Effectiveness of Medical Treatment on Survivability in Canine Cushing’s Syndrome: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
2.6. Sensitivity and Cross-Framework Analyses
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
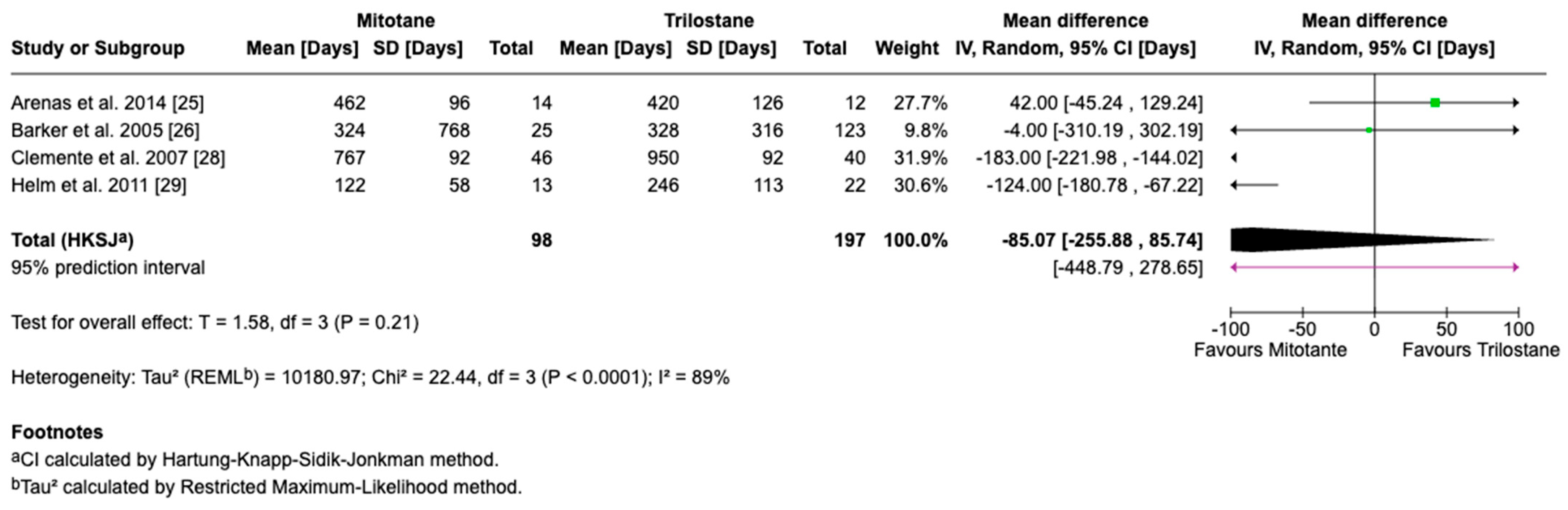
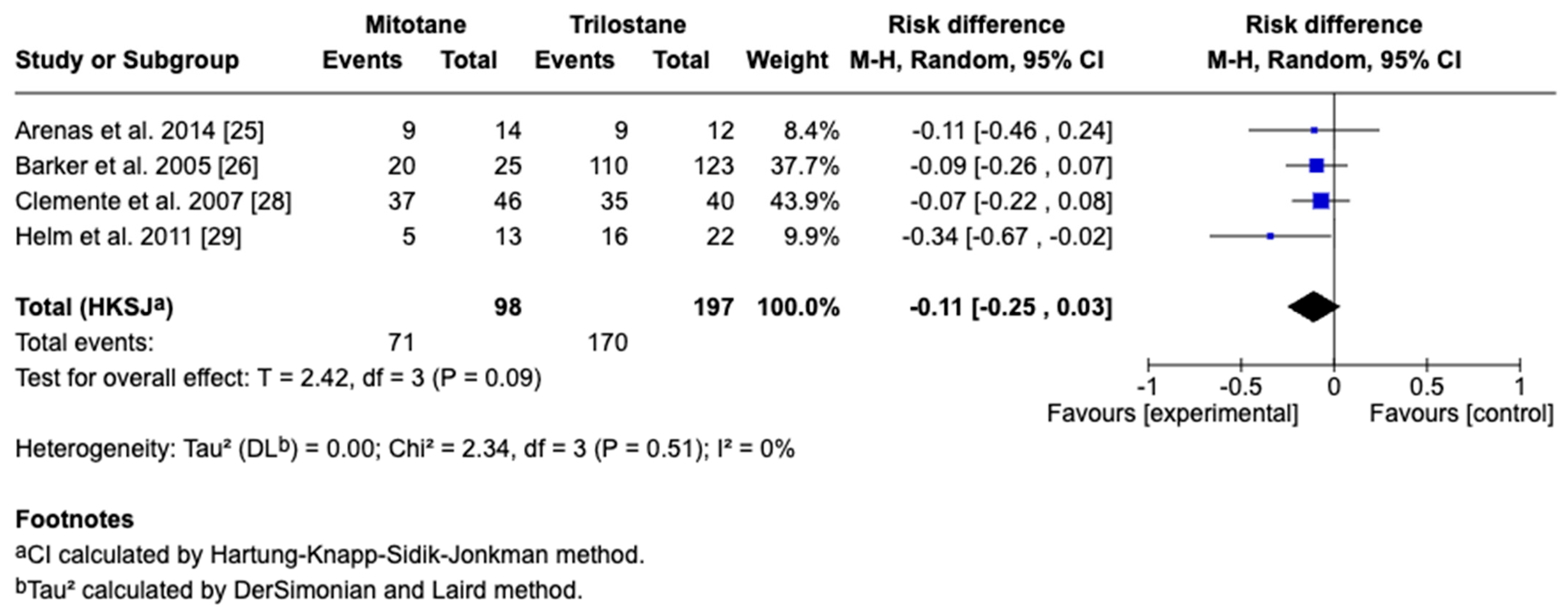
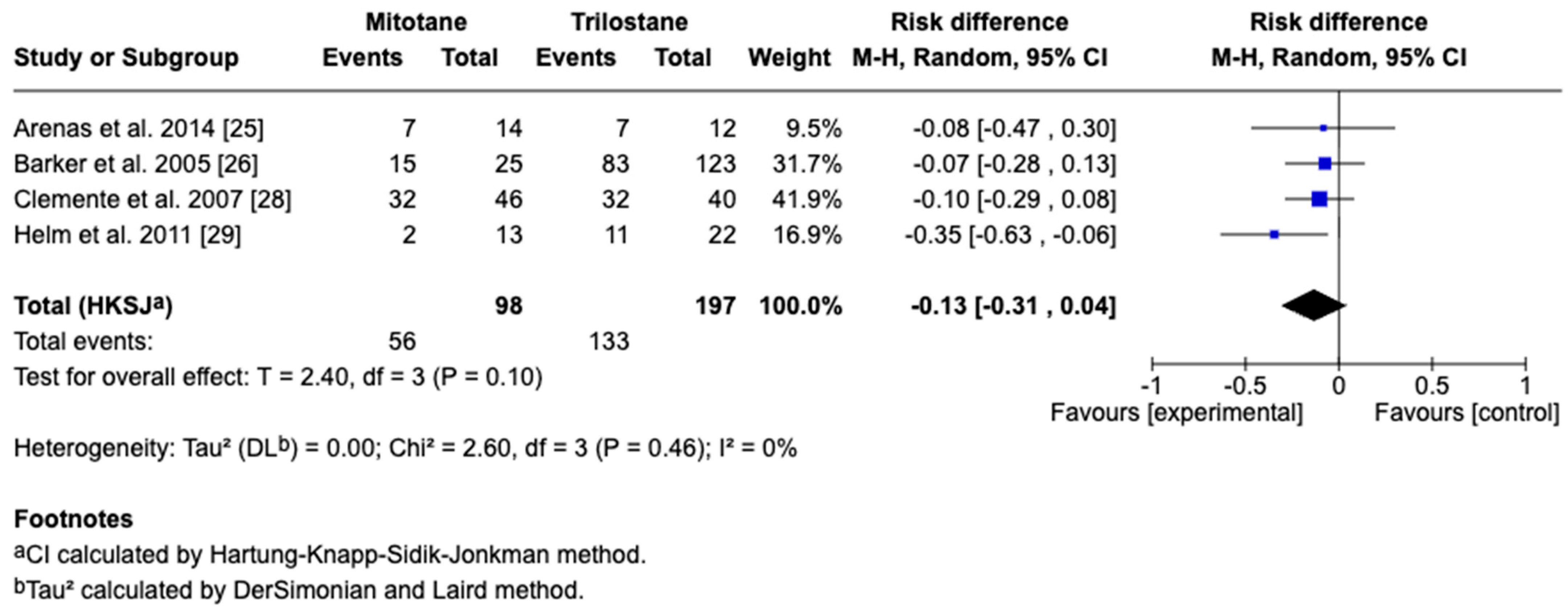
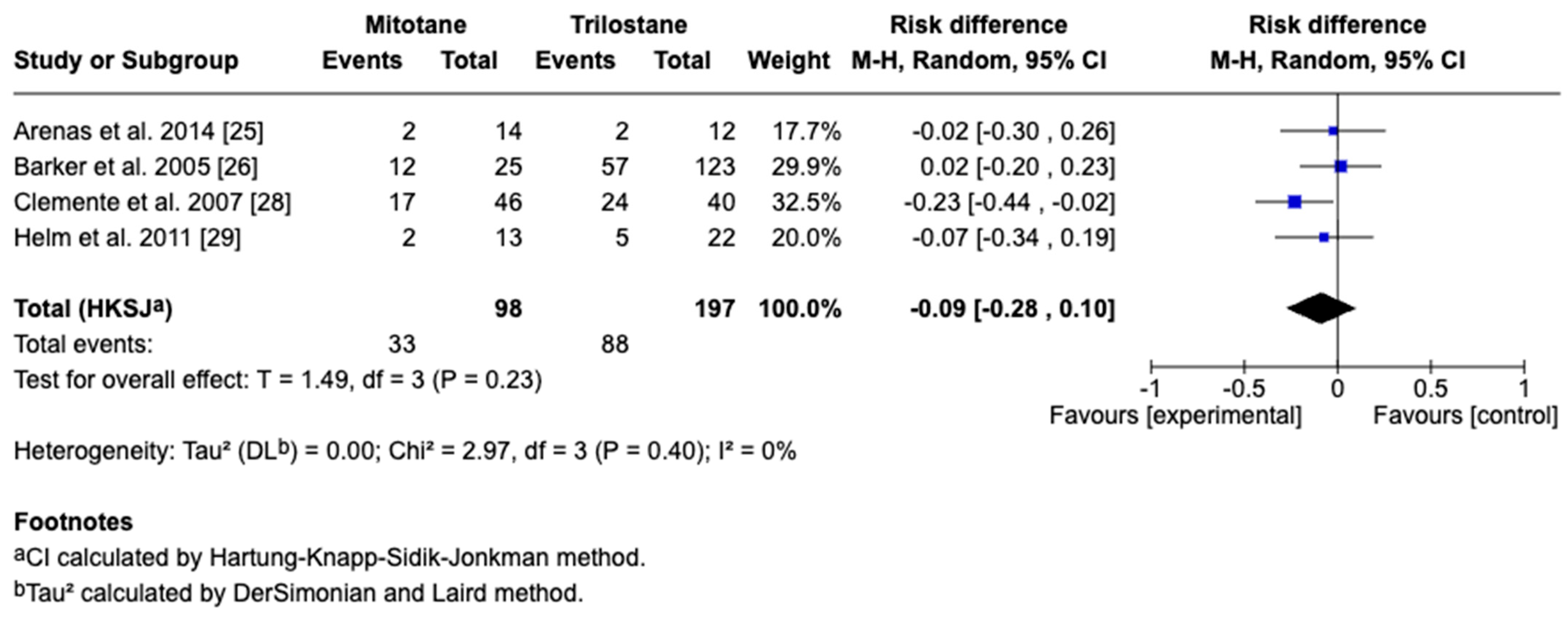
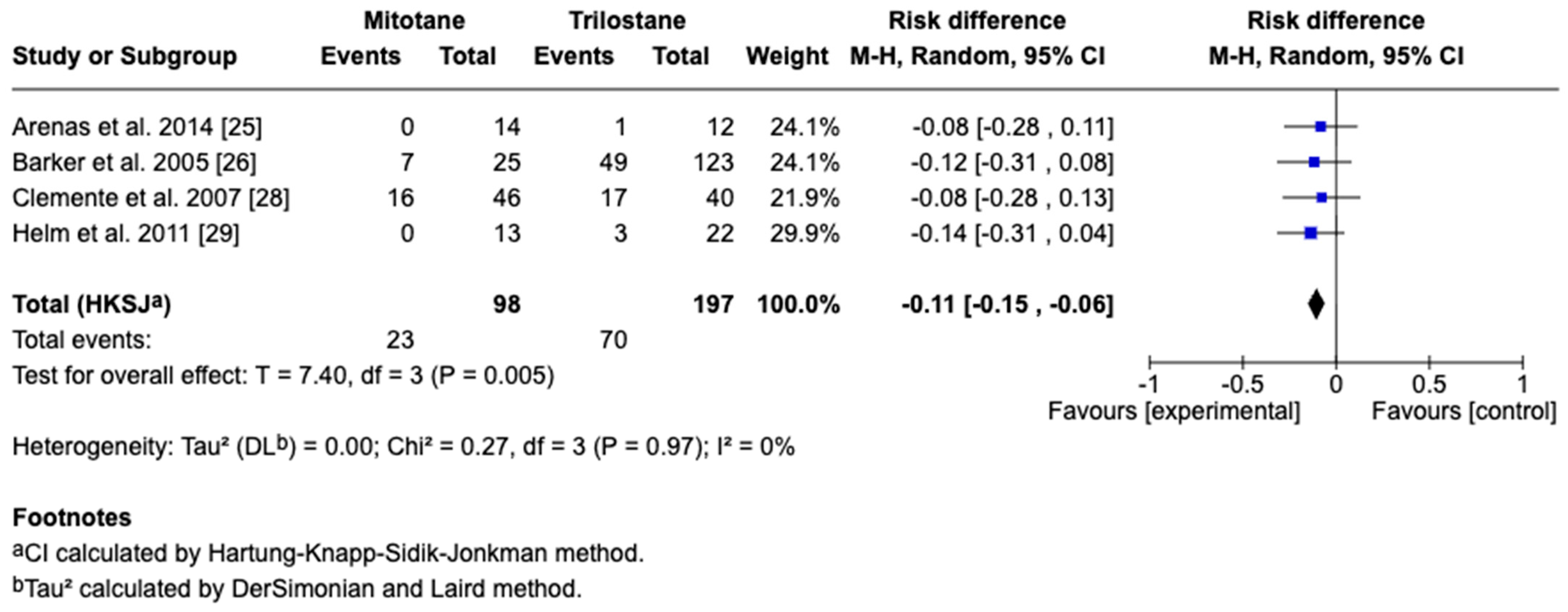
3.4. Survival Outcomes
3.5. Sensitivity and Cross-Framework Comparison
3.6. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| ADH | Adrenal-dependent hypercortisolism |
| BW | Body weight |
| CI | Confidence interval |
| CS | Cushing’s syndrome |
| d | Days |
| F | Female |
| HKSJ | Hartung–Knapp–Sidik–Jonkman |
| kg | Kilogram |
| M | Male |
| mg | Milligram |
| MRI | Magnetic resonance imaging |
| n | Number of animals |
| nmol/L | Nanomoles per liter |
| NR | Not reported |
| PHD | Pituitary-dependent hypercortisolism |
| PO | Per os (oral) |
| PRIMSA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| q12 | Twice daily |
| q24 | Once daily |
| q48 | Every other day |
| ROBINS-I | Risk of Bais in Non-Randomized Studies of Interventions |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| τ2 | Between-study variance |
| µg/dL | Micrograms per deciliter |
| y | years |
References
- Cushing, H. The Basophil Adenomas of the Pituitary Body and Their Clinical Manifestations (Pituitary Basophilism). J. Neurosurg. 1964, 21, 318–347. [Google Scholar] [CrossRef]
- Sanders, K.; Kooistra, H.S.; Galac, S. Treating Canine Cushing’s Syndrome: Current Options and Future Prospects. Vet. J. 2018, 241, 42–51. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.G.; Scudder, C.; Faire, J.M.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Epidemiology of Hyperadrenocorticism among 210,824 Dogs Attending Primary-Care Veterinary Practices in the UK from 2009 to 2014: Hyperadrenocorticism in Dogs. J. Small Anim. Pract. 2016, 57, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Labelle, P.; Kyles, A.E.; Farver, T.B.; De Cock, H.E.V. Indicators of Malignancy of Canine Adrenocortical Tumors: Histopathology and Proliferation Index. Vet. Pathol. 