Variability in Morphological Traits and Nutritional Profiles of Adult Eriocheir sinensis in Different Aquacultural Regions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Measurements of Morphometric Attributes
2.3. Measurements of Proximate Composition
2.4. Amino Acid Analysis and Assessments of Protein Quality
2.5. Fatty Acids Analysis
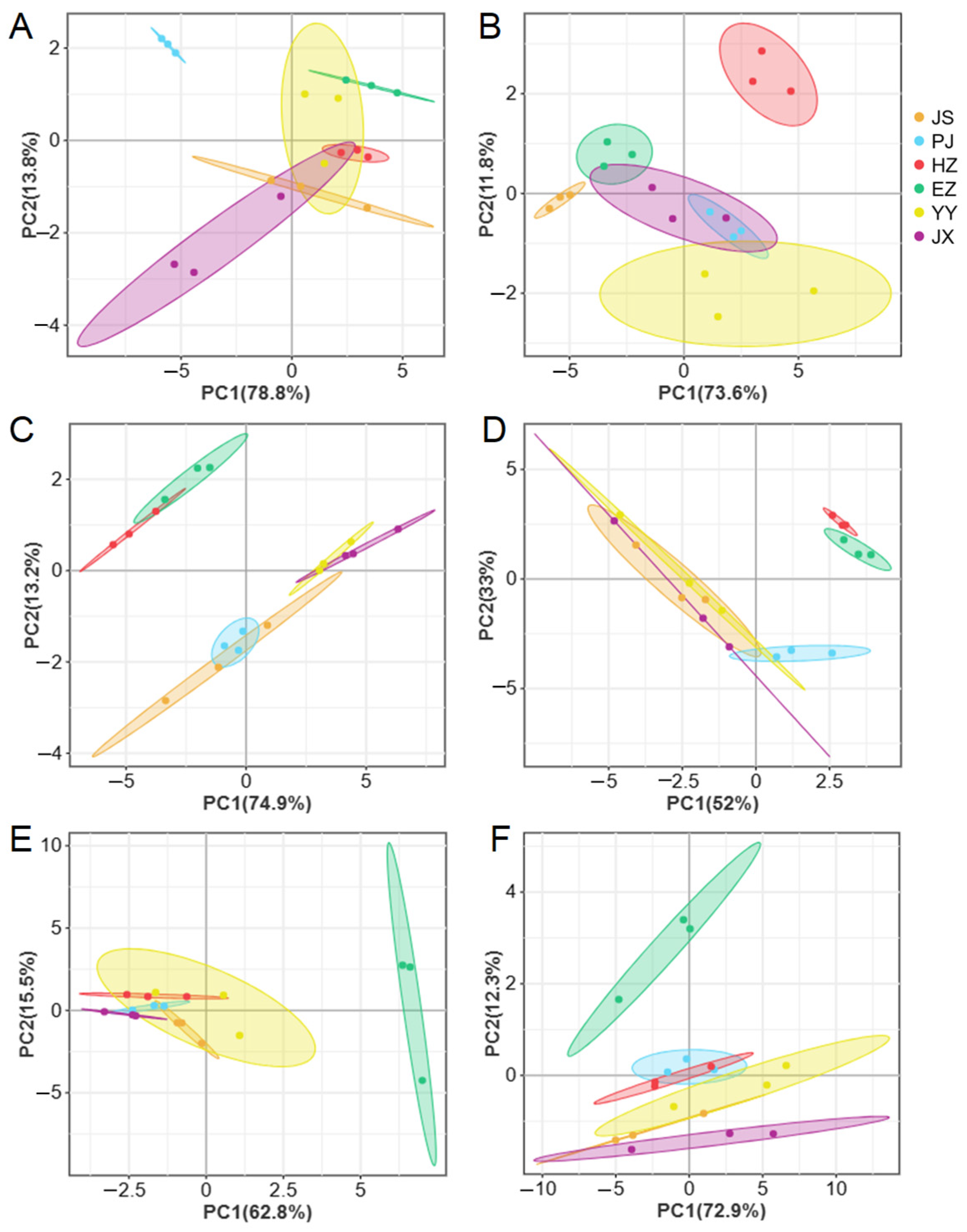
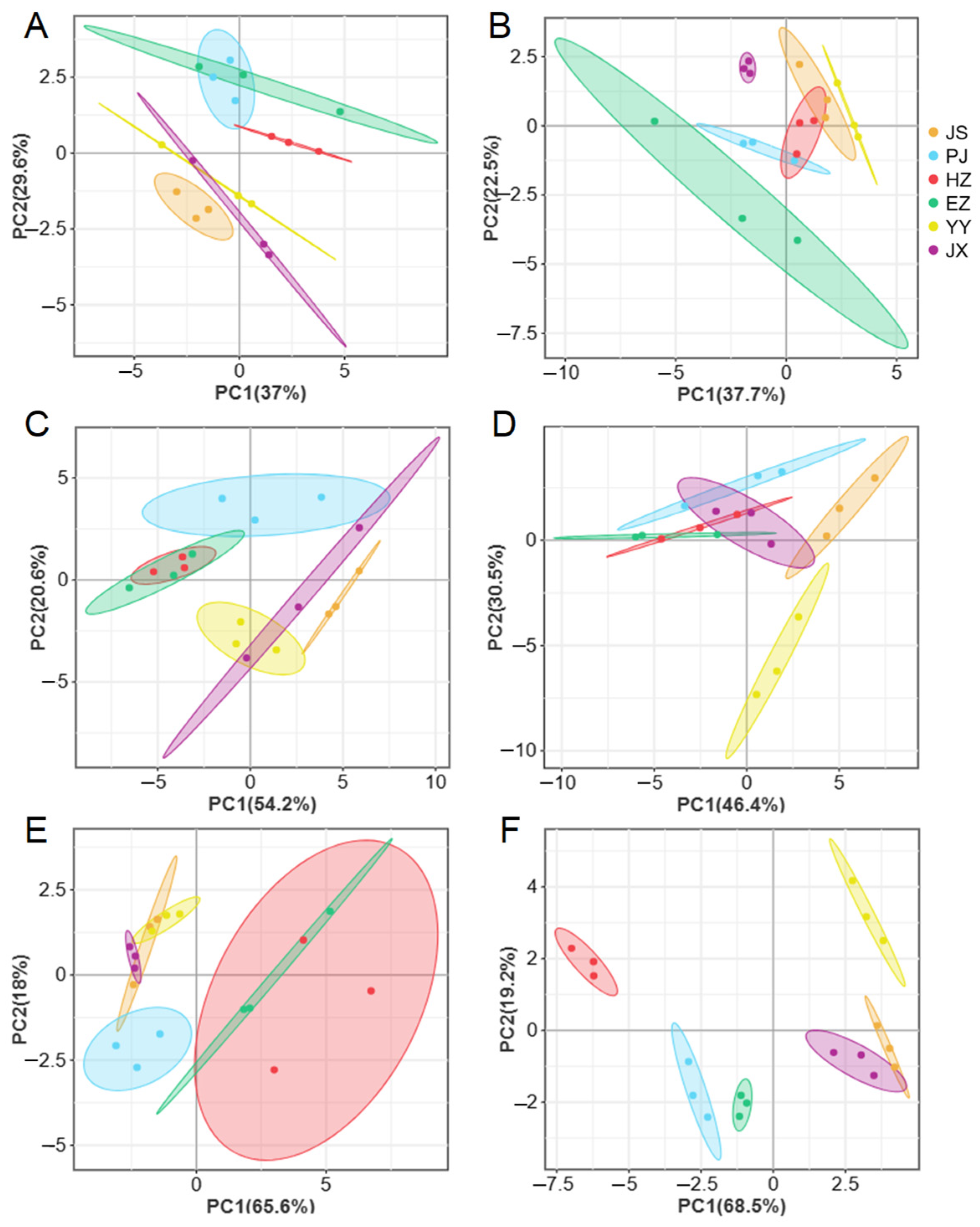
2.6. Principal Component Analysis
2.7. Statistical Analysis
3. Results
3.1. The Morphological Parameters
3.2. Proximate Composition
3.3. Variation in Amino Acids Composition
3.4. Variation in Fatty Acids Profiles
4. Discussion
4.1. The Effect of Different Regions on the Morphological Parameters
4.2. The Effect of Different Regions on Basic Nutrients in the Three Edible Parts
4.3. The Effect of Different Regions on Amino Acids Contents
4.4. The Effect of Different Regions on Fatty Acids Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.; Xu, W.; Lu, K. Preliminary discussions on some problems about artificial propagation of E. sinensis in earth ponds. J. Zhejiang Coll. Fish. 1995, 03, 177–181. [Google Scholar]
- Cheng, Y.; Wu, X.; Yang, X.; Hines, A.H. Current trends in hatchery techniques and stock enhancement for Chinese mitten crab, Eriocheir japonica sinensis. Rev. Fish. Sci. 2008, 16, 377–384. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, X.; Cui, Y. Stocking models of Chinese mitten crab (Eriocheir japonica sinensis) in Yangtze lakes. Aquaculture 2006, 255, 456–465. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Ma, X. Ecological Aquaculture of Chinese Mitten Crab; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. China Fishery Statistics Yearbook; China Agricultural Press: Beijing, China, 2020. [Google Scholar]
- Wu, X.; Cheng, Y.; Sui, L.; Yang, X.; Nan, T.; Wang, J. Biochemical composition of pond-reared and lake-stocked Chinese mitten crab Eriocheir sinensis (H. Milne-Edwards) broodstock. Aquac. Res. 2007, 38, 1459–1467. [Google Scholar] [CrossRef]
- Wang, X.; Han, G.; Ma, B.; Song, J.; Mu, Y.; Meng, D. Differences of Nutritional Components in Chinese Eriocheir sinensis: Research Progress. Chin. Agric. Sci. Bull. 2019, 35, 122–128. [Google Scholar]
- Chen, Q.Q. Nutritional Quality Analysis of Listed Chinese Mitten Crab (Eriocheir sinensis) in Yangtze River Delta. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2023. [Google Scholar]
- Zhang, D.; Jiang, X.; Zu, L.; Zhou, W.; Luo, M.; Cheng, Y.; Wu, X. A comparative evaluation of the nutritional quality of three wild populations of female mitten crabs (Eriocheir sensu stricto) in northern China. Crustaceana 2021, 94, 309–324. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Long, X.; Zhu, W.; Ma, T.; Cheng, Y. Nutritional quality of different grades of adult male chinese mitten crab, Eriocheir sinensis. J. Food Sci. Technol. 2018, 55, 944–955. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Cheng, Y.; Wu, X. Growth performance, gonad development and nutritional composition of Chinese mitten crab Eriocheir sinensis selected for growth and different maturity time. Aquaculture 2020, 523, 735194. [Google Scholar] [CrossRef]
- Yi, X.; Gao, J.; Li, L.; Du, J.; Nie, Z.; Zhang, X.; Xu, G. Effects of fattening diets on the nutritional quality and flavor of the adult female Chinese mitten crab (Eriocheir sinensis). Aquac. Rep. 2022, 25, 101223. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, S.; Zhang, H.; Liu, M.; Wu, N.; Pan, J.; Luo, M.; Wang, X.; Cheng, Y. Fattening culture improves the gonadal development and nutritional quality of male Chinese mitten crab Eriocheir sinensis. Aquaculture 2020, 518, 734865. [Google Scholar] [CrossRef]
- Li, Q.; Zu, L.; Cheng, Y.; Wade, N.M.; Liu, J.; Wu, X. Carapace color affects carotenoid composition and nutritional quality of the Chinese mitten crab, Eriochier sinensis. LWT-Food Sci. Technol. 2020, 126, 109286. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, W.; Zhu, R.; Zhang, D.; Wang, N.; Zheng, N.; Wang, S.; Liu, H.; Wan, J.; Chen, Y.; et al. Comparative study on growth performance and edible portion nutritional composition of male Eriocheir sinensis at different growth stages in rice-crab culture systems. J. Food Compos. Anal. 2024, 130, 106156. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, W.; Zhu, R.; Wang, N.; Yao, Q.; Liu, H.; Wan, J.; Chen, Y.; Wang, Q.; Zhang, D. Comparative Study on Growth Index and Nutritional Quality of Female Chinese Mitten Crab Eriocheir sinensis Selected at Different Growth Periods in Rice-Crab Culture Systems. Aquac. Nutr. 2023, 2023, 4805919. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ge, M.; Chen, H.; Jiang, S.; Lin, L.; Lu, J. Comparison between the nutritional qualities of wild-caught and rice-field male Chinese mitten crabs (Eriocheir sinensis). LWT-Food Sci. Technol. 2020, 117, 108663. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.U.; Wang, Y.; Tao, N.; Wu, X.; Wang, X.; Qiu, W.; Ma, M. Comparison of flavour qualities of three sourced Eriocheir sinensis. Food Chem. 2016, 200, 24–31. [Google Scholar] [CrossRef]
- Tang, M.; Qu, Z.; Shi, W.; Wang, X.; Wu, X. A comparative study on the flavour of wild Chinese mitten crab (Eriocheir sinensis) along the eastern coast of China. J. Food Compos. Anal. 2024, 127, 105949. [Google Scholar] [CrossRef]
- Wang, S.; Luo, L.; Zhang, R.; Guo, K.; Zhang, X.; Kong, L.; Zhao, Z. Biochemical composition and quality of wild-caught adult mitten crabs from three river basins. J. Food Compos. Anal. 2022, 110, 104574. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Li, J.; Huang, Q.; Huang, Z.; Cheng, Y. Comparison of the culture performance and profitability of wild-caught and captive pond-reared Chinese mitten crab (Eriocheir sinensis) juveniles reared in grow-out ponds: Implications for seed selection and genetic selection programs. Aquaculture 2014, 434, 48–56. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Wu, X.; Zhang, X.; Zhao, J.; Yang, J.; Cheng, Y. Gonadal development and biochemical composition of Chinese mitten crabs (Eriocheir sinensis) from four sources. J. Food Sci. 2021, 86, 1066–1080. [Google Scholar] [CrossRef]
- Mei, J.; Liang, X.; Yu, Y.; Lang, Y.; Li, X. The comparison and analysis of nutritional qualities of Chinese mitten crabs (Eriocheir sinensis) in rice field and pond culture modes. Food Chem. X 2023, 20, 100937. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Tang, C.; Sun, Y.; Cheng, Y. Nutritional quality of adult Eriocheir sinensis from the Yangtze River and Yellow River populations cultured in ponds at the Yellow River estuary area. Aquac. Fish. 2024. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Zhang, W.B.; Ma, X.Z. Comparative studies on morphological differences among four local populations of mitten crab. Chin. Agric. Sci. Bull. 2007, 23, 648–653. [Google Scholar]
- Wang, X.A.; Ma, A.J.; Zhuang, Z.M. Judgment of selection index of morphological traits in Fugu rubripes. Prog. Fish. Sci. 2012, 33, 10–15. [Google Scholar]
- Wang, Q.; Li, X.D.; Dai, W.; Jin, T.T.; Li, L.; Wang, X.M. The morphological discrimination of populations of mitten crab in different river systems. Fish. Sci. 2006, 25, 125–128. [Google Scholar]
- He, J.; Xu, P.; Zhu, J. Morphological variations and species validity of Eriocheir sinensis in the north and south drainage systems of China. Trans. Oceanol. Limnol. 2009, 3, 79–86. [Google Scholar]
