Influence of Eimeria spp. and Clostridium perfringens Infection on Growth Performance and Toltrazuril Residues in Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Growth Performance
2.3. Determination of Toltrazuril and Metabolites in Liver and Muscle Samples
2.3.1. Sample Preparation
2.3.2. Mass Spectrometry
2.3.3. Quality Assurance of the Results
2.4. Data Analysis
3. Results
3.1. Growth Performance
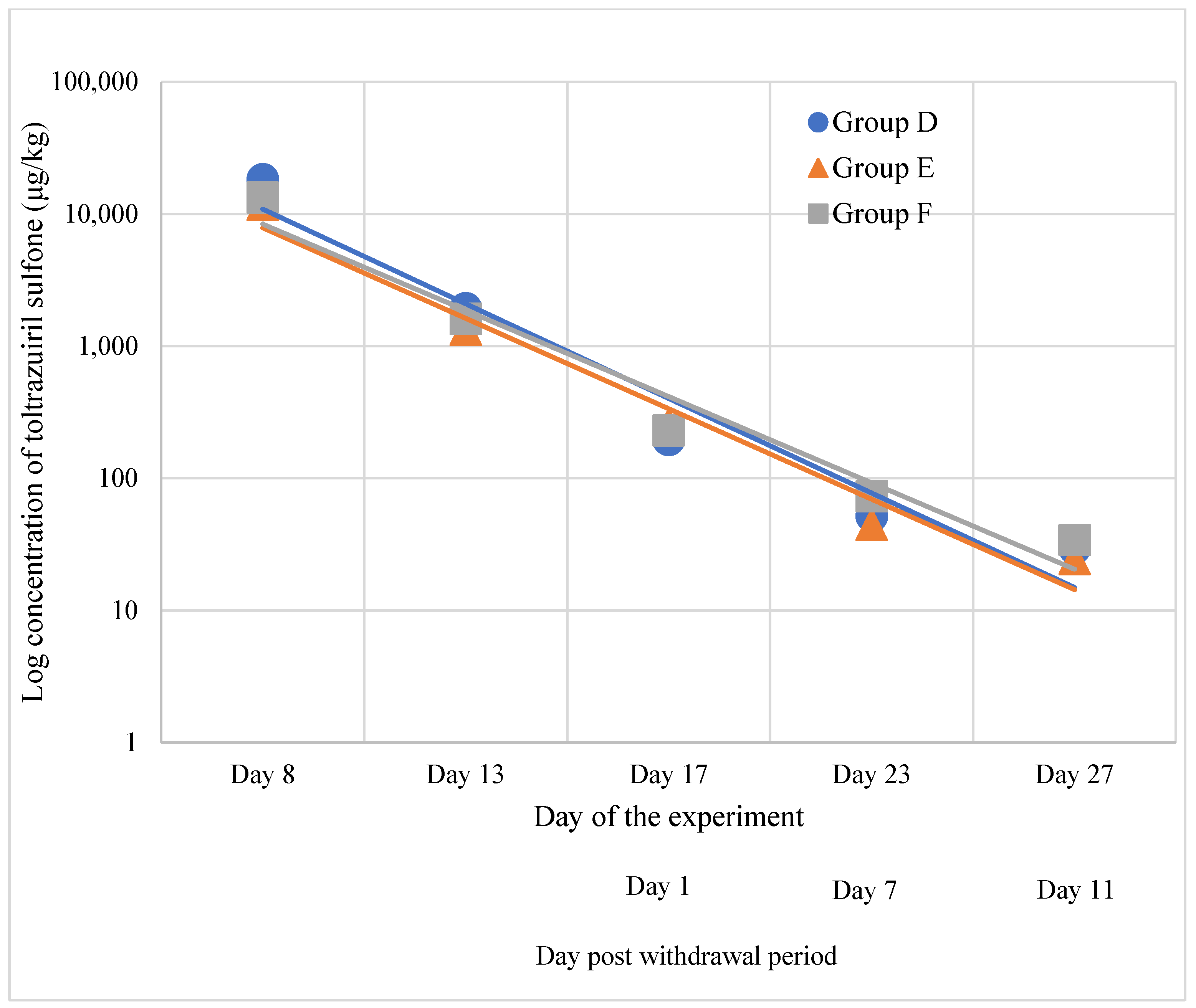
3.2. Residues of Toltrazuril and Metabolites in Tissues
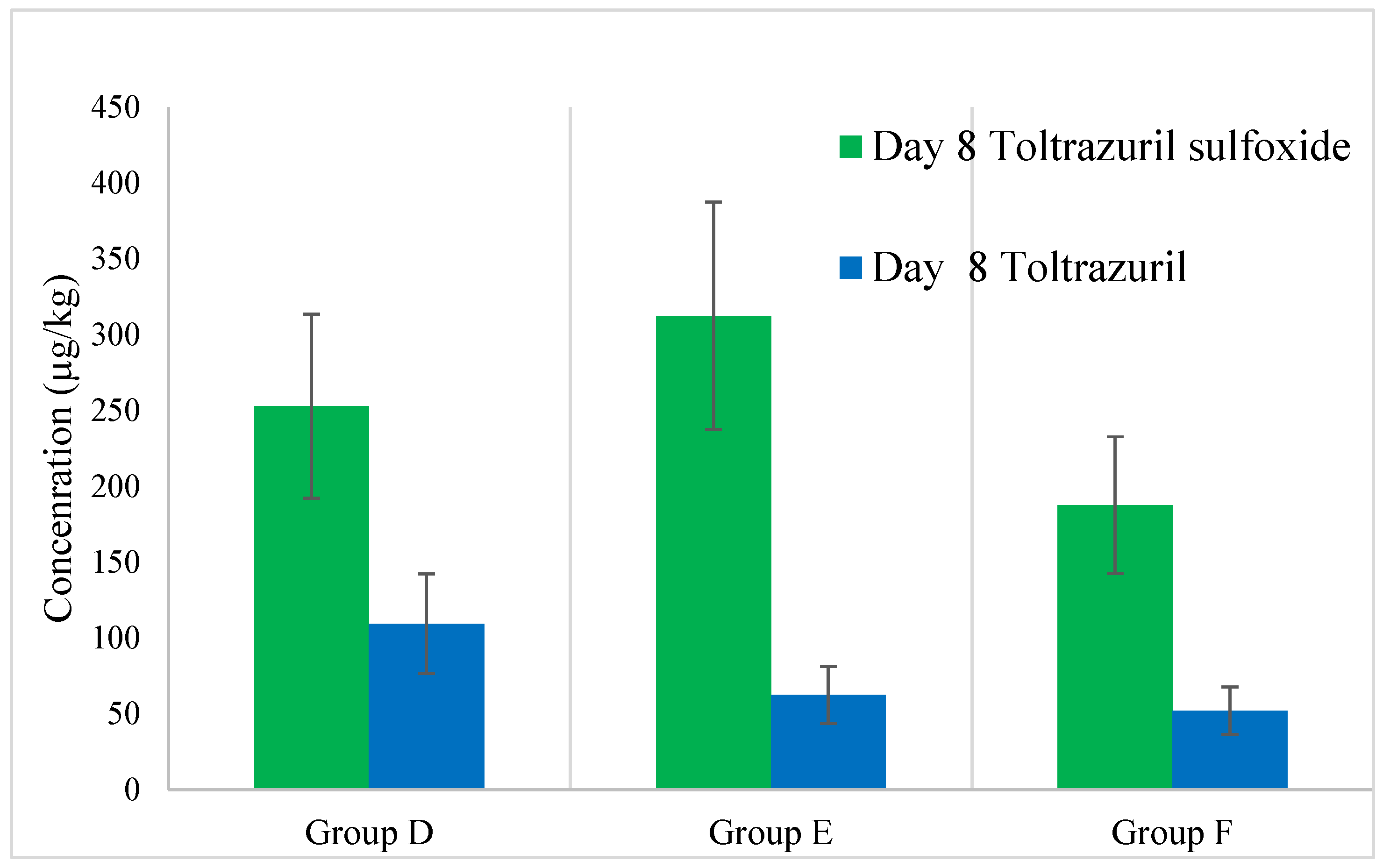
3.2.1. Liver
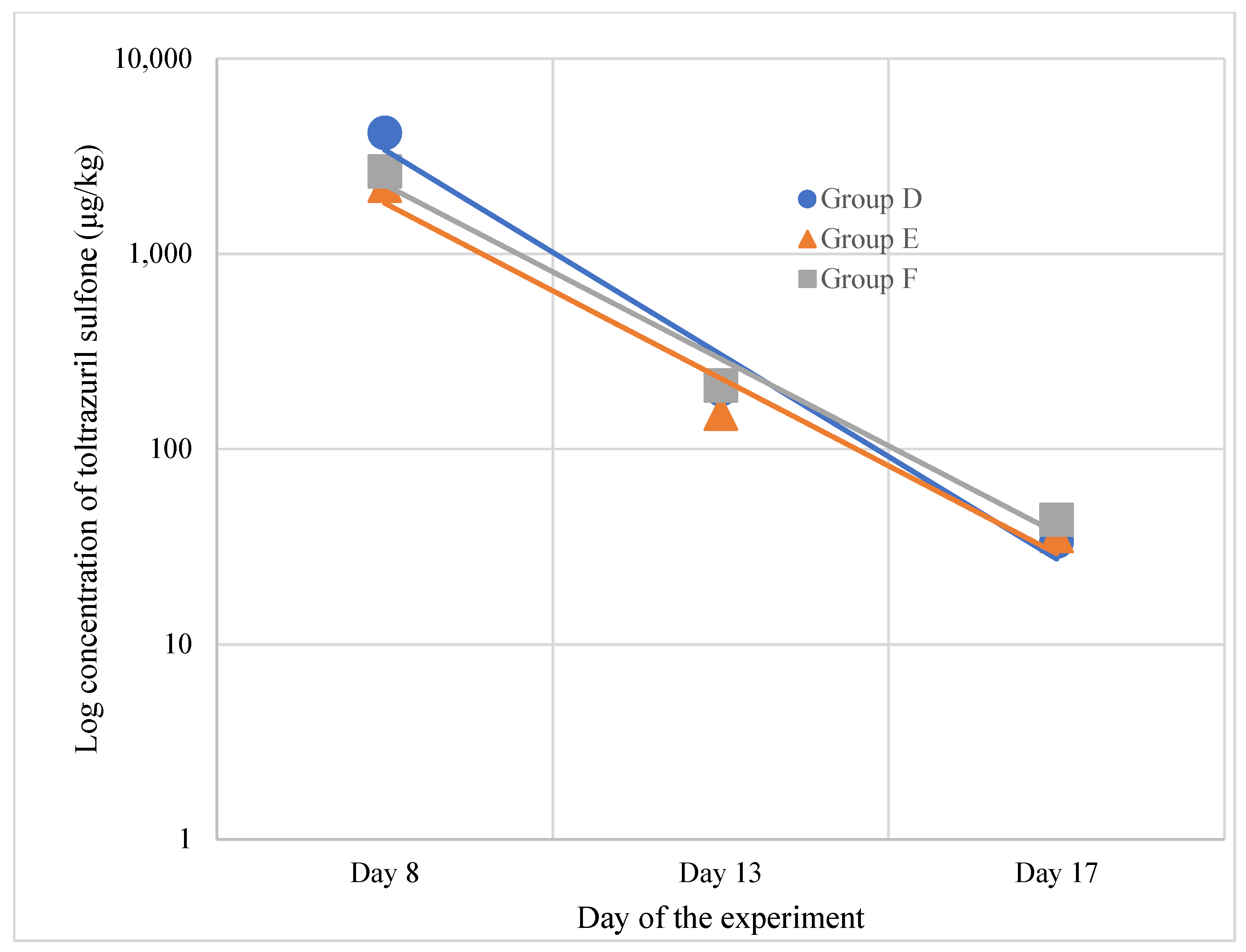
- Toltrazuril Sulfone
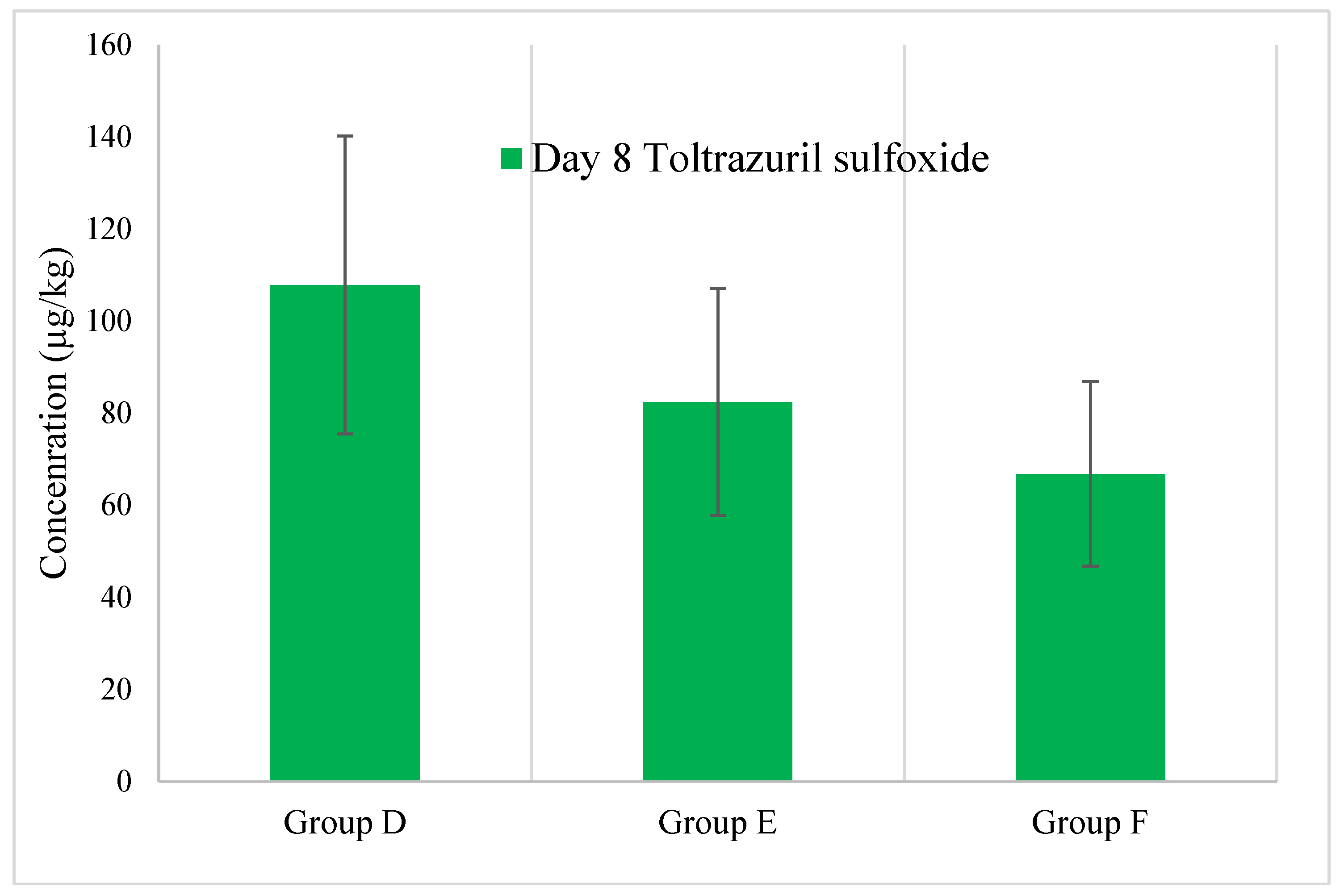
- Toltrazuril and Toltrazuril Sulfoxide
3.2.2. Muscle
- Toltrazuril Sulfone
- Toltrazuril and Toltrazuril Sulfoxide
4. Discussion
4.1. Growth Performance