2004, 41, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Schofield, I.; Brodbelt, D.C.; Niessen, S.J.M.; Church, D.B.; Geddes, R.F.; O’Neill, D.G. Frequency and Risk Factors for Naturally Occurring Cushing’s Syndrome in Dogs Attending UK Primary-Care Practices. J. Small Anim. Pract. 2022, 63, 265–274. [Google Scholar] [CrossRef]
- Behrend, E.N.; Kooistra, H.S.; Nelson, R.; Reusch, C.E.; Scott-Moncrieff, J.C. Diagnosis of Spontaneous Canine Hyperadrenocorticism: 2012 ACVIM Consensus Statement (Small Animal). J. Vet. Intern. Med. 2013, 27, 1292–1304. [Google Scholar] [CrossRef]
- Peterson, M.E. Medical Treatment of Canine Pituitary-Dependent Hyperadrenocorticism (Cushing’s Disease). Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 1005–1014. [Google Scholar] [CrossRef]
- Feelders, R.A.; Hofland, L.J. Medical Treatment of Cushing’s Disease. J. Clin. Endocrinol. Metab. 2013, 98, 425–438. [Google Scholar] [CrossRef]
- Lien, Y.-H.; Huang, H.-P. Use of Ketoconazole to Treat Dogs with Pituitary-Dependent Hyperadrenocorticism: 48 Cases (1994–2007). J. Am. Vet. Med. Assoc. 2008, 233, 1896–1901. [Google Scholar] [CrossRef]
- Kuroha, M.; Kuze, Y.; Shimoda, M.; Kokue, E. In Vitro Characterization of the Inhibitory Effects of Ketoconazole on Metabolic Activities of Cytochrome P-450 in Canine Hepatic Microsomes. Am. J. Vet. Res. 2002, 63, 900–905. [Google Scholar] [CrossRef]
- Pérez-López, L.; Mendoza, P.; Melián, C. Effects of Concurrent Canine Cushing’s Syndrome and Diabetes Mellitus on Insulin Requirements, Trilostane Dose, and Survival Time. Res. Vet. Sci. 2023, 161, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, F.N.; Vargas, A.M.; Guimarães, E.C.; Crivellenti, L.Z.; Pennacchi, C.S.; de Cerqueira, H.D.B.; Branco Lde, O.; Reis, N.S.; Borin-Crivellenti, S. Association between Post-ACTH Cortisol and Trilostane Dosage in Dogs with Pituitary-Dependent Hypercortisolism. Domest. Anim. Endocrinol. 2024, 89, 106871. [Google Scholar] [CrossRef] [PubMed]
- Reusch, C.E.; Steffen, T.; Hoerauf, A. The Efficacy of L-Deprenyl in Dogs with Pituitary-Dependent Hyperadrenocorticism. J. Vet. Intern. Med. 1999, 13, 291. [Google Scholar] [CrossRef]