- Lu, Y.; Wu, X.; He, J.; Wang, C.; Li, X.; Liu, N.; Wang, Y.; Cheng, Y. Comparative studies of the morphology and biochemical composition of wild juvenile Chinese mitten crabs from the Yangtze River, Yellow River and Liaohe River systems. J. Fish. Sci. China 2016, 23, 382–395. [Google Scholar]
- Xue, J.; Liu, H.; Jiang, T.; Chen, X.; Yang, J. Shape variation in the carapace of Chinese mitten crabs (Eriocheir sinensis H. Milne Edwards, 1853) in Yangcheng Lake during the year-long culture period. Eur. Zool. J. 2022, 89, 217–228. [Google Scholar] [CrossRef]
- Zhao, H. Comparative Studies on Morphology, Culture Performance and Biochemical Composition Among Yangtze, Huang, and Liao River Populations of Adult Eriocheir sinensis; Shanghai Ocean University: Shanghai, China, 2016. [Google Scholar]
- GB/T 19957-2005; AQSIQ (General Administration of Quality Supervision, Inspection, and Quarantine of the People’s Republic of China). Product of Geographical Indication—Yangcheng Lake Chinese Mitten Crab. Standards Press of China: Beijing, China, 2005. (In Chinese)
- Feng, W.; Feng, W.; Ge, J.; Li, J.; Su, S.; Jia, R.; Yu, J.; Xu, P.; Tang, Y. Alterations of amino acid metabolism and intestinal microbiota in Chinese mitten crab (Eriocheir sinensis) fed on formulated diet and iced trash fish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100924. [Google Scholar] [CrossRef]
- Food and Agricultural Organization. Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAO Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Millward, D.J. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr. 2012, 108, S31–S43. [Google Scholar] [CrossRef]
- Seligson, F.H.; Mackey, L.N. Variable predictions of protein quality by chemical score due to amino acid analysis and reference pattern. J. Nutr. 1984, 114, 682–691. [Google Scholar] [CrossRef]
- Guo, Y.; Gu, S.; Wang, X.; Zhao, L.; Zheng, J. Comparison of fatty acid and amino acid profiles of steamed Chinese mitten crab. Fish. Sci. 2014, 80, 621–633. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Wu, Y.; Luo, X.; Xu, Z.; Pan, J.; Zou, G.; Liang, H. Comparison of Body Characteristics, Carotenoid Composition, and Nutritional Quality of Chinese Mitten Crab (Eriocheir sinensis) with Different Hepatopancreas Redness. Foods 2024, 13, 993. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ren, M.; Li, S. Morphological identification of population of Eriocheir sinensis from Changjiang, Liaohe, and Oujiang rivers. J. Fish. China 1997, 21, 269–274. [Google Scholar]
- Jiang, X.; Wu, X.; Wang, H.; Yang, Y.; Cheng, Y. Can morphological characters be used to identify the pond-reared offspring of wild Chinese mitten crabs, Eriocheir sinensis, from different basins? Crustaceana 2019, 92, 957–978. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, X.; He, J.; Deng, D.; Xiang, C.; Cheng, Y. Effects of selective breeding on the morphological characteristics of wild and cultured juvenile Chinese mitten crab (Eriocheir sinensis). J. Fish. China 2018, 42, 1285–1298. [Google Scholar]
- Chen, J.; Ma, X.; Wang, W.; Yang, Y.; Tao, C. The study of relationships between growth, molt and accumulated temperature of the Chinese mitten crab (Eriocheir sinensis). J. Shanghai Ocean. Univ. 2016, 25, 675–683. [Google Scholar]
- Yi, P.; Fan, C.; Jiang, X.; Wu, X. Morphological differences of adult Chinese mitten crabs Eriocheir sinensis from different selected strains. Fish. Sci. Technol. Inf. 2023, 50, 218–223. [Google Scholar]
- Ikpe, E.; Ubong, U.; Archibong, U. Proximate analysis, heavy metals and total hydrocarbon content of Callinectes sapidus obtained from Ibaka River, Akwa Ibom State, Nigeria. Res. J. Sci. Technol. 2022, 2, 32–43. [Google Scholar]
- Wang, S.; Luo, L.; Zhang, R.; Guo, K.; Zhao, Z. The Biochemical Composition and Quality of Adult Chinese Mitten Crab Eriocheir sinensis Reared in Carbonate-Alkalinity Water. Foods 2024, 13, 362. [Google Scholar] [CrossRef] [PubMed]
- Nasef, A.M. Effect of Variation in Aquatic Environment Type on Biochemical Composition and Protein Quality in Some Fishes. Egypt. Acad. J. Biol. Sci. B Zool. 2021, 13, 207–223. [Google Scholar] [CrossRef]
- Jiang, H.; Yin, Y.; Zhang, X.; Hu, S.; Wang, Q. Chasing relationships between nutrition and reproduction: A comparative transcriptome analysis of hepatopancreas and testis from Eriocheir sinensis. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2009, 4, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Boucard, C.G.V.; Patrois, J.; Ceccaldi, H.J. Exhaustion of lipid reserves in the hepatopancreas of Fenneropenaeus indicus broodstock in relation to successive spawnings. Aquaculture 2004, 236, 523–537. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Cheng, Y.; Zeng, C.; Yang, X. Ovarian re-maturation following the first spawning in the Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards). Aquac. Res. 2011, 42, 417–426. [Google Scholar] [CrossRef]
- Gallo, A.; Costantini, M. Glycobiology of reproductive processes in marine animals: The state of the art. Mar. Drugs 2012, 10, 2861–2892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yao, Q.; Zhang, D.; Wang, N.; Liu, H.; Wan, J.; Chen, Y.; Wang, Q.; Guo, Z. Comparative study on growth, digestive function and intestinal microbial composition of female Chinese mitten crab Eriocheir sinensis selected at different growth stages in rice-crab culture systems. Aquaculture 2022, 554, 738120. [Google Scholar] [CrossRef]
- Gao, J.; Tai, X.; Shao, N.; Sun, Y.; Nie, Z.; Wang, Y.; Li, Q.; Xu, P.; Xu, G. Effects of effective microorganisms on the growth performance, nutritional composition and flavour quality of the pond-cultured Eriocheir sinensis. Aquac. Res. 2021, 52, 871–880. [Google Scholar] [CrossRef]
- Xu, J.Q.; Wu, X.G.; Zhang, P.C.; He, J.; Fan, Y.W.; Liu, M.M.; Cheng, Y.X. Growth, gonadal development and secondary sexual characteristics of pond-reared male Chinese mitten crab (Eriocheir sinensis) during the second year culture. Chin. J. Zool. 2016, 51, 434–448. [Google Scholar]
- Shao, L.; Wang, C.; He, J.; Wu, X.; Cheng, Y. Meat quality of Chinese mitten crabs fattened with natural and formulated diets. J. Aquat. Food Prod. Technol. 2014, 23, 59–72. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, S.; Sun, B.; Xiong, L.; Cheng, Y. Composition and nutritional qualities of edible tissues of Chinese mitten crab (Eriocheir sinensis) from Ya Lake over different months. J. Food Compos. Anal. 2022, 105, 104199. [Google Scholar] [CrossRef]
- Xing, S.; Liang, X.; Zhang, X.; Oliva Teles, A.; Peres, H.; Li, M.; Wang, H.; Mai, K.; Kaushik, S.J.; Xue, M. Essential amino acid requirements of fish and crustaceans, a meta-analysis. Rev. Aquac. 2024, 16, 1069–1086. [Google Scholar] [CrossRef]
- World Health Organization; United Nations University. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Brestenský, M.; Nitrayová, S.; Patráš, P.; Nitray, J. Dietary requirements for proteins and amino acids in human nutrition. Curr. Nutr. Food Sci. 2019, 15, 638–645. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, X.; Zhou, W.; Chen, W.; Levy, T.; Wu, X. Stocking density affects culture performance and economic profit of adult all-female Chinese mitten crabs (Eriocheir sinensis) reared in earthen ponds. Aquaculture 2024, 581, 740352. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Y.; Jeppesen, E.; Gu, X.; Mao, Z.; Chen, H. Farming practices affect the amino acid profiles of the aquaculture Chinese mitten crab. Peerj 2021, 9, e11605. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wang, M.; Li, J.; Song, C.; Ge, J.; Su, S.; Tang, Y. Studies on amino acid composition, nutritional scores and speciesidentification of muscle tissues of Eriocheir sinensis from six differentgeographical lakes. Freshw. Fish. 2021, 51, 28–37. [Google Scholar]