4.2. Residues of Toltrazuril and Metabolites in Tissues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirley, M.W.; Smith, A.L.; Tomley, F.M. The Biology of Avian Eimeria with an Emphasis on Their Control by Vaccination. Adv. Parasitol. 2005, 60, 285–330. [Google Scholar]
- Quiroz-Castañeda, R.E.; Dantán-González, E. Control of Avian Coccidiosis: Future and Present Natural Alternatives. Biomed. Res. Int. 2015, 2015, 430610. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.M.; Teixeira Soratto, T.A.; Cardinal, K.M.; Wagner, G.; Hauptli, L.; Ferreira Lima, A.L.; Dahlke, F.; Netto, D.P.; de Oliveira Moraes, P.; Leal Ribeiro, A.M. Modulation of the Intestinal Microbiota of Broilers Supplemented with Monensin or Functional Oils in Response to Challenge by Eimeria spp. PLoS ONE 2020, 15, e0237118. [Google Scholar] [CrossRef]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A Review on Prebiotics and Probiotics for the Control of Dysbiosis: Present Status and Future Perspectives. Animal 2014, 9, 43–48. [Google Scholar] [CrossRef]
- Teirlynck, E.; Haesebrouck, F.; Pasmans, F.; Dewulf, J.; Ducatelle, R.; Van Immerseel, F. The Cereal Type in Feed Influences Salmonella Enteritidis Colonization in Broilers. Poult. Sci. 2009, 88, 2108–2112. [Google Scholar] [CrossRef]
- Prescott, J.F.; Parreira, V.R.; Mehdizadeh Gohari, I.; Lepp, D.; Gong, J. The Pathogenesis of Necrotic Enteritis in Chickens: What We Know and What We Need to Know: A Review. Avian Pathol. 2016, 45, 288–294. [Google Scholar] [CrossRef]
- Wu, S.B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two Necrotic Enteritis Predisposing Factors, Dietary Fishmeal and Eimeria Infection, Induce Large Changes in the Caecal Microbiota of Broiler Chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Wade, B.; Keyburn, A. The True Cost of Necrotic Enteritis. World Poult. 2015, 31, 16–17. [Google Scholar]