- Pérez, A.M.D.; Guerrero, B.; Melián, C.; Ynaraja, E.; Peña, L. Use of Aminoglutethimide in the Treatment of Pituitary-Dependent Hyperadrenocorticism in the Dog. J. Small Anim. Pract. 2002, 43, 104–108. [Google Scholar] [CrossRef]
- Eastwood, J.M.; Elwood, C.M.; Hurley, K.J. Trilostane Treatment of a Dog with Functional Adrenocortical Neoplasia. J. Small Anim. Pract. 2003, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Langlois, D.K.; Fritz, M.C.; Schall, W.D.; Bari Olivier, N.; Smedley, R.C.; Pearson, P.G.; Bailie, M.B.; Hunt, S.W., 3rd. ATR-101, a Selective ACAT1 Inhibitor, Decreases ACTH-Stimulated Cortisol Concentrations in Dogs with Naturally Occurring Cushing’s Syndrome. BMC Endocr. Disord. 2018, 18, 24. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G.; on behalf of the Cochrane Statistical Methods Group. Analysing Data and Undertaking Meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G. On Tests of the Overall Treatment Effect in Meta-Analysis with Normally Distributed Responses. Stat. Med. 2001, 20, 1771–1782. [Google Scholar] [CrossRef]
- Sidik, K.; Jonkman, J.N. A Simple Confidence Interval for Meta-Analysis. Stat. Med. 2002, 21, 3153–3159. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for Routinely Presenting Prediction Intervals in Meta-Analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Arenas, C.; Melián, C.; Pérez-Alenza, M.D. Long-Term Survival of Dogs with Adrenal-Dependent Hyperadrenocorticism: A Comparison between Mitotane and Twice Daily Trilostane Treatment. J. Vet. Intern. Med. 2014, 28, 473–480. [Google Scholar] [CrossRef]
- Barker, E.N.; Campbell, S.; Tebb, A.J.; Neiger, R.; Herrtage, M.E.; Reid, S.W.J.; Ramsey, I.K. A Comparison of the Survival Times of Dogs Treated with Mitotane or Trilostane for Pituitary-Dependent Hyperadrenocorticism. J. Vet. Intern. Med. 2005, 19, 810. [Google Scholar] [CrossRef]