- Zhao, L.; Long, Y.; Zhang, L.; Zhang, C.; Xu, L.; Liu, Q.; Luo, J.; Du, Z.; Yang, S. Effects of paddyfield and pond culture on intestinal microbiota, immunecapacity and muscle free amino acids of Eriocheir sinensis. J. Shanghai Ocean Univ. 2022, 31, 1404–1412. [Google Scholar]
- Han, W.; Sun, Y.; Liu, J.; Zhang, Y.; Lu, Z.; Cheng, Y. Effect of different feeding modes on the growth, biochemical composition, and living environment of the juvenile Chinese mitten crab Eriocheir sinensis. Aquaculture 2021, 541, 736687. [Google Scholar] [CrossRef]
- Long, X.; Guo, Q.; Wang, X.; Francis, D.S.; Cheng, Y.; Wu, X. Effects of fattening period on ovarian development and nutritional quality of adult female Chinese mitten crab Eriocheir sinensis. Aquaculture 2020, 519, 734748. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Liu, P.; Chen, J.; Zhang, C. Uncovering the nutritive profiles of adult male chinese mitten crab (E. sinensis) harvested from the pond and natural water area of Qin lake based on metabolomics. Foods 2023, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Liu, Y. Study on the nutritional quality of ecologically bred Chinese mitten crabs with different body weights. Aquac. Res. 2020, 51, 2948–2961. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Chen, J.; Sun, B.; Zhang, D. Association of Dietary n3 and n6 Fatty Acids Intake with Hypertension: NHANES 2007–2014. Nutrients 2019, 11, 1232. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: Relationship to tissue development and aging. Prostaglandins Leukot. Essent. Fat. Acids 2016, 114, 28–34. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 polyunsaturated fatty acids, obesity and cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- FAO; WHO. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; FAO: Rome, Italy, 2008; Volume 10, p. 14. [Google Scholar]
- Barrento, S.; Marques, A.; Teixeira, B.; Mendes, R.; Bandarra, N.; Vaz-Pires, P.; Nunes, M.L. Chemical composition, cholesterol, fatty acid and amino acid in two populations of brown crab Cancer pagurus: Ecological and human health implications. J. Food Compos. Anal. 2010, 23, 716–725. [Google Scholar] [CrossRef]
- Yu, J.Y.; Yan, P.S. Fatty acid composition evaluation of edible parts of Eriocheir sinensis in intensive ponds of Gucheng waters. Genet. Mol. Res. 2015, 14, 5334–5345. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Kasyanov, S.P.; Kharlamenko, V.I.; Svetashev, V.I. Lipids and of fatty acids of edible crabs of the north-western Pacific. Food Chem. 2009, 116, 657–661. [Google Scholar] [CrossRef]
- Lee, M.; Park, J.C.; Lee, J. Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquat. Toxicol. 2018, 200, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, S.; Zhang, C.; Zhu, J.; Feng, W.; Chen, H.; Jiang, J.; Lu, Z.; Liu, W.; Gan, J. The Role of Biogeography in Shaping Intestinal Flora and Influence on Fatty Acid Composition in Red Swamp Crayfish (Procambarus clarkii). Microb. Ecol. 2023, 86, 3111–3127. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.C. Comparison of gender differences in nutritional value and key odor profile of hepatopancreas of Chinese mitten crab (Eriocheir sinensis). J. Food Sci. 2017, 82, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zheng, J.; Chen, C.; Wu, K.; Lin, F.; Ning, L.; Rong, H.; Chen, C.; Xiao, F.; Zhang, H. Differences in lipid accumulation and mobilization in the hepatopancreas and ovary of female mud crab (Scylla paramamosain, Estampador, 1949) during ovarian development. Aquaculture 2023, 564, 739046. [Google Scholar] [CrossRef]
- Wen, X.; Chen, L.; Ai, C.; Zhou, Z.; Jiang, H. Variation in lipid composition of Chinese mitten-handed crab, Eriocheir sinensis during ovarian maturation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 95–104. [Google Scholar] [CrossRef]
- Ying, X.; Yang, W.; Zhang, Y. Comparative studies on fatty acid composition of the ovaries and hepatopancreas at different physiological stages of the Chinese mitten crab. Aquaculture 2006, 256, 617–623. [Google Scholar] [CrossRef]
- Jia, L. Comparison of Environmental Factors and Muscle Quality in Rice-Shrimp Integrated Culture System in Different Areas and Establishment of Geographica Origin Traceability System; Shanghai Ocean University: Shanghai, China, 2022. [Google Scholar]



| Sampling Sites | Sex | Size | Body Weight (g) | Carapace Length (mm) |
|---|---|---|---|---|
| Yongyan Anhui (YY) | Male | 15 | 120.85 ± 5.98 | 56.99 ± 0.84 |
| Female | 15 | 112.27 ± 4.06 | 57.52 ± 0.64 | |
| Panjin Liaoning (PJ) | Male | 15 | 86.20 ± 1.82 | 50.75 ± 0.23 |
| Female | 15 | 57.90 ± 2.08 | 45.86 ± 0.50 | |
| Huzhou Zhejiang (HZ) | Male | 15 | 150.97 ± 1.72 | 61.22 ± 0.29 |
| Female | 15 | 146.42 ± 2.37 | 63.50 ± 0.35 | |
| Jinxian Jiangxi (JX) | Male | 15 | 246.04 ± 4.46 | 70.93 ± 0.27 |
| Female | 15 | 166.29 ± 1.97 | 65.61 ± 0.37 | |
| Changzhou Jiangsu (CZ) | Male | 15 | 212.97 ± 3.84 | 67.07 ± 0.49 |
| Female | 15 | 140.24 ± 1.24 | 61.53 ± 0.24 | |
| Ezhou Hubei (EZ) | Male | 15 | 174.12 ± 2.65 | 62.38 ± 0.28 |
| Female | 15 | 122.89 ± 2.03 | 59.06 ± 0.33 |
| HZ | PJ | EZ | YY | JX | CZ | |
|---|---|---|---|---|---|---|
| Male | ||||||
| T/C1 | 0.5394 ± 0.0051 c | 0.5442 ± 0.0052 bc | 0.5446 ± 0.0043 bc | 0.5426 ± 0.002 c | 0.5551 ± 0.0051 b | 0.5686 ± 0.0037 a |
| C2/C1 | 1.1345 ± 0.0205 | 1.1221 ± 0.0041 | 1.1319 ± 0.0058 | 1.1337 ± 0.0035 | 1.1381 ± 0.0055 | 1.1270 ± 0.0039 |
| C3/C1 | 0.2416 ± 0.0023 bc | 0.2442 ± 0.0025 c | 0.2458 ± 0.0034 c | 0.2426 ± 0.0035 c | 0.2344 ± 0.0021 a | 0.2328 ± 0.0018 a |
| C4/C1 | 0.6347 ± 0.0029 a | 0.6237 ± 0.0033 ab | 0.6140 ± 0.0044 bc | 0.6097 ± 0.0039 c | 0.6166 ± 0.0048 bc | 0.6215 ± 0.0047 b |
| C5/C1 | 0.5416 ± 0.0031 ab | 0.5370 ± 0.0023 b | 0.5405 ± 0.0023 ab | 0.5464 ± 0.0021 a | 0.5381 ± 0.0025 b | 0.5443 ± 0.0026 ab |
| L1/C1 | 0.7909 ± 0.0084 a | 0.7973 ± 0.0043 a | 0.7685 ± 0.0072 b | 0.7893 ± 0.0073 a | 0.7708 ± 0.0060 b | 0.8022 ± 0.0061 a |
| L2/C1 | 0.5544 ± 0.0078 ab | 0.5560 ± 0.0033 ab | 0.5706 ± 0.0336 a | 0.5519 ± 0.0057 ab | 0.5281 ± 0.0049 b | 0.5504 ± 0.0049 ab |
| L3/C1 | 0.4623 ± 0.0072 b | 0.5183 ± 0.0044 a | 0.4462 ± 0.0049 bc | 0.4424 ± 0.0067 c | 0.4457 ± 0.0064 bc | 0.4564 ± 0.0070 bc |
| Female | ||||||
| T/C1 | 0.5714 ± 0.0054 ab | 0.5764 ± 0.0082 ab | 0.5678 ± 0.0036 b | 0.5707 ± 0.0065 ab | 0.5868 ± 0.0039 a | 0.5828 ± 0.0057 ab |
| C2/C1 | 1.1120 ± 0.0202 | 1.1105 ± 0.0037 | 1.1236 ± 0.0019 | 1.0503 ± 0.0711 | 1.1236 ± 0.0022 | 1.1261 ± 0.0041 |
| C3/C1 | 0.2394 ± 0.0025 ab | 0.2467 ± 0.0033 a | 0.2347 ± 0.0027 bc | 0.2336 ± 0.0023 bc | 0.2377 ± 0.0033 b | 0.2301 ± 0.0017 c |
| C4/C1 | 0.6071 ± 0.0133 b | 0.6382 ± 0.0040 a | 0.6028 ± 0.0039 b | 0.6053 ± 0.0042 b | 0.6062 ± 0.0034 b | 0.6171 ± 0.0031 b |
| C5/C1 | 0.5343 ± 0.0028 bc | 0.5272 ± 0.0025 d | 0.5293 ± 0.0024 cd | 0.5426 ± 0.0022 a | 0.5369 ± 0.0020 ab | 0.5314 ± 0.0023 bcd |
| L1/C1 | 0.7148 ± 0.0090 b | 0.7240 ± 0.0086 b | 0.7161 ± 0.0053 b | 0.7278 ± 0.0040 ab | 0.7141 ± 0.0060 b | 0.7473 ± 0.0096 a |
| L2/C1 | 0.5117 ± 0.0079 | 0.5173 ± 0.0060 | 0.5056 ± 0.0051 | 0.5199 ± 0.0041 | 0.5092 ± 0.0055 | 0.5196 ± 0.0076 |
| L3/C1 | 0.4194 ± 0.0087 b | 0.4854 ± 0.0088 a | 0.4248 ± 0.0063 b | 0.4251 ± 0.0043 b | 0.4293 ± 0.0115 b | 0.4259 ± 0.0051 b |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | PJ | HZ | EZ | YY | JX | CZ | PJ | HZ | EZ | YY | JX | |
