 105845. [Google Scholar] [CrossRef]
- Massa, A.E.; Paredi, M.E.; Crupkin, M. A chemical assessment of freshness in stored adductor muscle from scallops. Braz. J. Chem. Eng. 2003, 20, 147–152. [Google Scholar] [CrossRef]
- Pacheco-Aguilar, R.; Marquez-Ríos, E.; Lugo-Sánchez, M.E.; García-Sanchez, G.; Maeda-Martínez, A.N.; Ocaño-Higuera, V.M. Postmortem changes in the adductor muscle of Pacific lions-paw scallop (Nodipecten subnodosus) during ice storage. Food Chem. 2008, 106, 253–259. [Google Scholar] [CrossRef]
- Kawashima, K.; Yamanaka, H. Effects of Storage Temperatures on the Post-mortem Biochemical Changes in Scallop Adductor Muscle. Nippon Suisan Gakkaishi 1992, 58, 2175–2180. [Google Scholar] [CrossRef]
- Ryder, J.M. Determination of adenosine triphosphate and its breakdown products in fish muscle by high performance liquid chromatography. J. Agric. Food Chem. 1985, 33, 678–680. [Google Scholar] [CrossRef]
- Guida, L.; Walker, T.I.; Reina, R.D. The adenylate energy charge as a new and useful indicator of capture stress in chondrichthyans. J. Comp. Physiol. B 2016, 186, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Racotta, I.S.; Palacios, E.; Ibarra, A.M.; Ramirez, J.L.; Arcos, F.; Arjona, O. Comparative biochemical composition of ploidy groups of the lion-paw scallop (Nodipecten subnodosus Sowerby) supports the physiological hypothesis for the lack of advantage in triploid mollusc’s growth in food-rich environments. Mar. Biol. 2008, 153, 1245–1256. [Google Scholar] [CrossRef]
- Viant, M.R.; Rosenblum, E.S.; Tjeerdema, R.S. Optimized method for the determination of phosphoarginine in abalone tissue by high-performance liquid chromatography. J. Chromatogr. B 2001, 765, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Takeuchi, M.; Kanno, N.; Nagahisa., E.; Sayo, Y. Determination of octopine by pre-column fluorescence derivatization using benzoin. Biochem. Int. 1991, 6, 1035–1039. [Google Scholar]
- Hintze, J. NCSS and PASS; Number Cruncher Statistical Systems (NCSS): Kaysvill, UT, USA, 2001. [Google Scholar]
- Chen, B.; Yan, Q.; Li, D.; Xie, J. Degradation mechanism and development of detection technologies of ATP-related compounds in aquatic products: Recent advances and remaining challenges. Crit. Rev. Food Sci. Nut. 2025, 65, 101–122. [Google Scholar] [CrossRef]
- Gao, S.; Li, Y.; Xu, F.; Zhu, J. Influence of seasonal variations on energy metabolism and quality deterioration of scallop adductor muscles during storage. LWT-Food Sci. Technol. 2023, 175, 114572. [Google Scholar]
- Zhang, Y.; Li, H.; Zhao, C.; Sun, F. Effect of temperature on ATP degradation and rigor mortis in the adductor muscle of Pecten maximus. Aquaculture 2022, 548, 737592. [Google Scholar]
- Liu, Y.; Wang, Y.; Wu, T.; Li, X.; Liu, B. Effects of seasonal environmental conditions on energy reserves and postmortem metabolism in bivalves. Aquac. Rep. 2021, 20, 100715. [Google Scholar]
- Salgado-García, R.L.; Kraffe, E.; Tripp-Valdez, M.A.; Ramírez-Arce, J.L.; Artigaud, S.; Flye-Sainte-Marie, J.; Mathieu-Resuge, M.; Sicard, M.T.; Arellano-Martínez, M.; Racotta, I.S. Energy metabolism of juvenile scallops Nodipecten subnodosus under acute increased temperature and low oxygen availability. Comp. Biochem. Physiol. Part A Mol. Int. Physiol. 2023, 278, 111373. [Google Scholar] [CrossRef]
- González-Estrada, F. Temperaturas Letales y Temperatura Óptima Para Crecimiento en una Población de Almeja Mano de León (Nodipecten subnodosus Sowerby, 1835). Master’s Thesis, Centro de Investigaciones Biológicas del Noroeste S.C., La Paz, BCS, Mexico, 2003. [Google Scholar]
