Blood Morphology and Hematology of Adult Baikal Seals (Pusa sibirica Gmelin, 1788) Under Professional Care †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
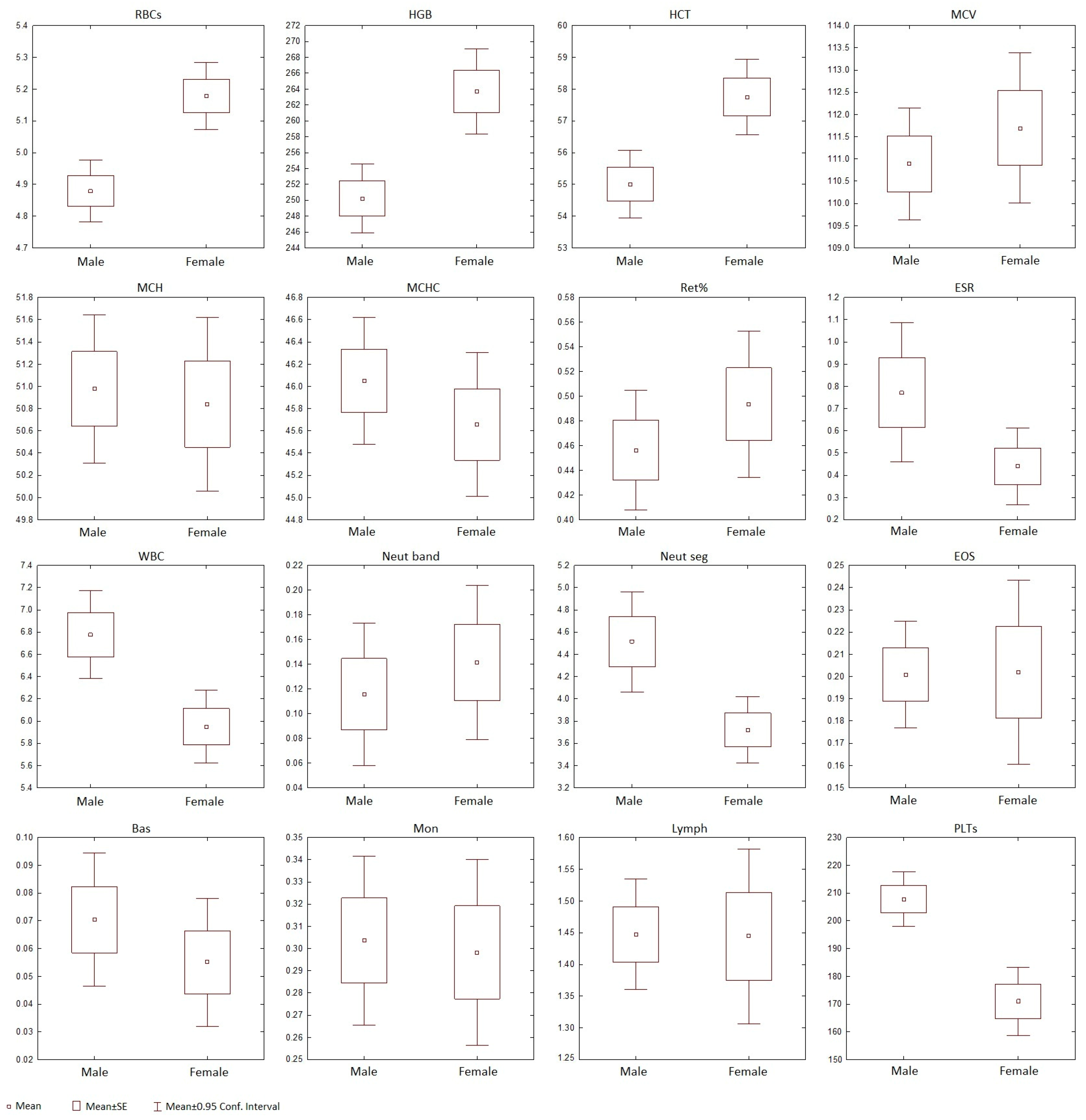
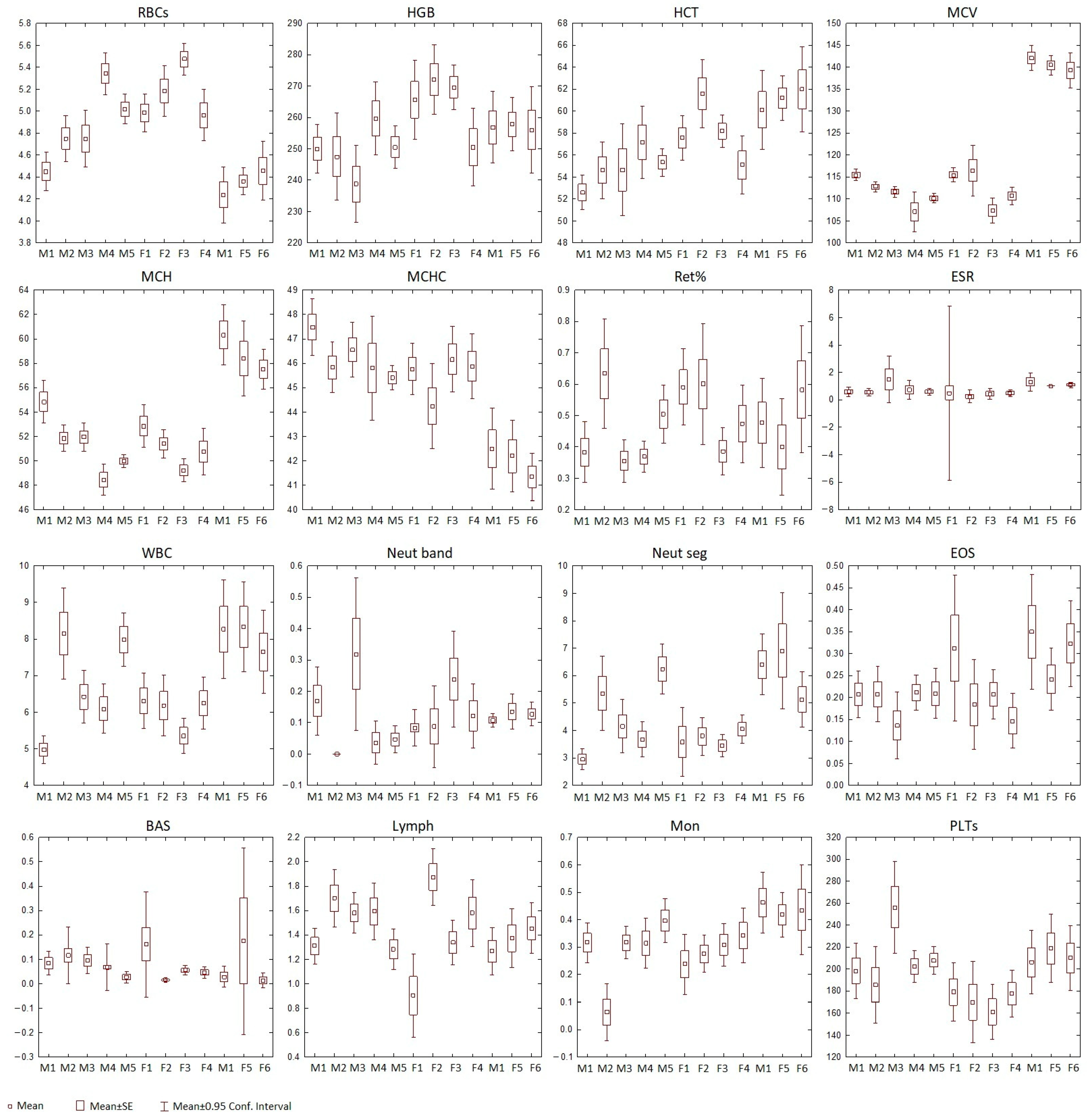
3.1. Erythrocytes
3.2. Leukocytes
3.3. Agranulocytes, Monocytes, and Lymphocytes
3.4. Platelets
3.5. Statistical Analysis of Baikal Seals’ Blood Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Häder, D.P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morán, A.; Banuet-Martínez, M.; Elorriaga-Verplancken, F.R.; Gar-cía-Ortuño, L.E.; Sandoval-Sierra, J.; Acevedo-Whitehouse, K. Atypical Red Blood Cells Are Prevalent in California Sea Lion Pups Born during Anomalous Sea Surface Temperature Events. Physiol. Biochem. Zool. 2017, 90, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Imaeda, D.; Nomiyama, K.; Kunisue, T.; Iwata, H.; Tsydenova, O.; Amano, M.; Petrov, E.A.; Batoev, V.B.; Tanabe, S. Blood levels of polychlorinated biphenyls and their hydroxylated metabolites in Baikal seals (Pusa sibirica): Emphasis on interspecies comparison, gender difference and association with blood thyroid hormone levels. Chemosphere 2014, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ozersky, T.; Pastukhov, M.V.; Poste, A.E.; Deng, X.Y.; Moore, M.V. Long-Term and Ontogenetic Patterns of Heavy Metal Contamination in Lake Baikal Seals (Pusa sibirica). Environ. Sci. Technol. 2017, 51, 10316–10325. [Google Scholar] [CrossRef]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine mammals and microplastics: A systematic review and call for standardization. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef]
- Barratclough, A.; Ferguson, S.H.; Lydersen, C.; Thomas, P.O.; Kovacs, K.M. A Review of Circumpolar Arctic Marine Mammal Health—A Call to Action in a Time of Rapid Environmental Change. Pathogens 2023, 12, 937. [Google Scholar] [CrossRef]