| Muscle | ||||||||||||
| Moisture | 78.90 ± 0.26 a | 78.47 ± 0.48 a | 73.60 ± 0.71 d | 76.13 ± 0.34 b | 75.83 ± 0.84 bd | 78.23 ± 0.20 ab | 78.03 ± 0.12 a | 76.7 ± 0.76 ab | 74.10 ± 1.21 b | 77.07 ± 1.38 a | 75.5 ± 0.21 ab | 77.83 ± 0.54 a |
| Ash | 1.73 ± 0.09 bc | 2.20 ± 0.06 a | 1.97 ± 0.09 ab | 1.63 ± 0.07 c | 1.67 ± 0.09 bc | 1.40 ± 0.06 c | 1.67 ± 0.09 b | 2.03 ± 0.03 a | 1.70 ± 0.06 b | 1.63 ± 0.15 b | 1.70 ± 0.06 b | 1.63 ± 0.09 b |
| Crude lipid | 0.83 ± 0.09 b | 0.90 ± 0.10 b | 1.07 ± 0.12 ab | 0.97 ± 0.03 b | 1.20 ± 0.21 a | 1.37 ± 0.12 a | 0.80 ± 0.06 c | 0.77 ± 0.09 cd | 0.90 ± 0.06 bc | 1.03 ± 0.09 b | 0.67 ± 0.03 d | 1.50 ± 0.06 a |
| Crude protein | 16.80 ± 0.46 c | 16.80 ± 0.23 c | 21.23 ± 0.79 a | 19.60 ± 0.21 b | 18.93 ± 0.37 b | 17.20 ± 0.10 c | 18.00 ± 0.25 b | 17.93 ± 0.64 b | 18.07 ± 0.84 b | 17.33 ± 0.23 b | 21.10 ± 0.20 a | 17.10 ± 0.36 b |
| Sugar | 0.70 ± 0.10 c | 1.07 ± 0.07 b | 1.53 ± 0.12 a | 0.70 ± 0.10 c | 1.60 ± 0.15 a | 1.10 ± 0.06 b | 0.47 ± 0.03 e | 1.33 ± 0.03 b | 2.27 ± 0.09 a | 1.03 ± 0.09 c | 0.67 ± 0.03 d | 1.27 ± 0.03 b |
| Hepatopancreas | ||||||||||||
| Moisture | 55.83 ± 1.43 b | 64.97 ± 2.53 a | 44.53 ± 0.87 c | 45.77 ± 1.49 c | 46.10 ± 2.17 c | 55.00 ± 3.10 b | 54.23 ± 4.69 a | 58.23 ± 3.67 a | 43.27 ± 0.49 b | 43.67 ± 1.79 b | 35.07 ± 2.78 b | 39.47 ± 2.14 b |
| Ash | 1.37 ± 0.15 | 1.50 ± 0.06 | 1.47 ± 0.09 | 1.43 ± 0.09 | 1.27 ± 0.09 | 1.30 ± 0.12 | 1.30 ± 0.12 | 1.33 ± 0.07 | 1.10 ± 0.06 | 1.37 ± 0.03 | 1.10 ± 0.15 | 1.10 ± 0.06 |
| Crude lipid | 29.27 ± 1.58 bc | 21.73 ± 2.67 c | 41.53 ± 1.59 a | 37.73 ± 3.62 ab | 40.9 ± 2.01 a | 32.97 ± 3.03 b | 29.53 ± 5.48 c | 27.80 ± 3.27 c | 37.93 ± 3.58 bc | 42.90 ± 2.52 ab | 53.20 ± 3.58 a | 48.47 ± 2.34 ab |
| Crude protein | 9.45 ± 0.23 ab | 10.09 ± 0.37 a | 8.08 ± 0.03 c | 9.56 ± 0.21 ab | 9.71 ± 0.25 ab | 8.97 ± 0.25 b | 11.47 ± 0.43 a | 10.63 ± 0.61 ab | 9.09 ± 0.04 b | 8.92 ± 0.21 b | 9.24 ± 1.14 b | 9.63 ± 0.24 b |
| Sugar | 0.83 ± 0.03 c | 1.20 ± 0.21 c | 2.33 ± 0.03 a | 1.77 ± 0.07 b | 1.23 ± 0.03 c | 0.87 ± 0.07 c | 1.50 ± 0.10 b | 1.50 ± 0.06 b | 2.40 ± 0.15 a | 1.23 ± 0.15 b | 0.73 ± 0.03 c | 0.60 ± 0.06 c |
| Gonad | ||||||||||||
| Moisture | 73.43 ± 0.55 a | 71.03 ± 0.55 b | 70.8 ± 0.80 b | 71.87 ± 0.73 ab | 73.47 ± 0.20 a | 73.30 ± 0.25 a | 52.77 ± 0.52 a | 52.97 ± 1.57 a | 48.70 ± 0.98 b | 50.13 ± 0.62 b | 44.37 ± 0.29 d | 51.53 ± 0.12 ab |
| Ash | 2.40 ± 0.10 a | 2.10 ± 0.06 ab | 1.90 ± 0.12 b | 2.13 ± 0.12 ab | 2.33 ± 0.15 a | 2.27 ± 0.15 a | 2.17 ± 0.12 cd | 2.40 ± 0.12 bc | 1.83 ± 0.34 cd | 2.80 ± 0.25 ab | 3.20 ± 0.17 a | 1.57 ± 0.09 d |
| Crude lipid | 0.80 ± 0.06 c | 1.10 ± 0.15 bc | 2.17 ± 0.20 a | 1.23 ± 0.07 b | 0.93 ± 0.19 bc | 0.83 ± 0.09 bc | 14.23 ± 0.62 b | 15.33 ± 0.77 ab | 16.8 ± 0.53 a | 14.60 ± 0.26 b | 16.30 ± 0.47 a | 15.60 ± 0.10 ab |
| Crude protein | 16.53 ± 0.15 d | 21.03 ± 1.12 a | 17.30 ± 0.65 cd | 21.17 ± 0.09 a | 18.47 ± 0.22 bc | 19.13 ± 0.13 b | 26.23 ± 0.72 b | 25.67 ± 2.44 b | 30.23 ± 0.72 a | 28.67 ± 0.33 ab | 31.33 ± 0.27 a | 29.70 ± 0.17 a |
| Sugar | 4.70 ± 0.10 a | 3.37 ± 0.13 c | 4.03 ± 0.15 b | 3.03 ± 0.09 c | 3.93 ± 0.03 b | 3.93 ± 0.13 b | 3.10 ± 0.12 a | 3.10 ± 0.10 a | 2.20 ± 0.35 b | 2.83 ± 0.19 a | 3.37 ± 0.03 a | 1.87 ± 0.03 c |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | PJ | HZ | EZ | YY | JX | CZ | PJ | HZ | EZ | YY | JX | |
| Aspartic acid | 1.406 ± 0.054 b | 1.099 ± 0.005 b | 1.582 ± 0.021 a | 1.618 ± 0.033 a | 1.435 ± 0.018 b | 1.189 ± 0.079 c | 1.218 ± 0.010 c | 1.417 ± 0.011 b | 1.542 ± 0.009 a | 1.392 ± 0.015 b | 1.432 ± 0.049 b | 1.368 ± 0.021 b |
| Threonine * | 0.668 ± 0.025 b | 0.506 ± 0.001 c | 0.760 ± 0.009 a | 0.750 ± 0.016 a | 0.687 ± 0.010 b | 0.558 ± 0.036 c | 0.596 ± 0.004 c | 0.677 ± 0.006 ab | 0.708 ± 0.004 a | 0.636 ± 0.007 c | 0.713 ± 0.032 a | 0.658 ± 0.014 b |
| Serine | 0.557 ± 0.021 c | 0.453 ± 0.002 d | 0.647 ± 0.008 ab | 0.676 ± 0.016 a | 0.616 ± 0.008 b | 0.454 ± 0.030 d | 0.468 ± 0.003 d | 0.590 ± 0.005 c | 0.641 ± 0.007 a | 0.553 ± 0.006 c | 0.606 ± 0.023 ab | 0.570 ± 0.027 b |
| Glutamic acid | 2.135 ± 0.080 b | 1.763 ± 0.009 c | 2.422 ± 0.029 a | 2.389 ± 0.049 a | 2.262 ± 0.046 ab | 1.790 ± 0.116 c | 1.805 ± 0.018 d | 2.286 ± 0.017 ab | 2.391 ± 0.016 a | 2.029 ± 0.022 c | 2.165 ± 0.082 bc | 2.099 ± 0.041 c |
| Glycine | 1.022 ± 0.039 b | 0.967 ± 0.006 b | 0.981 ± 0.011 b | 1.170 ± 0.024 a | 1.097 ± 0.016 ab | 0.866 ± 0.062 c | 0.864 ± 0.006 d | 1.060 ± 0.010 b | 0.904 ± 0.009 cd | 0.942 ± 0.010 c | 1.136 ± 0.042 a | 0.946 ± 0.015 c |
| Alanine | 1.242 ± 0.048 b | 1.047 ± 0.007 c | 1.350 ± 0.018 ab | 1.433 ± 0.025 a | 1.272 ± 0.026 b | 1.121 ± 0.034 c | 1.145 ± 0.008 c | 1.330 ± 0.011 ab | 1.311 ± 0.010 ab | 1.197 ± 0.009 bc | 1.332 ± 0.063 a | 1.237 ± 0.036 b |
| Cysteine | 0.103 ± 0.004 ab | 0.044 ± 0.003 d | 0.084 ± 0.003 bc | 0.067 ± 0.004 c | 0.072 ± 0.018 c | 0.114 ± 0.007 a | 0.069 ± 0.001 b | 0.085 ± 0.002 ab | 0.109 ± 0.003 a | 0.064 ± 0.005 b | 0.094 ± 0.020 ab | 0.098 ± 0.008 a |
| Valine * | 0.703 ± 0.027 a | 0.538 ± 0.004 b | 0.739 ± 0.010 a | 0.745 ± 0.013 a | 0.707 ± 0.012 a | 0.604 ± 0.043 b | 0.611 ± 0.003 b | 0.699 ± 0.005 b | 0.731 ± 0.006 a | 0.639 ± 0.006 c | 0.716 ± 0.022 a | 0.681 ± 0.009 b |
| Methionine * | 0.295 ± 0.015 b | 0.154 ± 0.002 e | 0.267 ± 0.005 bc | 0.229 ± 0.006 d | 0.258 ± 0.011 cd | 0.336 ± 0.014 a | 0.214 ± 0.001 cd | 0.325 ± 0.004 ab | 0.246 ± 0.005 bc | 0.155 ± 0.009 d | 0.339 ± 0.042 a | 0.275 ± 0.020 b |
| Isoleucine * | 0.640 ± 0.028 b | 0.501 ± 0.003 c | 0.706 ± 0.013 a | 0.637 ± 0.013 b | 0.644 ± 0.011 b | 0.543 ± 0.036 c | 0.560 ± 0.005 c | 0.660 ± 0.009 ab | 0.687 ± 0.007 a | 0.528 ± 0.003 c | 0.649 ± 0.021 b | 0.633 ± 0.012 b |
| Leucine * | 1.131 ± 0.047 b | 0.910 ± 0.006 c | 1.254 ± 0.019 a | 1.216 ± 0.023 a | 1.146 ± 0.018 ab | 0.948 ± 0.063 c | 0.987 ± 0.006 c | 1.181 ± 0.009 a | 1.210 ± 0.015 a | 1.020 ± 0.008 c | 1.160 ± 0.045 ab | 1.110 ± 0.016 b |
| Tyrosine | 0.575 ± 0.023 b | 0.408 ± 0.006 d | 0.624 ± 0.008 a | 0.636 ± 0.010 a | 0.556 ± 0.008 bc | 0.529 ± 0.008 c | 0.503 ± 0.003 c | 0.565 ± 0.008 ab | 0.616 ± 0.011 a | 0.537 ± 0.007 b | 0.616 ± 0.026 a | 0.566 ± 0.028 ab |
| Phenylalanine * | 0.626 ± 0.026 b | 0.477 ± 0.002 d | 0.694 ± 0.012 a | 0.650 ± 0.014 ab | 0.634 ± 0.008 b | 0.534 ± 0.030 c | 0.543 ± 0.003 c | 0.639 ± 0.008 ab | 0.661 ± 0.013 a | 0.542 ± 0.008 c | 0.649 ± 0.019 ab | 0.614 ± 0.013 b |
| Lysine * | 1.195 ± 0.047 a | 0.953 ± 0.007 b | 1.264 ± 0.017 a | 1.300 ± 0.022 a | 1.214 ± 0.025 a | 1.013 ± 0.061 b | 1.039 ± 0.008 c | 1.241 ± 0.009 ab | 1.265 ± 0.010 a | 1.128 ± 0.006 c | 1.231 ± 0.044 ab | 1.183 ± 0.021 bc |
| Histidine & | 0.322 ± 0.026 a | 0.230 ± 0.009 b | 0.317 ± 0.003 a | 0.313 ± 0.011 ab | 0.326 ± 0.009 a | 0.268 ± 0.018 b | 0.270 ± 0.002 c | 0.311 ± 0.007 b | 0.369 ± 0.028 a | 0.270 ± 0.006 c | 0.335 ± 0.013 ab | 0.325 ± 0.005 b |
| Arginine & | 1.424 ± 0.061 b | 1.178 ± 0.010 c | 1.458 ± 0.016 ab | 1.561 ± 0.033 a | 1.420 ± 0.024 b | 1.234 ± 0.075 c | 1.222 ± 0.009 d | 1.437 ± 0.005 b | 1.541 ± 0.020 a | 1.354 ± 0.011 c | 1.357 ± 0.043 c | 1.346 ± 0.019 c |
| Proline | 0.818 ± 0.034 ab | 0.551 ± 0.003 c | 0.680 ± 0.025 b | 0.869 ± 0.016 a | 0.745 ± 0.0140 b | 0.675 ± 0.057 b | 0.666 ± 0.006 c | 0.767 ± 0.017 b | 0.659 ± 0.044 c | 0.697 ± 0.009 c | 0.847 ± 0.027 a | 0.763 ± 0.021 b |
| EAA | 5.257 ± 0.211 a | 4.040 ± 0.022 b | 5.683 ± 0.08 a | 5.528 ± 0.105 a | 5.290 ± 0.090 a | 4.536 ± 0.257 b | 4.548 ± 0.029 bc | 4.269 ± 0.031 c | 6.000 ± 0.083 a | 5.841 ± 0.115 a | 5.616 ± 0.089 a | 4.804 ± 0.275 b |