- Hematyar, N.; Policar, T.; Rustad, T. Importance of proteins and mitochondrial changes as freshness indicators in fish muscle post--mortem. J. Sci. Food Agric. 2025, 105, 5163–5172. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Yañez, F.J.; Jimenez-Ruiz, E.I.; Canizales-Rodriguez, D.F.; Marquez-Rios, E.; Montoya-Camacho, N.; Ruiz-Cruz, S.; Ocaño-Higuera, V.M. Postmortem biochemical changes and evaluation of the freshness in the muscle of tilapia (Oreochromis niloticus) during the storage in ice. J. Fish. Aquac. Sci. 2014, 9, 435. [Google Scholar]
- Gornik, S.G.; Albalat, A.; Atkinson, R.J.; Coombs, G.H.; Neil, D.M. The influence of defined ante-mortem stressors on the early post-mortem biochemical processes in the abdominal muscle of the Norway lobster, Nephrops norvegicus (Linnaeus, 1758). Mar. Biol. Res. 2010, 6, 223–238. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Scheffler, T.L.; Gerrard, D.E. The conversion of muscle to meat. In Lawrie’s Meat Science, 9th ed.; Toldra, F., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2022; pp. 159–194. [Google Scholar]
- Viant, M.R.; Rosenblum, E.S.; Tjeerdema, R.S. NMR-based metabolomics: A powerful approach for characterizing the effects of environmental stressors on aquatic organisms. Aquat. Toxicol. 2001, 65, 153–170. [Google Scholar] [CrossRef]
- Dong, S. Effect of Shell Shape, Post-Harvest Rearing, and Super-Chilling Storage on the Changes in Biochemical Properties of Pacific Oysters (Crassostrea gigas). Doctoral Dissertation, Iwate University, Morioka, Japan, 2023. [Google Scholar]
- Smits, S. Structural Analysis of Octopine Dehydrogenase from Pecten maximus. Ph.D. Thesis, Mathematisch-Naturwissenschaftlichen Fakultät der Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany, 2008; 98p. [Google Scholar]
- Skjoldal, H.R.; Barkati, S. ATP content and adenylate energy charge of the mussel Mytilus edulis during the annual reproductive cycle in Lindaspollene, Western Norway. Mar. Biol. 1982, 70, 1–6. [Google Scholar] [CrossRef]
- Wicks, J.C.; Bodmer, J.S.; Yen, C.N.; Zumbaugh, M.D.; Matarneh, S.K.; Scheffler, T.L.; Silva, S.L.; Shi, H.; Gerrard, D.E. Postmortem muscle metabolism and meat quality. In New Aspects of Meat Quality, 2nd ed.; Purslow, P., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2022; pp. 67–93. [Google Scholar]
- Tejada, M. ATP—derived products and K—value determination. In Fishery Products: Quality, Safety and Authenticity; Blackwell Publishing: Oxford, UK, 2009; pp. 68–88. [Google Scholar]
- Lopes Da Costa, V.D. Effects of Storage on Product Quality of Bivalves (Oysters, Scallops, and Clams). Master’s Thesis, The University of Bergen, Bergen, Norway, 2018. [Google Scholar]
- Hiltz, D.F.; Dyer, W.J. Octopine in postmortem adductor muscle of the sea scallop (Placopecten magellanicus). J. Fish. Board Can. 1971, 28, 869–874. [Google Scholar] [CrossRef]
- Atkinson, D.E. The energy charge of the adenylate pool as a regulatory parameter: Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef]
- Fleury, P.G.; Mingant, C.; Castillo, A. A preliminary study of the behavior of reseeded juvenile great scallops of three sizes in three seasons. Aquac. Int. 1997, 4, 325–337. [Google Scholar] [CrossRef]
- Woll, A.K.; Bakke, S. Stress and mortality in the supply chain of live scallops Pecten maximus L., from scuba diver to market. Aquac. Res. 2017, 48, 594–607. [Google Scholar] [CrossRef]
- Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Lugo-Sánchez, M.E.; García-Sánchez, G.; Márquez-Ríos, E.; Gómez-Jimenez, S.; Pacheco-Aguilar, R. Effect of emerged shipment on the physiological condition of the adductor muscle in adult giant lion’s paw scallop Nodipecten subnodosus (Sowerby 1835). Aquac. Res. 2011, 42, 1087–1095. [Google Scholar] [CrossRef]