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C.; et al. Evaluation of the Epithelial Barrier Function and Ileal Microbiome in an Established Necrotic Enteritis Challenge Model in Broiler Chickens. Front. Vet. Sci. 2018, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kim, H.G.; Kim, J.S.; Oh, D.G.; Um, Y.J.; Seo, C.S.; Han, J.W.; Cho, H.J.; Kim, G.H.; Jeong, T.C.; et al. The Effect of Gut Microbiota on Drug Metabolism. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Wilson, I.D. Understanding “global” Systems Biology: Metabonomics and the Continuum of Metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Mathis, G.F.; McDougald, L.R. Drug Responsiveness of Field Isolates of Chicken Coccidia. Poult. Sci. 1982, 61, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H.; Ortmann-Falkenstein, G.; Haberkorn, A. The Effects of Sym. Triazinones on Developmental Stages of Eimeria Tenella, E. Maxima and E. Acervulina: A Light and Electron Microscopical Study. Z. Parasitenkd. 1984, 70, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Mathis, G.F.; Froyman, R.; Kennedy, T. Coccidiosis Control by Administering Toltrazuril in the Drinking Water for a 2-Day Period. Vet. Parasitol. 2004, 121, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, M.S.; Hwang, Y.H.; Song, I.B.; Park, B.K.; Yun, H.I. Pharmacokinetics of Toltrazuril and Its Metabolites, Toltrazuril Sulfoxide and Toltrazuril Sulfone, after a Single Oral Administration to Pigs. J. Vet. Med. Sci. 2010, 72, 1085–1087. [Google Scholar] [CrossRef]
- European Medicines Agency Toltrazuril Summary Report. 1998; pp. 1–5. Available online: https://www.ema.europa.eu/en/documents/mrl-report/toltrazuril-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 11 November 2024).