| SEAA | 1.746 ± 0.087 ab | 1.407 ± 0.017 bc | 1.775 ± 0.019 a | 1.874 ± 0.043 a | 1.746 ± 0.024 ab | 1.502 ± 0.094 b | 1.492 ± 0.011 d | 1.749 ± 0.011 b | 1.911 ± 0.029 a | 1.624 ± 0.017 c | 1.692 ± 0.056 bc | 1.671 ± 0.022 bc |
| NEAA | 7.857 ± 0.302 b | 6.335 ± 0.038 c | 8.370 ± 0.074 ab | 8.860 ± 0.175 a | 8.055 ± 0.116 b | 6.738 ± 0.365 c | 6.737 ± 0.044 d | 8.099 ± 0.074 ab | 8.171 ± 0.083 a | 7.411 ± 0.073 c | 8.226 ± 0.280 a | 7.647 ± 0.183 bc |
| TAA | 14.860 ± 0.600 b | 11.781 ± 0.076 c | 15.827 ± 0.171 ab | 16.261 ± 0.323 a | 15.091 ± 0.228 ab | 12.775 ± 0.716 ab | 12.778 ± 0.084 b | 11.781 ± 0.076 c | 15.827 ± 0.171 ab | 16.261 ± 0.323 a | 15.091 ± 0.228 b | 12.775 ± 0.716 c |
| EAA/TAA | 0.375 | 0.362 | 0.379 | 0.359 | 0.372 | 0.376 | 0.356 | 0.362 | 0.379 | 0.359 | 0.372 | 0.376 |
| EAA/NEAA | 0.710 | 0.674 | 0.717 | 0.659 | 0.697 | 0.713 | 0.675 | 0.527 | 0.734 | 0.788 | 0.685 | 0.629 |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | PJ | HZ | EZ | YY | JX | CZ | PJ | HZ | EZ | YY | JX | |
| Aspartic acid | 0.548 ± 0.017 c | 0.759 ± 0.015 b | 1.033 ± 0.008 a | 1.036 ± 0.012 a | 0.592 ± 0.046 c | 0.515 ± 0.023 d | 0.682 ± 0.052 c | 0.650 ± 0.011 c | 1.288 ± 0.021 a | 1.053 ± 0.018 b | 0.468 ± 0.016 d | 0.424 ± 0.019 d |
| Threonine * | 0.353 ± 0.012 c | 0.428 ± 0.010 b | 0.548 ± 0.002 a | 0.559 ± 0.008 a | 0.339 ± 0.019 c | 0.335 ± 0.019 c | 0.406 ± 0.027 c | 0.396 ± 0.006 c | 0.628 ± 0.012 a | 0.556 ± 0.012 b | 0.310 ± 0.008 d | 0.268 ± 0.015 d |
| Serine | 0.222 ± 0.006 c | 0.350 ± 0.011 b | 0.379 ± 0.001 ab | 0.392 ± 0.005 a | 0.261 ± 0.019 c | 0.212 ± 0.022 d | 0.300 ± 0.023 c | 0.301 ± 0.005 c | 0.487 ± 0.009 a | 0.431 ± 0.008 b | 0.222 ± 0.004 d | 0.173 ± 0.004 e |
| Glutamic acid | 0.737 ± 0.025 c | 0.901 ± 0.008 b | 1.031 ± 0.003 a | 0.962 ± 0.016 ab | 0.757 ± 0.050 c | 0.666 ± 0.037 d | 0.863 ± 0.056 c | 0.791 ± 0.015 d | 1.177 ± 0.018 a | 1.022 ± 0.011 b | 0.577 ± 0.015 e | 0.526 ± 0.031 e |
| Glycine | 0.377 ± 0.016 b | 0.411 ± 0.008 a | 0.425 ± 0.002 a | 0.450 ± 0.005 a | 0.350 ± 0.022 b | 0.369 ± 0.020 b | 0.430 ± 0.023 ab | 0.410 ± 0.004 b | 0.458 ± 0.008 a | 0.453 ± 0.012 a | 0.326 ± 0.006 c | 0.305 ± 0.019 c |
| Alanine | 0.516 ± 0.025 d | 0.497 ± 0.006 d | 0.685 ± 0.005 b | 0.761 ± 0.007 a | 0.415 ± 0.022 e | 0.579 ± 0.035 c | 0.512 ± 0.02 cd | 0.539 ± 0.007 c | 0.735 ± 0.012 a | 0.643 ± 0.014 b | 0.450 ± 0.010 d | 0.444 ± 0.050 d |
| Cysteine | 0.058 ± 0.005 bc | 0.085 ± 0.002 a | 0.052 ± 0.001 c | 0.058 ± 0.002 b | 0.066 ± 0.004 b | 0.064 ± 0.006 b | 0.066 ± 0.005 a | 0.044 ± 0.002 b | 0.061 ± 0.003 a | 0.064 ± 0.002 a | 0.048 ± 0.003 b | 0.045 ± 0.002 b |
| Valine * | 0.375 ± 0.010 b | 0.427 ± 0.008 a | 0.433 ± 0.003 a | 0.461 ± 0.006 a | 0.367 ± 0.023 b | 0.383 ± 0.021 b | 0.420 ± 0.022 c | 0.407 ± 0.004 c | 0.521 ± 0.011 a | 0.466 ± 0.010 b | 0.320 ± 0.012 d | 0.301 ± 0.014 d |
| Methionine * | 0.153 ± 0.009 b | 0.239 ± 0.011 a | 0.175 ± 0.007 b | 0.173 ± 0.004 b | 0.189 ± 0.008 b | 0.178 ± 0.014 b | 0.162 ± 0.010 ab | 0.170 ± 0.020 ab | 0.150 ± 0.009 b | 0.212 ± 0.008 a | 0.157 ± 0.002 b | 0.152 ± 0.010 b |
| Isoleucine * | 0.293 ± 0.009 b | 0.330 ± 0.003 a | 0.276 ± 0.002 b | 0.290 ± 0.001 b | 0.280 ± 0.020 b | 0.296 ± 0.016 ab | 0.337 ± 0.022 a | 0.328 ± 0.003 a | 0.341 ± 0.007 a | 0.291 ± 0.009 b | 0.241 ± 0.008 c | 0.23 ± 0.0120 c |
| Leucine * | 0.519 ± 0.018 b | 0.588 ± 0.010 a | 0.505 ± 0.003 b | 0.532 ± 0.004 b | 0.509 ± 0.026 b | 0.534 ± 0.028 ab | 0.585 ± 0.034 a | 0.569 ± 0.008 ab | 0.588 ± 0.013 a | 0.529 ± 0.015 b | 0.443 ± 0.013 c | 0.419 ± 0.021 c |
| Tyrosine | 0.267 ± 0.014 a | 0.287 ± 0.004 a | 0.240 ± 0.003 b | 0.259 ± 0.001 ab | 0.259 ± 0.009 ab | 0.268 ± 0.024 ab | 0.254 ± 0.013 b | 0.263 ± 0.004 b | 0.304 ± 0.010 a | 0.254 ± 0.010 b | 0.173 ± 0.007 c | 0.157 ± 0.010 c |
| Phenylalanine* | 0.327 ± 0.017 a | 0.336 ± 0.004 a | 0.224 ± 0.003 b | 0.246 ± 0.003 b | 0.317 ± 0.022 a | 0.332 ± 0.023 a | 0.364 ± 0.023 a | 0.344 ± 0.004 a | 0.278 ± 0.014 b | 0.236 ± 0.014 b | 0.265 ± 0.009 b | 0.255 ± 0.007 b |
| Lysine * | 0.493 ± 0.016 a | 0.490 ± 0.005 a | 0.455 ± 0.002 ab | 0.457 ± 0.005 ab | 0.437 ± 0.028 b | 0.476 ± 0.028 ab | 0.552 ± 0.033 a | 0.505 ± 0.008 ab | 0.530 ± 0.011 a | 0.456 ± 0.011 bc | 0.411 ± 0.009 c | 0.373 ± 0.026 c |
| Histidine & | 0.167 ± 0.009 c | 0.213 ± 0.019 a | 0.208 ± 0.004 ab | 0.227 ± 0.004 a | 0.188 ± 0.010 bc | 0.172 ± 0.006 c | 0.188 ± 0.010 bc | 0.169 ± 0.003 c | 0.253 ± 0.008 a | 0.207 ± 0.010 b | 0.165 ± 0.005 c | 0.138 ± 0.004 d |
| Arginine & | 0.398 ± 0.007 c | 0.526 ± 0.021 a | 0.487 ± 0.004 ab | 0.491 ± 0.006 ab | 0.397 ± 0.014 c | 0.450 ± 0.039 c | 0.478 ± 0.027 b | 0.574 ± 0.006 a | 0.598 ± 0.013 a | 0.558 ± 0.014 a | 0.354 ± 0.010 c | 0.360 ± 0.034 c |
| Proline | 0.319 ± 0.019 c | 0.403 ± 0.014 b | 0.486 ± 0.006 a | 0.430 ± 0.013 b | 0.356 ± 0.005 c | 0.324 ± 0.015 c | 0.383 ± 0.033 ab | 0.375 ± 0.011 b | 0.433 ± 0.010 a | 0.375 ± 0.019 b | 0.337 ± 0.011 b | 0.234 ± 0.007 c |
| EAA | 2.512 ± 0.067 | 2.837 ± 0.026 | 2.615 ± 0.017 | 2.718 ± 0.028 | 2.437 ± 0.137 | 2.534 ± 0.148 | 2.826 ± 0.168 a | 2.719 ± 0.036 a | 3.037 ± 0.070 a | 2.747 ± 0.076 a | 2.146 ± 0.059 b | 1.998 ± 0.098 b |
| SEAA | 0.565 ± 0.016 b | 0.739 ± 0.039 a | 0.695 ± 0.008 ab | 0.718 ± 0.004 a | 0.585 ± 0.023 b | 0.622 ± 0.045 ab | 0.666 ± 0.037 b | 0.743 ± 0.009 ab | 0.850 ± 0.018 a | 0.765 ± 0.024 ab | 0.519 ± 0.015 c | 0.498 ± 0.038 c |
| NEAA | 3.043 ± 0.124 c | 3.693 ± 0.054 b | 4.330 ± 0.025 a | 4.347 ± 0.054 a | 3.055 ± 0.161 c | 2.996 ± 0.179 c | 3.489 ± 0.223 c | 3.373 ± 0.047 c | 4.943 ± 0.089 a | 4.294 ± 0.089 b | 2.601 ± 0.056 d | 2.308 ± 0.127 d |
| TAA | 6.120 ± 0.206 b | 7.269 ± 0.110 a | 7.640 ± 0.041 a | 7.783 ± 0.084 a | 6.077 ± 0.321 b | 6.152 ± 0.372 b | 6.981 ± 0.427 b | 6.835 ± 0.082 b | 8.830 ± 0.176 a | 7.806 ± 0.183 ab | 5.266 ± 0.126 d | 4.804 ± 0.259 c |
| EAA/TAA | 0.411 | 0.390 | 0.342 | 0.349 | 0.401 | 0.412 | 0.405 | 0.398 | 0.344 | 0.352 | 0.408 | 0.416 |
| EAA/NEAA | 0.827 | 0.768 | 0.604 | 0.625 | 0.797 | 0.846 | 0.811 | 0.806 | 0.614 | 0.640 | 0.825 | 0.867 |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | PJ | HZ | EZ | YY | JX | CZ | PJ | HZ | EZ | YY | JX | |
| Aspartic acid | 1.788 ± 0.014 a | 1.755 ± 0.015 a | 1.722 ± 0.031 ab | 1.812 ± 0.042 a | 1.723 ± 0.080 a | 1.618 ± 0.022 b | 2.051 ± 0.052 b | 2.184 ± 0.021 ab | 2.156 ± 0.037 ab | 2.174 ± 0.038 ab | 2.230 ± 0.063 a | 2.170 ± 0.079 ab |
| Threonine * | 1.372 ± 0.005 a | 1.257 ± 0.023 b | 1.160 ± 0.028 c | 1.228 ± 0.032 bc | 1.318 ± 0.055 ab | 1.213 ± 0.016 bc | 1.321 ± 0.033 a | 1.365 ± 0.013 ab | 1.330 ± 0.022 ab | 1.367 ± 0.025 ab | 1.466 ± 0.041 a | 1.418 ± 0.051 ab |
| Serine | 0.708 ± 0.005 ab | 0.696 ± 0.015 ab | 0.638 ± 0.019 c | 0.694 ± 0.005 c | 0.743 ± 0.025 a | 0.628 ± 0.010 c | 1.354 ± 0.036 c | 1.484 ± 0.014 ab | 1.425 ± 0.023 bc | 1.455 ± 0.027 abc | 1.557 ± 0.041 a | 1.449 ± 0.051 bc |
| Glutamic acid | 1.949 ± 0.004 b | 1.967 ± 0.025 b | 2.024 ± 0.040 ab | 2.110 ± 0.035 a | 2.075 ± 0.047 a | 1.824 ± 0.023 c | 2.846 ± 0.076 b | 3.046 ± 0.033 ab | 2.970 ± 0.049 b | 2.946 ± 0.050 b | 3.200 ± 0.094 a | 2.993 ± 0.113 ab |
| Glycine | 0.588 ± 0.008 a | 0.583 ± 0.010 a | 0.585 ± 0.006 a | 0.594 ± 0.006 a | 0.594 ± 0.020 a | 0.549 ± 0.006 b | 1.182 ± 0.028 ab | 1.190 ± 0.016 ab | 1.159 ± 0.019 ab | 1.125 ± 0.019 b | 1.242 ± 0.035 a | 1.231 ± 0.047 a |