- Castillo, V.A.; Gómez, N.V.; Lalia, J.C.; Cabrera Blatter, M.F.; García, J.D. Cushing’s Disease in Dogs: Cabergoline Treatment. Res. Vet. Sci. 2008, 85, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; De Andrés, P.J.; Arenas, C.; Melián, C.; Morales, M.; Pérez-Alenza, M.D. Comparison of Non-selective Adrenocorticolysis with Mitotane or Trilostane for the Treatment of Dogs with Pituitary-dependent Hyperadrenocorticism. Vet. Rec. 2007, 161, 805–809. [Google Scholar] [CrossRef]
- Helm, J.R.; McLauchlan, G.; Boden, L.A.; Frowde, P.E.; Collings, A.J.; Tebb, A.J.; Elwood, C.M.; Herrtage, M.E.; Parkin, T.D.H.; Ramsey, I.K. A Comparison of Factors That Influence Survival in Dogs with Adrenal-Dependent Hyperadrenocorticism Treated with Mitotane or Trilostane: Medical Management of ADH. J. Vet. Intern. Med. 2011, 25, 251–260. [Google Scholar] [CrossRef]
- de Carvalho, G.L.C.; Meirelles, L.; da Silva, C.C.; Neto, W.S.; Furtado, P.V.; Machado, L.; de Moura Martins, F.S.; da Silva Mello, F.P.; de Faria Valle, S.; Pöppl, Á.G. Assessment of Selegiline and Trilostane Combined Therapy Efficacy for Canine Pituitary-Dependent Hypercortisolism Treatment: A Pilot Randomized Clinical Trial. Res. Vet. Sci. 2022, 150, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, G.; Malerba, E.; Dolfini, C.; Brugnoli, F.; Giannuzzi, P.; Semprini, G.; Tosolini, P.; Fracassi, F. Cushing’s Syndrome-an Epidemiological Study Based on a Canine Population of 21,281 Dogs. Open Vet. J. 2019, 9, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Langner, K.; Foster, S.F.; Duff, B.C. Patient Signalment and Aetiology of Hypercortisolism in Australian Dogs with Cushing’s Syndrome. Aust. Vet. J. 2025, 103, 206–214. [Google Scholar] [CrossRef]
- Nagata, N.; Sawamura, H.; Ikenaka, Y.; Morishita, K.; Hosoya, K.; Sasaki, N.; Nakamura, K.; Takiguchi, M. Urinary Steroid Profiling Using Liquid Chromatography-Tandem Mass Spectrometry for the Diagnosis of Canine Cushing’s Syndrome. Vet. J. 2024, 306, 106151. [Google Scholar] [CrossRef] [PubMed]
- McConnell, B.M.; Cortes, Y.; Bailey, D. Retrospective Evaluation of Shock Index and Mortality in Dogs with Head Trauma (2015–2020): 86 Cases. J. Vet. Emerg. Crit. Care 2024, 34, 387–392. [Google Scholar] [CrossRef]