- Kydyrmanov, A.; Karamendin, K.; Kassymbekov, Y.; Kumar, M.; Mazkirat, S.; Suleimenova, S.; Baimukanov, M.; Carr, I.M.; Goodman, S.J. Exposure of wild Caspian seals (Pusa caspica) to parasites, bacterial and viral pathogens, evaluated via molecular and serological assays. Front. Mar. Sci. 2023, 10, 1087997. [Google Scholar] [CrossRef]
- Boily, F.; Beaudoin, S.; Measures, L.N. Hematology and serum chemistry of harp (Phoca groenlandica) and hooded seals (Cystophora cristata) during the breeding season, in the Gulf of St. Lawrence, Canada. J. Wildl. Dis. 2006, 42, 115–132. [Google Scholar] [CrossRef]
- Greig, D.J.; Gulland, F.M.; Rios, C.A.; Hall, A.J. Hematology and serum chemistry in stranded and wild-caught harbor seals in central California: Reference intervals, predictors of survival, and parameters affecting blood variables. J. Wildl. Dis. 2010, 46, 1172–1184. [Google Scholar] [CrossRef]
- Reidarson, T.H.; Duffield, D.; McBain, J. Hematology of Marine Mammals. In Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 950–957. [Google Scholar]
- Reagan, W.J.; Rovira, A.R.; DeNicola, D.B. Veterinary Hematology: Atlas common Domestic and Non-Domestic Species, 3rd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2019; 144p. [Google Scholar]
- Satyaningtijas, A.S.; Indrawati, A.; Syarafina, R.F.; Milani, T.F.; Suryaputra, M.; Saleema, A.K.; Hanadhita, D. Erythrocytes and leukocytes profiles of bottlenose dolphins (Tursiops aduncus) at conservation site. Biodiversitas 2020, 21, 3359–3363. [Google Scholar] [CrossRef]
- Stacy, N.I.; Nollens, H. Hematology of Marine Mammals. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M.B., Harr, K.E., Seelig, D.M., Wardrop, K.J., Weiss, D.J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2022; pp. 1104–1113. [Google Scholar]