| Alanine | 1.070 ± 0.017 | 1.098 ± 0.034 | 1.036 ± 0.035 | 1.107 ± 0.018 | 1.038 ± 0.055 | 1.015 ± 0.013 | 1.310 ± 0.033 | 1.322 ± 0.014 | 1.336 ± 0.023 | 1.310 ± 0.024 | 1.391 ± 0.041 | 1.370 ± 0.050 |
| Cysteine | 0.288 ± 0.015 a | 0.229 ± 0.003 b | 0.192 ± 0.002 b | 0.236 ± 0.009 b | 0.232 ± 0.034 b | 0.234 ± 0.004 b | 0.226 ± 0.005 b | 0.224 ± 0.005 b | 0.254 ± 0.005 b | 0.236 ± 0.001 ab | 0.246 ± 0.007 a | 0.240 ± 0.010 ab |
| Valine * | 0.518 ± 0.001 b | 0.496 ± 0.013 b | 0.565 ± 0.005 a | 0.575 ± 0.019 a | 0.592 ± 0.027 a | 0.494 ± 0.007 b | 1.481 ± 0.035 ab | 1.496 ± 0.015 ab | 1.472 ± 0.023 b | 1.444 ± 0.025 b | 1.589 ± 0.045 a | 1.554 ± 0.058 ab |
| Methionine * | 0.026 ± 0.016 ab | 0.028 ± 0.003 b | 0.040 ± 0.006 a | 0.019 ± 0.002 b | 0.048 ± 0.008 a | 0.012 ± 0.001 b | 0.585 ± 0.012 b | 0.441 ± 0.004 c | 0.564 ± 0.009 c | 0.407 ± 0.007 c | 0.601 ± 0.015 b | 0.743 ± 0.032 a |
| Isoleucine * | 0.703 ± 0.007 ab | 0.650 ± 0.002 b | 0.617 ± 0.014 b | 0.730 ± 0.035 a | 0.680 ± 0.033 ab | 0.620 ± 0.010 b | 1.088 ± 0.026 ab | 1.107 ± 0.013 ab | 1.098 ± 0.014 b | 1.034 ± 0.025 b | 1.133 ± 0.033 a | 1.127 ± 0.039 a |
| Leucine * | 1.018 ± 0.016 ab | 0.923 ± 0.012 c | 0.915 ± 0.023 c | 1.046 ± 0.035 a | 1.005 ± 0.050 ab | 0.934 ± 0.012 bc | 1.886 ± 0.045 | 1.945 ± 0.020 | 1.924 ± 0.031 | 1.878 ± 0.042 | 2.001 ± 0.056 | 1.990 ± 0.072 |
| Tyrosine | 0.402 ± 0.018 a | 0.343 ± 0.003 b | 0.373 ± 0.009 ab | 0.387 ± 0.015 ab | 0.382 ± 0.026 ab | 0.364 ± 0.007 ab | 1.005 ± 0.026 | 1.025 ± 0.009 | 1.053 ± 0.011 | 1.094 ± 0.049 | 1.046 ± 0.033 | 1.051 ± 0.034 |
| Phenylalanine * | 0.564 ± 0.018 a | 0.508 ± 0.004 ab | 0.504 ± 0.017 ab | 0.480 ± 0.019 b | 0.540 ± 0.042 ab | 0.517 ± 0.012 ab | 1.133 ± 0.033 | 1.159 ± 0.010 | 1.120 ± 0.010 | 1.200 ± 0.077 | 1.190 ± 0.036 | 1.168 ± 0.039 |
| Lysine * | 0.678 ± 0.006 cd | 0.690 ± 0.020 cd | 0.829 ± 0.015 a | 0.730 ± 0.026 bc | 0.760 ± 0.038 b | 0.653 ± 0.011 d | 1.585 ± 0.037 | 1.668 ± 0.021 | 1.637 ± 0.030 | 1.617 ± 0.028 | 1.703 ± 0.051 | 1.669 ± 0.061 |
| Histidine & | 0.339 ± 0.001 b | 0.327 ± 0.013 b | 0.327 ± 0.014 b | 0.333 ± 0.006 b | 0.400 ± 0.012 a | 0.312 ± 0.004 b | 0.518 ± 0.013 b | 0.544 ± 0.015 ab | 0.508 ± 0.006 b | 0.454 ± 0.010 c | 0.572 ± 0.016 a | 0.545 ± 0.016 ab |
| Arginine & | 0.532 ± 0.007 b | 0.541 ± 0.013 b | 0.619 ± 0.021 a | 0.531 ± 0.014 b | 0.620 ± 0.025 a | 0.534 ± 0.009 b | 1.634 ± 0.035 b | 1.755 ± 0.013 ab | 1.646 ± 0.028 b | 1.653 ± 0.018 b | 1.804 ± 0.054 a | 1.743 ± 0.064 ab |
| Proline | 2.078 ± 0.035 a | 1.871 ± 0.009 b | 1.524 ± 0.065 c | 1.900 ± 0.021 ab | 1.960 ± 0.126 ab | 1.755 ± 0.030 b | 1.113 ± 0.029 bc | 1.160 ± 0.038 bc | 1.095 ± 0.049 c | 1.233 ± 0.034 b | 1.309 ± 0.053 a | 1.184 ± 0.034 bc |
| EAA | 4.879 ± 0.048 ab | 4.551 ± 0.069 cd | 4.629 ± 0.095 bcd | 4.808 ± 0.075 abc | 4.942 ± 0.172 a | 4.443 ± 0.068 d | 9.079 ± 0.22 ab | 9.181 ± 0.093 ab | 9.145 ± 0.139 ab | 8.948 ± 0.229 b | 9.683 ± 0.275 a | 9.670 ± 0.351 a |
| SEAA | 0.872 ± 0.006 c | 0.868 ± 0.022 c | 0.947 ± 0.035 b | 0.863 ± 0.008 c | 1.019 ± 0.036 a | 0.846 ± 0.012 c | 2.152 ± 0.047 bc | 2.299 ± 0.028 ab | 2.153 ± 0.034 bc | 2.107 ± 0.028 c | 2.376 ± 0.069 a | 2.288 ± 0.080 ab |
| NEAA | 8.872 ± 0.097 a | 8.542 ± 0.057 ab | 8.094 ± 0.206 b | 8.84 ± 0.066 a | 8.746 ± 0.396 a | 7.987 ± 0.113 b | 11.086 ± 0.279 b | 11.634 ± 0.147 ab | 11.449 ± 0.216 ab | 11.572 ± 0.202 b | 12.222 ± 0.363 a | 11.688 ± 0.418 ab |
| TAA | 14.623 ± 0.149 a | 13.962 ± 0.136 abc | 13.669 ± 0.335 bc | 14.511 ± 0.131 | 14.707 ± 0.564 a | 13.277 ± 0.193 c | 22.317 ± 0.546 b | 23.114 ± 0.268 ab | 22.747 ± 0.388 ab | 22.627 ± 0.458 b | 24.28 ± 0.706 a | 23.647 ± 0.849 ab |
| EAA/TAA | 0.334 | 0.326 | 0.339 | 0.331 | 0.336 | 0.335 | 0.407 | 0.397 | 0.402 | 0.395 | 0.399 | 0.409 |
| EAA/NEAA | 0.550 | 0.533 | 0.572 | 0.544 | 0.566 | 0.556 | 0.819 | 0.789 | 0.799 | 0.773 | 0.792 | 0.827 |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | PJ | HZ | EZ | YY | JX | CZ | PJ | HZ | EZ | YY | JX | |
| C16:0 | 95.95 ± 2.30 | 80.94 ± 3.28 | 104.46 ± 6.44 | 86.5 ± 13.37 | 89.89 ± 9.32 | 103.70 ± 12.36 | 93.01 ± 3.96 | 83.07 ± 5.07 | 82.15 ± 1.32 | 97.54 ± 18.96 | 93.52 ± 6.89 | 105.23 ± 1.46 |
| C18:0 | 74.4 ± 1.32 ab | 59.13 ± 4.01 bc | 82.44 ± 3.58 a | 56.71 ± 8.01 c | 68.84 ± 2.9 abc | 71.83 ± 8.36 abc | 76.60 ± 3.16 a | 66.85 ± 1.85 ab | 74.6 ± 1.52 a | 57.96 ± 8.00 b | 66.36 ± 2.24 ab | 72.57 ± 2.37 a |
| C20:0 | 8.37 ± 0.11 b | 8.01 ± 0.25 b | 8.55 ± 0.26 b | 10.38 ± 1.1 a | 7.45 ± 0.64 b | 7.79 ± 0.18 b | 8.49 ± 0.14 b | 7.91 ± 0.48 b | 7.46 ± 0.23 b | 10.83 ± 0.72 a | 7.89 ± 0.24 b | 7.54 ± 0.22 b |
| C22:0 | 7.33 ± 0.04 ab | 6.75 ± 0.11 b | 8.01 ± 0.25 ab | 9.56 ± 1.38 a | 8.71 ± 0.96 ab | 8.56 ± 0.94 ab | 7.95 ± 0.17 b | 7.51 ± 0.35 b | 7.38 ± 0.26 b | 10.23 ± 0.77 a | 7.49 ± 0.23 b | 8.19 ± 0.52 b |
| ΣSFA | 186.04 ± 3.33 ab | 154.33 ± 6.25 b | 203.47 ± 10.52 a | 163.15 ± 23.69 ab | 174.89 ± 11.6 ab | 191.89 ± 21.56 ab | 186.04 ± 6.55 | 165.34 ± 2.42 | 171.59 ± 2.80 | 176.56 ± 27.81 | 175.27 ± 8.60 | 193.53 ± 3.11 |
| C16:1 | 14.01 ± 0.63 b | 10.89 ± 0.13 b | 22.67 ± 3.65 ab | 25.65 ± 3.44 a | 17.16 ± 4.00 ab | 18.67 ± 3.05 ab | 17.51 ± 1.61 b | 17.08 ± 1.73 b | 16.63 ± 3.18 b | 31.06 ± 8.88 a | 20.99 ± 1.70 ab | 23.72 ± 0.49 ab |
| C18:1n9c | 133.92 ± 4.68 bc | 107.51 ± 3.38 c | 174.35 ± 4.94 a | 125.13 ± 20.73 bc | 140.68 ± 13.05 abc | 157.03 ± 17.92 ab | 146.92 ± 4.27 ab | 127.29 ± 2.68 b | 132.17 ± 8.17 ab | 144.33 ± 31.89 ab | 132.31 ± 5.19 ab | 169.78 ± 1.39 a |
| C20:1 | 12.60 ± 0.62 a | 4.26 ± 0.11 c | 5.11 ± 0.42 c | 5.76 ± 1.01 c | 9.04 ± 1.05 b | 10.89 ± 1.31 ab | 10.15 ± 0.40 b | 4.64 ± 0.22 d | 5.15 ± 0.45 d | 5.41 ± 0.76 d | 14.56 ± 0.30 a | 7.19 ± 0.48 c |
| C22:1n9 | 12.36 ± 0.74 cd | 18.33 ± 1.84 ab | 19.55 ± 0.72 a | 15.52 ± 0.83 bc | 11.46 ± 1.41 d | 15.57 ± 0.71 bc | 11.91 ± 1.50 ab | 16.15 ± 2.15 ab | 9.91 ± 2.17 b | 17.29 ± 2.58 a | 11.06 ± 1.04 ab | 13.20 ± 2.55 ab |
| ΣMUFA | 172.89 ± 6.10 bc | 141.00 ± 1.75 c | 221.67 ± 3.52 a | 172.06 ± 25.69 bc | 178.34 ± 18.23 abc | 202.15 ± 19.69 ab | 186.48 ± 6.35 | 165.15 ± 4.62 | 163.86 ± 12.94 | 198.09 ± 40.55 | 178.91 ± 4.85 | 213.89 ± 3.73 |
| C18:2n6c | 39.02 ± 5.79 d | 88.61 ± 15.11 ab | 112.55 ± 5.34 a | 74.66 ± 13.25 bc | 56.39 ± 9.50 cd | 53.77 ± 8.25 cd | 35.06 ± 2.80 b | 94.06 ± 15.22 a | 70.58 ± 2.27 a | 73.39 ± 11.25 a | 26.35 ± 3.55 b | 90.8 ± 6.66 a |
| C18:3n3 | 5.09 ± 0.80 b | 13.45 ± 3.16 a | 11.01 ± 2.88 ab | 8.97 ± 1.13 ab | 7.10 ± 1.94 b | 5.99 ± 0.79 b | 5.27 ± 0.76 d | 13.26 ± 0.57 ab | 11.00 ± 0.99 b | 14.21 ± 0.91 a | 5.87 ± 0.33 d | 7.79 ± 0.30 c |
| C20:2 | 7.52 ± 0.61 c | 13.59 ± 1.06 ab | 15.49 ± 0.24 a | 16.77 ± 2.95 a | 10.62 ± 1.29 bc | 10.85 ± 1.26 bc | 9.79 ± 0.70 b | 13.13 ± 1.68 ab | 10.99 ± 0.38 ab | 13.18 ± 2.73 ab | 5.99 ± 0.78 c | 14.52 ± 0.56 a |
| C20:4n6 | 17.77 ± 2.70 c | 38.72 ± 2.89 ab | 38.86 ± 1.00 ab | 48.15 ± 6.92 a | 26.03 ± 6.07 b | 26.54 ± 2.49 b | 19.45 ± 2.15 c | 34.12 ± 5.78 a | 20.93 ± 2.54 bc | 31.03 ± 7.15 ab | 14.48 ± 1.36 c | 32.16 ± 0.7 ab |
| C20:5n3 | 56.8 ± 10.60 b | 75.58 ± 6.12 ab | 85.18 ± 2.38 ab | 52.14 ± 7.57 b | 75.47 ± 23.02 ab | 101.5 ± 13.81 a | 73.50 ± 8.20 a | 71.28 ± 3.95 a | 42.27 ± 3.60 b | 41.09 ± 5.81 b | 58.35 ± 7.47 ab | 83.44 ± 3.46 a |
| C22:6n3 | 75.44 ± 17.21 b | 51.05 ± 7.14 bc | 74.92 ± 5.01 bc | 30.42 ± 4.59 c | 78.91 ± 25.82 ab | 121.97 ± 14.16 a | 92.63 ± 11.72 a | 44.14 ± 6.52 b | 50.11 ± 7.01 b | 26.68 ± 3.41 b | 44.08 ± 13.23 b | 93.31 ± 7.11 a |