- Arenas Bermejo, C.; Pérez Alenza, D.; García San José, P.; Llauet, L.; Pérez-López, L.; Melián, C.; C Feldman, E. Laboratory Assessment of Trilostane Treatment in Dogs with Pituitary-Dependent Hyperadrenocorticism. J. Vet. Intern. Med. 2020, 34, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-D.; Kang, J.-H.; Chang, D.; Na, K.-J.; Yang, M.-P. Efficacy of Low- and High-Dose Trilostane Treatment in Dogs (< 5 Kg) with Pituitary-Dependent Hyperadrenocorticism. J. Vet. Intern. Med. 2013, 27, 91–98. [Google Scholar] [CrossRef]
- Burkhardt, W.A.; Boretti, F.S.; Reusch, C.E.; Sieber-Ruckstuhl, N.S. Evaluation of Baseline Cortisol, Endogenous ACTH, and Cortisol/ACTH Ratio to Monitor Trilostane Treatment in Dogs with Pituitary-Dependent Hypercortisolism. J. Vet. Intern. Med. 2013, 27, 919–923. [Google Scholar] [CrossRef]
- Di Paolo, V.; Ferrari, F.M.; Poggesi, I.; Dacasto, M.; Capolongo, F.; Quintieri, L. A Genetic Algorithm-Based Approach for Quantitative Prediction of Drug-Drug Interactions Caused by Cytochrome P450 3A Inhibitors and Inducers in Dogs and Cats. Chem. Biol. Interact. 2025, 416, 111537. [Google Scholar] [CrossRef] [PubMed]
- Cain, C.L.; White, E.; Citron, L.E.; Zheng, Q.; Morris, D.O.; Grice, E.A.; Bradley, C.W., 2nd. Longitudinal Evaluation of the Cutaneous and Rectal Microbiota of German Shepherd Dogs with Perianal Fistulas Undergoing Therapy with Ciclosporin and Ketoconazole. Vet. Dermatol. 2024, 35, 375–385. [Google Scholar] [CrossRef]
- García San José, P.; Pérez-Alenza, M.D.; Alonso-Miguel, D.; González Sanz, S.; Arenas Bermejo, C. Prevalence of Systemic Hypertension and Control of Systolic Blood Pressure in a Cohort of 14 Dogs with Adrenal-Dependent Hypercortisolism during the First Year of Trilostane Treatment or after Adrenalectomy. Animals 2024, 14, 511. [Google Scholar] [CrossRef]
- Yoshida, K.; Kobatake, Y.; Takashima, S.; Nishii, N. Evaluation of Muscle Mass and Intramuscular Fatty Infiltration in Dogs with Hypercortisolism and Their Association with Prognosis. J. Vet. Intern. Med. 2024, 38, 1334–1344. [Google Scholar] [CrossRef]
- Kumble, D.; Badiadka, N. Novel Spectrophotometric Methods for the Determination of Selegiline Hydrochloride in Bulk and Its Pharmaceutical Preparation. ISRN Spectrosc. 2014, 2014, 541970. [Google Scholar] [CrossRef]