- European Commission. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Off. J. Eur. Union 2009, 15, 1–72. [Google Scholar]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current Trends in Drug Metabolism and Pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef]
- Bladek, T.; Posyniak, A.; Jablonski, A.; Gajda, A. Pharmacokinetics of Tulathromycin in Edible Tissues of Healthy and Experimentally Infected Pigs with Actinobacillus Pleuropneumoniae. Food Addit. Contam. Part A 2015, 32, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Gbylik-Sikorska, M.; Gajda, A.; Posyniak, A. Pharmacokinetic Depletion Phase of Doxycycline in Healthy and Mycoplasma Gallisepticum-Infected Chicken Broilers after Coadministration of Enrofloxacin Traces. J. Vet. Pharmacol. Ther. 2018, 41, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B. Quantification of the Crowding Effect during Infections with the Seven Eimeria Species of the Domesticated Fowl: Its Importance for Experimental Designs and the Production of Oocyst Stocks. Int. J. Parasitol. 2001, 31, 1056–1069. [Google Scholar] [CrossRef]
- ISO 15213-2:2023; Microbiology of the Food Chain—Horizontal Method for the Determination and Enumeration of Clostridium spp. International Organization for Standardization: Geneva, Switzerland, 2023.
- European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residue of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods To. Off. J. Eur. Union 2021, 33, 1–31. [Google Scholar]
- Lu, M.; Li, R.W.; Zhao, H.; Yan, X.; Lillehoj, H.S.; Sun, Z.; Oh, S.T.; Wang, Y.; Li, C. Effects of Eimeria Maxima and Clostridium Perfringens Infections on Cecal Microbial Composition and the Possible Correlation with Body Weight Gain in Broiler Chickens. Res. Vet. Sci. 2020, 132, 142–149. [Google Scholar] [CrossRef]