| ΣPUFA | 201.64 ± 35.96 bc | 281.00 ± 23.58 ab | 338 ± 16.49 a | 231.11 ± 36.11 ab | 254.51 ± 65.29 ab | 320.63 ± 38.28 ab | 235.7 ± 24.91 a | 269.99 ± 31.60 ab | 205.89 ± 10.24 b | 199.58 ± 30.35 b | 165.12 ± 18.12 b | 322.03 ± 4.05 a |
| Σn-3 PUFA | 137.32 ± 27.12 b | 140.08 ± 11.07 b | 171.11 ± 10.09 ab | 91.53 ± 13.11 b | 161.47 ± 50.06 ab | 229.46 ± 26.76 a | 171.4 ± 19.8 a | 128.68 ± 10.32 ab | 103.38 ± 9.63 b | 81.98 ± 9.38 b | 118.3 ± 13.94 b | 184.54 ± 10.01 a |
| Σn-6 PUFA | 56.79 ± 8.39 c | 127.33 ± 16.87 a | 151.4 ± 6.30 a | 122.82 ± 20.06 ab | 82.42 ± 14.34 bc | 80.31 ± 10.62 c | 51.17 ± 6.58 b | 128.18 ± 20.42 a | 91.51 ± 0.46 a | 104.42 ± 18.31 a | 40.83 ± 4.73 b | 122.96 ± 7.36 a |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | PJ | HZ | EZ | YY | JX | CZ | PJ | HZ | EZ | YY | JX | |
| C12:0 | 23.56 ± 2.54 c | 31.77 ± 14.08 c | 79.41 ± 5.13 a | 56.11 ± 1.47 b | 38.49 ± 2.23 bc | 29.16 ± 5.91 c | 22.26 ± 0.52 c | 69.90 ± 12.26 b | 110.95 ± 12.64 a | 60.06 ± 6.06 b | 46.55 ± 3.57 bc | 53.01 ± 4.20 b |
| C13:0 | 11.34 ± 0.78 b | 18.47 ± 7.71 ab | 28.78 ± 2. 07 a | 29.03 ± 1.96 a | 20.19 ± 0.63 ab | 15.49 ± 3.34 b | 10.08 ± 0.66 b | 34.10 ± 5.87 a | 29.94 ± 3.03 a | 31.04 ± 4.25 a | 26.85 ± 2.76 a | 26.27 ± 5.58 a |
| C14:0 | 489.17 ± 62.06 bc | 205.24 ± 31.38 c | 510.42 ± 75.82 b | 532.89 ± 63.9 ab | 816.00 ± 54.53 a | 602.27 ± 194.98 ab | 332.39 ± 76.84 bc | 263.31 ± 30.05 c | 571.52 ± 8.93 b | 591.07 ± 12.27 b | 1569.32 ± 171.52 a | 540.97 ± 83.38 b |
| C15:0 | 127.79 ± 14.5 c | 174.56 ± 25.61 bc | 202.79 ± 10.56 b | 301.82 ± 13.36 a | 209.16 ± 9.14 b | 150.09 ± 40.93 b | 119.65 ± 24.74 c | 186.23 ± 32.68 b | 220.26 ± 10.59 b | 301.60 ± 21.03 a | 320.76 ± 26.56 a | 207.48 ± 7.75 b |
| C16:0 | 4071.8 ± 628.5 c | 4706.5 ± 605.1 c | 9518.7 ± 759.1 a | 7710.6 ± 955.6 ab | 6450.8 ± 281.2 bc | 5381.6 ± 1592.3 bc | 5263.7 ± 1201.4 c | 5635.6 ± 747.3 bc | 8212.2 ± 643.9 a | 9282.2 ± 373.8 a | 8256.9 ± 561.9 a | 7673.3 ± 233.6 ab |
| C17:0 | 105.95 ± 11.10 c | 139.48 ± 28.32 b | 191.88 ± 9.82 ab | 241.02 ± 15.94 a | 153.88 ± 10.32 bc | 135.09 ± 32.46 bc | 103.6 ± 23.21 c | 167.45 ± 25.52 bc | 188.33 ± 15.36 b | 259.54 ± 29.39 a | 201.02 ± 11.27 ab | 163.61 ± 14.56 bc |
| C18:0 | 621.77 ± 72.56 c | 824.66 ± 152.2 c | 1532.31 ± 128.38 a | 1275.07 ± 93.06 ab | 947.54 ± 41.94 bc | 852.47 ± 225.25 c | 733.35 ± 83.95 c | 1219.5 ± 214.78 ab | 1441.05 ± 110.24 ab | 1590.57 ± 134.6 a | 1152.79 ± 31.26 b | 1233.58 ± 64.22 ab |
| C20:0 | 79.26 ± 6.04 d | 102.75 ± 11.81 bcd | 162.80 ± 5.70 a | 133.74 ± 10.5 abc | 136.05 ± 3.45 ab | 96.50 ± 23.59 cd | 64.97 ± 8.55 b | 145.45 ± 29.70 a | 150.16 ± 12.10 a | 161.98 ± 29.92 a | 168.83 ± 18.31 a | 127.92 ± 8.02 a |
| C21:0 | 19.70 ± 1.09 c | 46.03 ± 5.47 b | 70.35 ± 4.83 a | 66.47 ± 1.06 a | 40.93 ± 4.55 b | 24.74 ± 5.18 c | 22.95 ± 3.98 c | 53.08 ± 11.08 ab | 76.25 ± 12.12 a | 72.62 ± 10.43 a | 38.48 ± 2.38 bc | 43.56 ± 0.54 bc |
| C22:0 | 52.20 ± 4.75 c | 130.80 ± 32.48 ab | 154.05 ± 14.89 a | 98.99 ± 10.15 bc | 86.90 ± 3.71 bc | 69.05 ± 13.61 c | 50.95 ± 7.33 c | 135.97 ± 22.20 a | 142.76 ± 11.99 a | 131.69 ± 29.05 a | 75.20 ± 2.08 bc | 108.01 ± 15 ab |
| C23:0 | 26.96 ± 1.72 d | 87.99 ± 20.32 ab | 98.29 ± 5.97 a | 58.68 ± 5.69 bc | 46.37 ± 3.78 cd | 34.27 ± 6.706 cd | 22.93 ± 2.60 c | 88.84 ± 20.26 a | 98.67 ± 6.36 a | 78.31 ± 17.41 ab | 30.27 ± 1.74 c | 55.33 ± 6.41 bc |
| C24:0 | 65.27 ± 5.81 c | 106.19 ± 23.38 bc | 121.54 ± 3.95 b | 173.88 ± 8.25 a | 119.51 ± 11.23 b | 86.75 ± 26.72 bc | 48.34 ± 8.60 c | 141.64 ± 18.4 ab | 120.49 ± 10.34 ab | 170.10 ± 31.79 a | 107.02 ± 6.10 b | 92.54 ± 15.09 bc |
| ΣSFA | 5694.7 ± 796.1 c | 6574.4 ± 850.1 c | 12,671.3 ± 952.7 a | 10,678.3 ± 1153.9 ab | 9065.8 ± 301.4 abc | 7477.4 ± 2160.6 bc | 6795.2 ± 1434.1 c | 8141.1 ± 1137.6 bc | 11,362.6 ± 836.7 a | 12,730.8 ± 683.8 a | 11,994.1 ± 794.9 a | 10,325.6 ± 322.9 ab |
| C14:1 | 43.61 ± 13.48 bc | 22.86 ± 4.99 c | 68.63 ± 16.21 ab | 102.20 ± 10.69 a | 89.36 ± 6.59 a | 63.51 ± 23.88 abc | 29.00 ± 0.91 d | 51.87 ± 12.17 cd | 83.28 ± 6.59 b | 100.41 ± 8.09 b | 163.79 ± 17.31 a | 70.25 ± 4.94 bc |
| C16:1 | 1268.3 ± 332.8 c | 1279.8 ± 261.1 c | 3391.9 ± 723.8 ab | 4030.9 ± 554.2 a | 2662.5 ± 142.4 abc | 1887.2 ± 669.7 bc | 1671.6 ± 92.4 c | 1819.1 ± 284.8 c | 2800.8 ± 292.6 b | 3919.3 ± 304.5 a | 3311.7 ± 377.7 ab | 2781.1 ± 155.5 b |
| C18:1n9c | 4115.4 ± 641.1 c | 6718.9 ± 762.9 c | 11,908.9 ± 559.6 a | 10,118.2 ± 1072.2 ab | 7179.1 ± 330.0 bc | 6790.9 ± 1978.4 c | 6845.5 ± 1049.5 c | 8473.5 ± 1285.9 bc | 10,285.3 ± 1252.1 ab | 11,845.4 ± 246.9 a | 8766.1 ± 441.2 bc | 11,146.9 ± 398.7 ab |
| C20:1 | 739.21 ± 57.09 a | 185.12 ± 30.15 d | 375.05 ± 75.76 c | 334.22 ± 20.77 cd | 573.12 ± 40.35 ab | 437.58 ± 93.47 bc | 558.99 ± 125.41 b | 243.83 ± 48.87 c | 421.38 ± 27.62 bc | 345.65 ± 33.88 bc | 1308.15 ± 174.78 a | 411.93 ± 31.9 bc |
| C22:1n9 | 102.56 ± 10.84 b | 78.37 ± 19.08 bc | 101.88 ± 21.92 b | 53.73 ± 3.68 c | 206.03 ± 10.72 a | 89.46 ± 14.26 bc | 101.37 ± 13.74 bc | 70.76 ± 11.06 c | 129.79 ± 9.99 ab | 53.75 ± 7.77 c | 142.75 ± 12.20 a | 65.45 ± 7.22 c |
| C24:1 | 142.40 ± 10.99 a | 30.14 ± 8.57 b | 46.97 ± 6.11 b | 23.94 ± 1.82 b | 140.49 ± 9.95 a | 174.47 ± 60.16 a | 88.70 ± 23.42 b | 37.62 ± 4.68 c | 55.02 ± 1.61 bc | 21.74 ± 3.77 c | 208.97 ± 9.08 a | 57.56 ± 15.48 bc |
| ΣMUFA | 6411.5 ± 1026.3 c | 8315.2 ± 1025.6 c | 15,893.3 ± 480.2 a | 14,663.1 ± 1646.9 ab | 10,850.7 ± 444.1 bc | 9443.1 ± 2726.3 c | 9295.2 ± 1292.8 c | 10,696.7 ± 1611.9 bc | 13,775.6 ± 1578.4 ab | 16,286.2 ± 337.8 a | 13,901.4 ± 1016.7 ab | 14,533.2 ± 344.5 a |
| C18:2n6c | 1937.4 ± 237.4 d | 4704.1 ± 459.1 bc | 8485.3 ± 644.5 a | 5946.8 ± 518.6 b | 4187.8 ± 942.2 bcd | 3387.6 ± 1181.8 cd | 1688.9 ± 382.9 c | 4751.0 ± 1420.1 ab | 6730.5 ± 514.3 a | 6200.2 ± 651.6 a | 2371.8 ± 478.3 bc | 7093.3 ± 1580.2 a |
| C18:3n6 | 9.15 ± 0.85 c | 23.25 ± 9.79 bc | 24.85 ± 2.50 b | 45.55 ± 3.93 a | 28.61 ± 2.99 b | 18.44 ± 3.93 bc | 7.44 ± 0.39 c | 29.20 ± 4.06 ab | 30.15 ± 2.67 ab | 35.52 ± 3.61 a | 22.09 ± 3.66 b | 25.38 ± 4.04 ab |
| C18:3n3 | 156.17 ± 13.53 c | 417.40 ± 54.99 bc | 1053.31 ± 196.72 a | 750.96 ± 160.4 ab | 762.58 ± 244.28 ab | 258.5 ± 81.21 c | 165.12 ± 35.98 b | 364.02 ± 92.67 b | 1518.20 ± 139.99 a | 1379.48 ± 426.25 a | 317.03 ± 44.06 b | 586.90 ± 10.96 b |
| C20:2 | 176.90 ± 17.30 d | 320.20 ± 64.21 cd | 496.44 ± 30.24 b | 874.20 ± 61.52 a | 335.27 ± 48.27 c | 243.13 ± 57.05 cd | 351.56 ± 47.21 c | 421.57 ± 36.76 bc | 545.56 ± 97.66 bc | 927.92 ± 68.46 a | 323.09 ± 54.26 c | 621.13 ± 127.83 b |
| C20:3n6 | 30.91 ± 4.48 c | 62.42 ± 17.41 b | 73.80 ± 2.67 b | 116.81 ± 17.24 a | 54.92 ± 5.38 bc | 42.45 ± 5.32 bc | 43.53 ± 8.99 c | 63.67 ± 4.68 bc | 78.66 ± 10.38 b | 109.40 ± 14.18 a | 62.68 ± 4.84 bc | 67.21 ± 7.32 bc |
| C20:3n3 | 68.72 ± 10.22 c | 95.47 ± 14.67 abc | 139.79 ± 9.19 a | 138.46 ± 19.94 a | 121.78 ± 21.81 ab | 82.04 ± 16.76 bc | 120.19 ± 22.16 bc | 116.07 ± 9.05 bc | 219.75 ± 26.28 ab | 261.94 ± 76.63 a | 103.91 ± 9.92 c | 147.12 ± 4.26 bc |
| C20:4n6 | 876.24 ± 72.01 a | 359.34 ± 75.63 b | 561.41 ± 36.90 b | 867.78 ± 64.5 a | 812.08 ± 56.56 a | 584.23 ± 111.85 b | 586.89 ± 118.81 b | 330.24 ± 75.40 b | 542.94 ± 46.38 b | 645.68 ± 99.1 b | 1753.32 ± 235.49 a | 507.46 ± 56.07 b |
| C22:2 | 12.50 ± 1.81 | 22.97 ± 10.19 | 21.92 ± 2.02 | 23.76 ± 3.20 | 18.25 ± 1.24 | 14.65 ± 1.38 | 15.66 ± 1.55 b | 32.72 ± 4.37 a | 22.80 ± 2.37 b | 21.67 ± 5.39 b | 15.49 ± 0.81 b | 16.77 ± 2.03 b |
| C20:5n3 | 494.37 ± 68.92 bc | 220.35 ± 39.34 c | 595.83 ± 91.71 b | 446.53 ± 55.94 bc | 992.87 ± 51.59 a | 781.91 ± 253.44 ab | 358.56 ± 62.34 b | 192.95 ± 54.65 b | 501.49 ± 25.85 b | 364.30 ± 21.92 b | 1520.38 ± 232.94 a | 553.87 ± 166.78 b |