- Wang, Y.; An, Y.; Hou, X.; Xu, Y.; Li, Z.; Liu, X.; Zheng, F.; Sun, M.; Han, R.; Lu, C.; et al. Cushing’s Syndrome in Pregnancy Secondary to Adrenocortical Adenoma: A Case Series and Review. Endocrinol. Diabetes Metab. 2024, 7, e00474. [Google Scholar] [CrossRef]
- Friede, T.; Röver, C.; Wandel, S.; Neuenschwander, B. Meta-Analysis of Two Studies in the Presence of Heterogeneity with Applications in Rare Diseases. Biom. J. 2017, 59, 658–671. [Google Scholar] [CrossRef]
- Bender, R.; Friede, T.; Koch, A.; Kuss, O.; Schlattmann, P.; Schwarzer, G.; Skipka, G. Methods for Evidence Synthesis in the Case of Very Few Studies. Res. Synth. Methods 2018, 9, 382–392. [Google Scholar] [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting Summary Statistics to Perform Meta-Analyses of the Published Literature for Survival Endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical Methods for Incorporating Summary Time-to-Event Data into Meta-Analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Barczak, S.; Hertz, Z.; Badura, B. Management of Psychosis in the Course of Cushing Syndrome: A Review. Drugs Ther. Perspect. 2024, 40, 371–382. [Google Scholar] [CrossRef]
- García San José, P.; Arenas Bermejo, C.; Alonso-Miguel, D.; González Sanz, S.; Clares Moral, I.; Portero Fuentes, M.; Pérez-Alenza, M.D. Survival of Dogs with Pituitary-Dependent Hyperadrenocorticism Treated Twice Daily with Low Doses of Trilostane. Vet. Rec. 2022, 191, e1630. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Kojima, K.; Yuki, M. Comparison of Survival Times for Dogs with Pituitary-Dependent Hyperadrenocorticism in a Primary-Care Hospital: Treated with Trilostane versus Untreated. J. Vet. Intern. Med. 2017, 31, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Hirst, T.C.; Sena, E.S.; Macleod, M.R. Using Median Survival in Meta-Analysis of Experimental Time-to-Event Data. Syst. Rev. 2021, 10, 292. [Google Scholar] [CrossRef]
- Hautekiet, J.; Prigge, E.-S.; Obermueller, T.; Arbyn, M.; Goetghebeur, E. A Framework for Meta-Analysis through Standardized Survival Curves. arXiv 2021, arXiv:2111.13132. [Google Scholar] [CrossRef]