- Clayton, L.; Arnold, J.; Long, S.; Mankowski, J.L.; Montali, R.J.; Hadfield, C.A.; Brown, D.E. Case report: Metarubricytosis in two Atlantic bottlenose dolphin (Tursiops truncatus truncatus) calves. In Proceedings of the 43rd Annual Conference of the International Association for Aquatic Animal Medicine, Atlanta, GA, USA, 12–16 May 2012. [Google Scholar]
- Harvey, J.W. Veterinary Hematology: A Diagnostic Guide and Colour Atlas; Saunders: St. Louis, MI, USA; Elsevier: St. Louis, MI, USA, 2012; 360p. [Google Scholar] [CrossRef]
- Clark, P.; Boardman, W.S.; Duignan, P.J. Cytology of haematological cells of otariid seals indigenous to Australasian waters. Aust. Vet. J. 2002, 80, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, U.; Heidemann, G.; Skirnisson, K.; Schumacher, W.; Pickering, R.M. Impact of captivity and contamination level on blood parameters of harbour seals (Phoca vitulina). Comp. Biochem. Physiol. 1995, 112A, 455–462. [Google Scholar] [CrossRef]
- Dierauf, L.A.; Gulland, F. CRC Handbook of Marine Mammal Medicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001; 1063p. [Google Scholar]
- Gulland, F.; Dierauf, L.A.; Whitman, K.L. CRC Handbook of Marine Mammal Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 1087. [Google Scholar]
- Vail, M.; Beaufrère, H.; Gallini, S.; Paluch, H.; Brandão, J.; Digeronimo, P. Hemato-logic and plasma biochemical prognostic indicators for stranded free-ranging phocids presented for rehabilitation. Sci. Rep. 2022, 12, 10546. [Google Scholar] [CrossRef] [PubMed]
- Esipova, P.V.; Yachmen, V.A.; Suvorova, I.V.; Pushchin, I.I. Hematology and cytomorphometric characteristics of peripheral blood of the Baikal seal (Pusa sibirica Gmelin, 1788). In Proceedings of the XII International Scientific and Practical Conference «Marine Research and Education—MARESEDU 2023», Moscow, Russia, 23–27 October 2024; pp. 237–242. [Google Scholar]