| C22:6n3 | 2817.9 ± 415.9 a | 203.5 ± 52.3 b | 872.5 ± 237.6 b | 255.8 ± 25.9 b | 2930.9 ± 382.2 a | 2715.9 ± 902.1 a | 1741.25 ± 458.7 b | 157.5 ± 32.9 c | 665.29 ± 40.4 c | 193.6 ± 25.9 c | 5382.6 ± 483.5 a | 1024.6 ± 473.3 bc |
| ΣPUFA | 6580.0 ± 519.7 b | 6429.1 ± 627.8 b | 12,325.1 ± 497.9 a | 9466.6 ± 887.6 ab | 10,245.1 ± 1152.7 ab | 8128.8 ± 2541.6 b | 5079.1 ± 1126.1 b | 6459.1 ± 1712.6 b | 10,855.4 ± 776.6 a | 10,139.7 ± 1073.5 a | 11,872.5 ± 1531.5 a | 10,643.8 ± 1050.7 a |
| Σn-3 | 3537.2 ± 504.6 ab | 936.8 ± 89.8 c | 2661.5 ± 211.4 bc | 1591.8 ± 248.1 c | 4808.2 ± 503.8 a | 3838.8 ± 1239.8 ab | 2385.1 ± 567.5 bc | 830.6 ± 186.3 c | 2904.7 ± 212.5 b | 2199.35 ± 474.9 bc | 7323.9 ± 769.2 a | 2312.5 ± 636.2 bc |
| Σn-6 | 2853.7 ± 161.6 c | 5149.2 ± 479.1 bc | 9145.3 ± 608.2 a | 6976.9 ± 582.2 ab | 5083.4 ± 898.5 bc | 4032.7 ± 1280.3 c | 2326.8 ± 510.8 c | 5174.1 ± 1494.5 abc | 7382.3 ± 570.8 a | 6990.8 ± 644.1 ab | 4209.9 ± 719.2 bc | 7693.4 ± 1544.5 a |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | PJ | HZ | EZ | YY | JX | CZ | PJ | HZ | EZ | YY | JX | |
| C14:0 | - | - | - | - | - | - | 117.99 ± 7.78 c | 111.03 ± 12.38 c | 169.75 ± 11.49 b | 94.34 ± 1.94 c | 215.56 ± 10.33 a | 105.7 ± 6.94 c |
| C15:0 | - | - | - | - | - | - | 38.48 ± 0.34 c | 81.21 ± 10.11 ab | 89.78 ± 4.22 a | 60.46 ± 2.25 b | 64.88 ± 2.68 b | 42.17 ± 3.26 c |
| C16:0 | 45.04 ± 4.31 c | 67.31 ± 10.20 c | 225.49 ± 20.53 a | 160.95 ± 13.22 b | 48.17 ± 8.68 c | 44.21 ± 4.21 c | 1698.15 ± 92.34 c | 2071.88 ± 77.61 b | 2716.76 ± 69.97 a | 1627.39 ± 54.99 c | 1909.47 ± 71.62 bc | 1750.53 ± 40.30 bc |
| C17:0 | - | - | - | - | - | - | 57.31 ± 2.05 c | 94.99 ± 1.49 b | 123.87 ± 10.30 a | 80.87 ± 2.05 bc | 74.36 ± 3.50 bc | 53.73 ± 4.45 c |
| C18:0 | 39.46 ± 3.92 c | 58.12 ± 8.97 bc | 123.78 ± 8.05 a | 103.88 ± 20.00 ab | 49.29 ± 6.62 c | 37.36 ± 2.01 c | 547.03 ± 28.86 c | 813.64 ± 14.41 b | 1167.53 ± 27.40 a | 627.74 ± 12.7 c | 540.28 ± 29.40 c | 598.15 ± 26.97 c |
| C20:0 | 29.89 ± 2.06 a | 18.36 ± 2.15 b | 20.28 ± 1.25 b | 23.68 ± 0.57 ab | 20.80 ± 1.66 b | 19.31 ± 0.99 b | 38.64 ± 1.34 d | 89.49 ± 5.74 b | 118.41 ± 5.26 a | 70.25 ± 1.57 c | 46.33 ± 3.17 d | 39.06 ± 1.50 d |
| C22:0 | 14.85 ± 0.86 | 15.61 ± 1.29 | 24.54 ± 1.18 | 20.12 ± 4.46 | 16.35 ± 0.58 | 18.23 ± 1.26 | 47.23 ± 4.53 c | 65.75 ± 3.06 ab | 78.19 ± 3.06 a | 46.29 ± 1.33 c | 59.66 ± 2.55 bc | 71.66 ± 4.29 ab |
| ΣSFA | 129.24 ± 2.14 c | 159.4 ± 22.29 c | 394.08 ± 15.8 a | 308.63 ± 12.54 b | 134.61 ± 15.5 c | 119.11 ± 7.3 c | 2544.84 ± 125.24 c | 3328 ± 107.56 b | 4464.27 ± 108.64 a | 2607.36 ± 66.74 c | 2910.55 ± 118.74 bc | 2661 ± 76.96 c |
| C16:1 | 33.16 ± 3.82 cd | 40.08 ± 2.65 bc | 62.01 ± 4.76 a | 52.59 ± 7.15 ab | 10.28 ± 0.81 d | 21.02 ± 0.53 d | 822.41 ± 81.19 d | 1761.89 ± 104.34 a | 1501.49 ± 41.72 ab | 1173.44 ± 50.18 bc | 1076.58 ± 94.23 cd | 1070.36 ± 8.83 cd |
| C18:1n9c | 79.89 ± 3.64 b | 66.47 ± 4.87 b | 276.15 ± 27.29 a | 233.07 ± 5.9 a | 84.12 ± 9.50 b | 81.76 ± 5.72 b | 2282.81 ± 136.66 c | 3647.52 ± 279.58 ab | 4393.19 ± 214.08 a | 2742.1 ± 136.18 c | 2619.72 ± 92.68 c | 2817.16 ± 155.04 bc |
| C20:1 | 13.26 ± 1.58 | 11.65 ± 0.57 | 15.55 ± 2.41 | 13.68 ± 2.1 | 10.79 ± 0.31 | 9.52 ± 0.74 | 111.86 ± 7.59 b | 65.83 ± 6.01 cd | 125.5 ± 3.60 b | 54.55 ± 3.77 d | 215.08 ± 6.29 a | 82.53 ± 4.33 c |
| C22:1n9 | 15.64 ± 1.69 c | 21.79 ± 1.93 bc | 30.25 ± 3.39 a | 25.47 ± 1.1 ab | 14.20 ± 2.04 c | 12.57 ± 1.39 c | 34.44 ± 4.14 c | 69.39 ± 3.75 b | 113.88 ± 2.82 a | 62.86 ± 3.71 b | 35.59 ± 1.22 c | 23.08 ± 1.86 c |
| C24:1 | 73.39 ± 11.45 | 41.69 ± 14.3 | 85.87 ± 12.93 | 91.35 ± 19.73 | 83.47 ± 33.69 | 45.95 ± 10.72 | 14.40 ± 1.47 a | 10.35 ± 0.22 bc | 16.09 ± 0.68 a | 10.26 ± 0.41 bc | 13.82 ± 0.76 ab | 7.72 ± 0.25 c |
| ΣMUFA | 215.33 ± 13.58 b | 181.67 ± 15.72 b | 489.82 ± 17.95 a | 422.82 ± 12.05 a | 201.88 ± 22.09 b | 170.82 ± 7.72 b | 3265.92 ± 208.6 b | 5554.99 ± 167.51 a | 6150.15 ± 248.41 a | 4043.22 ± 111.24 b | 3960.80 ± 173.44 b | 4000.84 ± 155.7 b |
| C18:2n6c | 26.24 ± 1.68 cd | 37.44 ± 1.83 c | 142.86 ± 5.39 a | 109.04 ± 5.17 b | 30.68 ± 2.40 cd | 20.74 ± 0.91 d | 1430.18 ± 88.68 c | 1871.2 ± 63.5 b | 2662.16 ± 115.42 a | 1592.32 ± 88.97 bc | 1845.95 ± 130.47 b | 1200.64 ± 50.82 c |
| C18:3n3 | - | - | - | - | - | - | 133.62 ± 8.82 c | 516.1 ± 32.79 ab | 666.35 ± 12.51 a | 494.01 ± 71.07 b | 107.56 ± 10.40 c | 184 ± 16.61 c |
| C20:2 | 13.11 ± 2.18 c | 14.55 ± 1.93 c | 30.49 ± 5.99 b | 48.56 ± 2.2 a | 15.49 ± 0.52 c | 18.89 ± 0.44 bc | 77.47 ± 4.60 c | 151.44 ± 24.27 b | 220.53 ± 14.55 a | 179.58 ± 3.78 ab | 74.76 ± 8.77 c | 119.63 ± 14.27 bc |
| C20:3n6 | - | - | - | - | - | - | 10.43 ± 0.15 b | 35.99 ± 3.79 a | 43.75 ± 1.91 a | 38.23 ± 1.54 a | 13.07 ± 0.43 b | 15.88 ± 1.93 b |
| C20:3n3 | - | - | - | - | - | - | 41.46 ± 3.53 c | 63.99 ± 3.56 bc | 135.47 ± 5.13 a | 79.61 ± 14.66 b | 32.44 ± 1.37 c | 45.38 ± 5.44 c |
| C20:4n6 | 47.16 ± 2.43 bc | 35.65 ± 3.29 c | 125.07 ± 13.05 a | 105.6 ± 1.91 a | 69.27 ± 4.00 b | 42.05 ± 4.30 bc | 279.87 ± 8.62 b | 279.84 ± 22.24 b | 534.06 ± 40.69 a | 437.41 ± 51.53 a | 407.1 ± 27.59 ab | 287.24 ± 5.65 b |
| C20:5n3 | 48.76 ± 0.20 bc | 41.86 ± 1.91 c | 68.46 ± 4.35 a | 45.18 ± 3.23 c | 58.37 ± 2.72 ab | 47.23 ± 1.97 bc | 618.36 ± 58.10 b | 325.28 ± 16.14 c | 739.33 ± 22.26 b | 412.30 ± 12.53 c | 1122.55 ± 58.68 a | 667.45 ± 28.59 b |
| C22:6n3 | 55.72 ± 0.52 ab | 48.89 ± 0.44 ab | 49.92 ± 4.86 ab | 57.19 ± 2.13 a | 48.88 ± 0.54 ab | 42.86 ± 3.90 b | 635.18 ± 71.84 b | 180.85 ± 11.54 c | 859.67 ± 45.04 b | 222.98 ± 17.15 c | 683.35 ± 102.76 b | 1025.17 ± 52.48 a |
| ΣPUFA | 190.99 ± 2.72 cd | 178.41 ± 6.63 cd | 416.8 ± 18.83 a | 365.58 ± 4.22 b | 222.69 ± 6.95 c | 171.77 ± 10.82 d | 3226.57 ± 230.55 c | 3424.68 ± 80.4 bc | 5861.33 ± 232.88 a | 3456.43 ± 113.53 b | 4286.77 ± 327.95 b | 3545.39 ± 79.84 bc |
| Σn-3 | 104.48 ± 0.59 ab | 90.75 ± 2.23 b | 118.38 ± 7.05 a | 102.38 ± 1.73 ab | 107.25 ± 2.56 ab | 90.09 ± 5.87 b | 1441.80 ± 155.53 c | 1086.21 ± 20.51 d | 2400.82 ± 73.67 a | 1208.90 ± 77.32 cd | 1945.90 ± 99.31 b | 1922 ± 75.66 b |
| Σn-6 | 73.41 ± 1.47 cd | 73.10 ± 4.97 cd | 267.93 ± 15.46 a | 214.65 ± 6.54 b | 99.95 ± 4.87 c | 62.79 ± 5.19 d | 1737.47 ± 77.18 cd | 2187.04 ± 69.35 b | 3239.97 ± 152.76 a | 2067.96 ± 39.78 b | 2266.10 ± 124.1 bc | 1503.76 ± 49.86 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; He, Q.; Li, J.; Zhou, J.; Hua, G.; Xu, Y.; Jiang, G.; Tang, Y. Variability in Morphological Traits and Nutritional Profiles of Adult Eriocheir sinensis in Different Aquacultural Regions. Animals 2025, 15, 243. https://doi.org/10.3390/ani15020243
Feng W, He Q, Li J, Zhou J, Hua G, Xu Y, Jiang G, Tang Y. Variability in Morphological Traits and Nutritional Profiles of Adult Eriocheir sinensis in Different Aquacultural Regions. Animals. 2025; 15(2):243. https://doi.org/10.3390/ani15020243
Chicago/Turabian StyleFeng, Wenrong, Qinghong He, Jianlin Li, Jun Zhou, Guoan Hua, Yuanfeng Xu, Gang Jiang, and Yongkai Tang. 2025. "Variability in Morphological Traits and Nutritional Profiles of Adult Eriocheir sinensis in Different Aquacultural Regions" Animals 15, no. 2: 243. https://doi.org/10.3390/ani15020243
APA StyleFeng, W., He, Q., Li, J., Zhou, J., Hua, G., Xu, Y., Jiang, G., & Tang, Y. (2025). Variability in Morphological Traits and Nutritional Profiles of Adult Eriocheir sinensis in Different Aquacultural Regions. Animals, 15(2), 243. https://doi.org/10.3390/ani15020243