- Salika, T.; Turner, R.M.; Fisher, D.; Tierney, J.F.; White, I.R. Implications of Analysing Time-to-Event Outcomes as Binary in Meta-Analysis: Empirical Evidence from the Cochrane Database of Systematic Reviews. BMC Med. Res. Methodol. 2022, 22, 73. [Google Scholar] [CrossRef] [PubMed]


| Author | Period | Study | Type | n | Age | Breed | Gender | Dose | Duration |
|---|---|---|---|---|---|---|---|---|---|
| Arenas et al., 2014 [25] | 1994–2009 | Retrospective | ADH | 26 | Mean 10.9 y (range 7–16) | 13 breeds various represented | 10 M 16 F | Mitotane: 50–75 mg/kg/day (9–30 days induction), then 75–100 mg/kg/week. Trilostane: 3 mg/kg PO q12. | Mitotane:15.4 months (range 2.0–37.0 months) Trilostane: 17.7 months (range 3.3–55.0 months) |
| Barker et al., 2005 [26] | 1998–2003 | Retrospective | PDH | 148 | Mean 9.6 ± 2.3 y (range 3.5–15.2) | 44 breeds; toy (26%), terrier (22%) over-represented vs. UK registrations | 75 M (45 intact, 30 neutered), 73 F (20 intact, 53 spayed) | Mitotane: Induction: 50 mg/kg PO q24 until clinical signs improved and post—ACTH cortisol < 120 nmol/L (4.32 µg/dL) Trilostane: No induction. 5–20 kg PO q24 = 60 mg 21–40 kg = 120 mg > 40 kg = 120–240 mg. Target: 4-h post-medication post-ACTH cortisol 40–120 nmol/L (1.44–4.32 µg/dL) | Mitotane: Continuous weekly maintenance after induction; continued lifelong or until death (median survival 708 d) Trilostane: Continuous once-daily (occasionally increased frequency) therapy lifelong or until death (median survival 662 d) |
| Castillo et al., 2008 [27] | NR | Prospective | PDH | 63 | Mean 9 y (range 3–14) | 63 breeds; 40% mixed breed; the remainder were pure breeds | 24 M 39 F | Ketoconazole: 20 mg kg−1 day−1 PO q24 Cabergoline: 0.07 mg kg−1 per week PO q48 | Ketoconazole: Follow-up reported to 1 year for endocrine and MRI outcomes; survival followed to 4 years Cabergoline: Same: outcomes assessed at 1 year; responders followed up to 4 years. |