- Moal, J.J.; Le Coz, R.; Samain, J.F.; Daniel, J.Y. Oyster adenylate energy charge (AEC) and its natural variability: Implications for environmental monitoring. Ser. De Doc. Oceanogr. 1991, 17, 279–280. [Google Scholar]
- Maguire, J.; Flury, P.; Burnell, G. Some methods for quantifying quality in the scallop Pecten maximus. J. Shellfish Res. 1999, 18, 56–66. [Google Scholar]
- Liu, M.; Li, T.; Zhang, H.; Ni, H.; Yuan, G.; Wang, Z.; Sun, J.H.; Dong, Z. Starvation alters lipid and glycogen metabolism in clam Cyclina sinensis. Aquac. Rep. 2024, 38, 102353. [Google Scholar] [CrossRef]
- Patterson, H.K.; Carmichael, R.H. The effect of lipid extraction on carbon and nitrogen stable isotope ratios in oyster tissues: Implications for glycogen--rich species. Rapid Commun. Mass Spectrom. 2016, 30, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.; Witte, F.; Terjung, N.; Heinz, V.; Juadjur, A.; Gibis, M. Metabolic, proteomic and microbial changes postmortem and during beef aging. Crit. Rev. Food Sci. Nut. 2024, 64, 1076–1109. [Google Scholar] [CrossRef] [PubMed]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New insights in muscle biology that alter meat quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Babita, M.; Kailasam, M.; Muralidhar, M.; Hussain, T.; Behera, A.; Jithendran, K.P. Effect of changing environmental factors on reproductive cycle and endocrinology of fishes. In Outlook of Climate Change and Fish Nutrition; Springer: Berlin/Heidelberg, Germany, 2023; pp. 377–396. [Google Scholar]
- Liu, M.J.; Gao, J.; Guo, H.Y.; Zhu, K.C.; Liu, B.S.; Zhang, N.; Sun, J.-H.; Zhang, D.C. Transcriptomics reveal the effects of breeding temperature on growth and metabolism in the early developmental stage of Platax teira. Biology 2023, 12, 1161. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Xu, C.; Yu, R. Seasonal changes of reproductive activity and biochemical composition of pen shell Atrina pectinata Linnaeus, 1767 in Bohai Sea, China. J. Ocean. Univ. China 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Ansell, A.D. Storage and utilization of reserves in Pectinid bivalves with particular reference to the adductor muscle. In Proceedings of the 2nd Scallop Workshop, Brest, France, 8–13 May 1978; 17p. [Google Scholar]
- Yokoyama, Y.; Sakaguchi, M.; Kawai, F.; Kanamori, M. Effects of storage temperature on postmortem changes of ATP and its related compounds and freshness indices in oyster tissues. Fish. Sci. 1994, 60, 217–223. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nut. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef]
- Rodrigues, E.; Santos, M.R.D.S.; Rodrigues Júnior, E.; Gannabathula, S.V.; Lavrado, H.P. Arginine metabolism of the Antarctic Bivalve Laternula elliptica (King & Broderip, 1831): An ecophysiological approach. Polar Biol. 2009, 32, 691–702. [Google Scholar] [CrossRef]
- Marzari, A.; Racotta, I.S.; Escobedo-Fregoso, C.; Artigaud, S.; Kraffe, E.; Salgado-García, R.L. Reproductive effort affects cellular response in the mantle of Nodipecten subnodosus scallops exposed to acute hyperthermia. Comp. Biochem. Physiol. Part A Mol. Int. Physiol. 2025, 299, 111766. [Google Scholar] [CrossRef] [PubMed]
- Salgado García, R.L. Respuestas Metabólicas de Nodipecten Subnodosus (Pectinidae) Expuesta a Hipertermia en Sinergia con Hipoxia y el Estado Reproductivo. Doctoral Dissertation, Instituto Politécnico Nacional. Centro Interdisciplinario de Ciencias Marinas, La Paz, BCS, Mexico, 2020. [Google Scholar]