- Mathis, G.F.; Froyman, R.; Irion, T.; Kennedy, T. Coccidiosis Control with Toltrazuril in Conjunction with Anticoccidial Medicated or Nonmedicated Feed. Avian Dis. 2003, 47, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J. Necrotic Enteritis Predisposing Factors in Broiler Chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-Induced Mucogenesis Promotes the Onset of Necrotic Enteritis by Supporting Clostridium Perfringens Growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Rood, J.I.; Moore, R.J.; Titball, R.W. Rethinking Our Understanding of the Pathogenesis of Necrotic Enteritis in Chickens. Trends Microbiol. 2009, 17, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, B.K.; Hwang, Y.H.; Song, I.B.; Kim, T.W.; Cho, J.H.; Ham, S.H.; Lim, J.H.; Yun, H.I. Pharmacokinetics and Metabolism of Toltrazuil and Its Major Metabolites after Oral Administration in Broilers. J. Poult. Sci. 2013, 50, 257–261. [Google Scholar] [CrossRef]
- Soliman, A. Pharmacokinetics and Tissue Residue of Toltrazuril in Broiler Chickens. Int. J. Basic Appl. Sci. 2015, 4, 310. [Google Scholar] [CrossRef]
- Kandeel, M. Pharmacokinetics and Oral Bioavailability of Amoxicillin in Chicken Infected with Caecal Coccidiosis. J. Vet. Pharmacol. Ther. 2015, 38, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M. Bioavailability and Pharmacokinetics of Ampicillin in Chicken Infected with Eimeria Tenella. Int. J. Pharmacol. 2014, 10, 340. [Google Scholar] [CrossRef]
- Haritova, A.M.; Lashev, L.D.; Koinarski, V.C. Sulfachloropyrazine Disposition in Eimeria Tenella Infected Chickens. Vet. Arh. 2013, 83, 211–222. [Google Scholar]
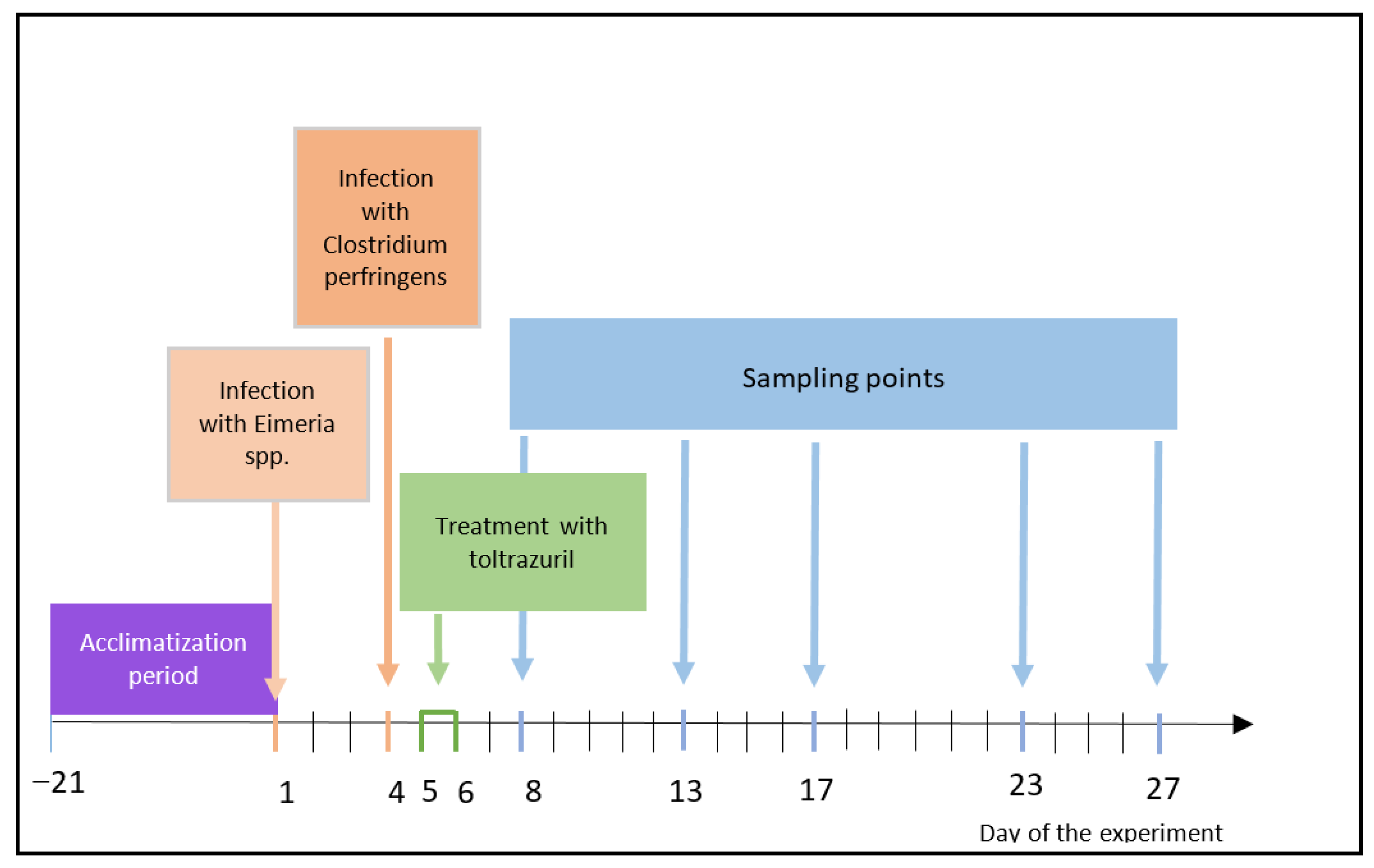
| Experimental Factor | Eimeria spp. | Clostridium Perfringens | Toltrazuril |
|---|---|---|---|
| Day of the experiment/ Day of life | 1/ 22 | 4/ 25 | 5 and 6/ 26 and 27 |
| Group A—10 chickens | - | - | - |
| Group B—15 chickens | + | - | - |
| Group C—15 chickens | + | + | - |
| Group D—30 chickens | - | - | + |
| Group E—30 chickens | + | - | + |
| Group F—30 chickens | + | + | + |