| Clemente et al., 2007 [28] | 1994–2006 | Retrospective | PDH | 86 | Mean 9.5 ± 2.0 y (range 6–13) | NR | 25 M (3 neutered), 21 F (6 spayed) | Mitotane: 75–100 mg/kg/day (25-day induction), followed by lifelong glucocorticoid and mineralocorticoid replacement Trilostane: 3 mg/kg PO q12 | Mitotane: 25-day induction with mitotane, followed by lifelong hormone-replacement therapy. Dogs were followed for six months until death (median survival 720 d) Trilostane: Treatment life-long; dose adjusted at re-checks based on clinical signs and post-ACTH cortisol. (median survival 900 d) |
| Helm et al., 2011 [29] | 1996–2008 | Retrospective | ADH | 37 | Mean 11 y (range 7–14) | 14 breeds; 8 labradors, 7 crossbreeds, others ≤ 4 | 13 M 24 F (Neutered = 24/37) | Mitotane: Induction: 50 mg/kg PO q24 until clinical signs improved and post—ACTH cortisol < 120 nmol/L (4.32 µg/dL). Trilostane: No induction. PO q24 | Mitotane: Continuous medical therapy after induction; weekly maintenance continued lifelong Trilostane: PO q24 (occasionally q12) therapy lifelong; dose adjusted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanlly, S.; Slessor, J.; Yan, W.; Thorlakson, J.J.D.; Bruce, H.L.; Uwiera, R.R.E. Effectiveness of Medical Treatment on Survivability in Canine Cushing’s Syndrome: A Systematic Review and Meta-Analysis. Animals 2025, 15, 2954. https://doi.org/10.3390/ani15202954
Shanlly S, Slessor J, Yan W, Thorlakson JJD, Bruce HL, Uwiera RRE. Effectiveness of Medical Treatment on Survivability in Canine Cushing’s Syndrome: A Systematic Review and Meta-Analysis. Animals. 2025; 15(20):2954. https://doi.org/10.3390/ani15202954
Chicago/Turabian StyleShanlly, Sophia, Jordan Slessor, Wenting Yan, Jessica J. D. Thorlakson, Heather L. Bruce, and Richard R. E. Uwiera. 2025. "Effectiveness of Medical Treatment on Survivability in Canine Cushing’s Syndrome: A Systematic Review and Meta-Analysis" Animals 15, no. 20: 2954. https://doi.org/10.3390/ani15202954
APA StyleShanlly, S., Slessor, J., Yan, W., Thorlakson, J. J. D., Bruce, H. L., & Uwiera, R. R. E. (2025). Effectiveness of Medical Treatment on Survivability in Canine Cushing’s Syndrome: A Systematic Review and Meta-Analysis. Animals, 15(20), 2954. https://doi.org/10.3390/ani15202954