- Salgado-García, R.L.; Kraffe, E.; Maytorena-Verdugo, C.I.; Rivera-Camacho, A.R.; Sicard, M.T.; Arellano-Martínez, M.; Racotta, I.S. Metabolic responses of adult lion’s paw scallops Nodipecten subnodosus exposed to acute hyperthermia in relation to seasonal reproductive effort. Sci. Rep. 2020, 10, 2449. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Clark, S.A.; Ellington, W.R.; Chapman, M.S. The role of phosphagen specificity loops in arginine kinase. Protein Sci. 2004, 13, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Götze, S.; Pörtner, H.O.; Lannig, G. Exploring the mechanisms behind swimming performance limits to ocean warming and acidification in the Atlantic king scallop, Pecten maximus. Front. Ecol. Evol. 2024, 12, 1347160. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Liao, H. Structure and functional analysis reveal an important regulated role of arginine kinase in Patinopecten yessoensis under low pH stress. Aquat. Toxicol. 2020, 222, 105452. [Google Scholar] [CrossRef]
- Alvarez, C.; Morán, L.; Keenan, D.F.; Mullen, A.M.; Delgado-Pando, G. Mechanical and biochemical methods for rigor measurement: Relationship with eating quality. J. Food Qual. 2019, 2019, 1894543. [Google Scholar] [CrossRef]
- Fung, T.S.; Ryu, K.W.; Thompson, C.B. Arginine: At the crossroads of nitrogen metabolism. EMBO J. 2025, 44, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Allen, K. Amino acids in the Mollusca. Am. Zool. 1961, 1, 253–261. [Google Scholar] [CrossRef]



| Stage | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Harvest | 0.58 ± 0.02 a | 0.84 ± 0.11 be | 0.65 ± 0.02 c | 0.85 ± 0.02 b |
| Post transport | 0.31 ± 0.02 d | 0.67 ± 0.08 e | 0.51 ± 0.05 f | 0.83 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Ruiz, E.I.; Ocaño-Higuera, V.M.; Sumaya-Martínez, M.T.; Márquez-Ríos, E.; Ruíz-Cruz, S.; Canizales-Rodríguez, D.F.; Tortoledo-Ortiz, O.; Garzón-García, A.M.; Ramos-Enríquez, J.R.; Valdez-Hurtado, S.; et al. Energy Processes During Rigor Mortis in the Adductor Muscle of the Lion’s Paw Scallop (Nodipecten subnodosus): Effects of Seasonality and Storage Temperature. Animals 2025, 15, 2953. https://doi.org/10.3390/ani15202953
Jiménez-Ruiz EI, Ocaño-Higuera VM, Sumaya-Martínez MT, Márquez-Ríos E, Ruíz-Cruz S, Canizales-Rodríguez DF, Tortoledo-Ortiz O, Garzón-García AM, Ramos-Enríquez JR, Valdez-Hurtado S, et al. Energy Processes During Rigor Mortis in the Adductor Muscle of the Lion’s Paw Scallop (Nodipecten subnodosus): Effects of Seasonality and Storage Temperature. Animals. 2025; 15(20):2953. https://doi.org/10.3390/ani15202953
Chicago/Turabian StyleJiménez-Ruiz, Edgar Iván, Víctor Manuel Ocaño-Higuera, María Teresa Sumaya-Martínez, Enrique Márquez-Ríos, Saúl Ruíz-Cruz, Dalila Fernanda Canizales-Rodríguez, Orlando Tortoledo-Ortiz, Alba Mery Garzón-García, José Rogelio Ramos-Enríquez, Santiago Valdez-Hurtado, and et al. 2025. "Energy Processes During Rigor Mortis in the Adductor Muscle of the Lion’s Paw Scallop (Nodipecten subnodosus): Effects of Seasonality and Storage Temperature" Animals 15, no. 20: 2953. https://doi.org/10.3390/ani15202953
APA StyleJiménez-Ruiz, E. I., Ocaño-Higuera, V. M., Sumaya-Martínez, M. T., Márquez-Ríos, E., Ruíz-Cruz, S., Canizales-Rodríguez, D. F., Tortoledo-Ortiz, O., Garzón-García, A. M., Ramos-Enríquez, J. R., Valdez-Hurtado, S., Silvas-García, M. I., & Montoya-Camacho, N. (2025). Energy Processes During Rigor Mortis in the Adductor Muscle of the Lion’s Paw Scallop (Nodipecten subnodosus): Effects of Seasonality and Storage Temperature. Animals, 15(20), 2953. https://doi.org/10.3390/ani15202953