| Day of the Experiment | |||||||
|---|---|---|---|---|---|---|---|
| −21 | 1 | 8 | 13 | 17 | 23 | 27 | |
| Group A | 38.7 ± 3.1 a | 132 ± 18.9 a | 184 ± 31.2 a | 206 ± 33.9 a | 261 ± 37.1 a | 281 ± 31.1 a | 301 ± 21.6 a |
| Group B | 42.4 ± 4.2 a | 103 ± 14.9 a | 110 ± 13.2 b | 128 ± 22.9 b | 142 ± 25.2 b | 161 ± 22.6 b | 182 ± 31.8 b |
| Group C | 41.2 ± 4.1 a | 125 ± 17.4 a | 162 ± 14.3 a | 192 ± 23.3 a | 223 ± 30.1 a | 279 ± 42.2 a | 299 ± 51.2 a |
| Group D | 41.9 ± 3.2 a | 129 ± 30.1 a | 182 ± 40.8 a | 212 ± 60.1 a | 251 ± 55.8 a | 291 ± 55.5 a | 302 ± 36.2 a |
| Group E | 39.4 ± 3.5 a | 132 ± 21.9 a | 153 ± 31.7 a | 182 ± 53.1 a | 249 ± 41.8 a | 271 ± 47.8 a | 301 ± 63.6 a |
| Group F | 42.5 ± 3.6 a | 136 ± 21.8 a | 162 ± 22.1 a | 191 ± 37.8 a | 249 ± 36.9 a | 281 ± 48.1 a | 302 ± 62.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietruk, K.; Karamon, J.; Jedziniak, P.; Tokarzewski, S.; Olejnik, M. Influence of Eimeria spp. and Clostridium perfringens Infection on Growth Performance and Toltrazuril Residues in Chickens. Animals 2025, 15, 216. https://doi.org/10.3390/ani15020216
Pietruk K, Karamon J, Jedziniak P, Tokarzewski S, Olejnik M. Influence of Eimeria spp. and Clostridium perfringens Infection on Growth Performance and Toltrazuril Residues in Chickens. Animals. 2025; 15(2):216. https://doi.org/10.3390/ani15020216
Chicago/Turabian StylePietruk, Konrad, Jacek Karamon, Piotr Jedziniak, Stanisław Tokarzewski, and Małgorzata Olejnik. 2025. "Influence of Eimeria spp. and Clostridium perfringens Infection on Growth Performance and Toltrazuril Residues in Chickens" Animals 15, no. 2: 216. https://doi.org/10.3390/ani15020216
APA StylePietruk, K., Karamon, J., Jedziniak, P., Tokarzewski, S., & Olejnik, M. (2025). Influence of Eimeria spp. and Clostridium perfringens Infection on Growth Performance and Toltrazuril Residues in Chickens. Animals, 15(2), 216. https://doi.org/10.3390/ani15020216