- Mamontov, A.A.; Mamontova, E.A.; Tarasova, E.N. Persistent Organic Pollutants in Baikal Seal (Pusa sibirica) Blubber. Russ. J. Gen. Chem. 2019, 89, 2791–2797. [Google Scholar] [CrossRef]
- Ronald, K.; Kay, J. Haematology and plasma chemistry of captive Baikal seals Pusa sibrica. Aquat. Mamm. 1982, 9, 83–94. [Google Scholar]
- Gordeev, I.I.; Boltnev, E.A.; Suvorova, T.A.; Mikryakov, D.V.; Balabanova, L.V. Pe-ripheral Blood Cell Composition of Baikal Seal Phoca sibirica. Inland Water Biol. 2023, 16, 1173–1177. [Google Scholar] [CrossRef]
- Kurashvili, L.V.; Mikhalkina, O.P. Skorost’ osedaniia éritrotsitov v klinicheskoĭ praktike [Erythrocyte sedimentation rate in clinical practice]. Klin. Lab. Diagn. 1994, 5, 56–57. (In Russian) [Google Scholar] [PubMed]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures, 5th ed.; Chapman and Hall: New York, NY, USA; CRC: New York, NY, USA, 2000; 1928p. [Google Scholar]
- Esipova, P.V.; Solovyeva, M.A.; Rozhnov, V.V.; Mamaev, E.G.; Suvorova, I.V.; Yachmen, V.A. Blood cell morphology of the harbor seal Phoca vitulina (Linnaeus, 1758) from the Commander Islands. Russ. J. Mar. Biol. 2025, in press.
- Mischenko, P.V.; Yachmen, V.A.; Andrianova, E.N.; Zakharenko, P.G. The Blood Cell Morphology of the Beluga Whale Delphinapterus leucas (Pallas, 1776). Russ. J. Mar. Biol. 2023, 49, 267–273. [Google Scholar] [CrossRef]
- Somporn, L.; Wangnaitham, S.; Sutanonpaiboon, J.; Chansue, N.; Sailasuta, A. Morphological, cytochemical and ultrastructural studies of blood cells in Irrawaddy river dolphin (Orcaella brevirostris): A case study. Thai J. Vet. Med. 2010, 40, 331–335. [Google Scholar] [CrossRef]
- Trumble, S.J.; Castellini, M.; Mau, T.; Castellini, M.J. Dietary and seasonal influences on blood chemistry and hematology in captive harbor seals. Mar. Mammal Sci. 2006, 22, 104–123. [Google Scholar] [CrossRef]
- Yakupova, A.; Tomarovsky, A.; Totikov, A.; Beklemisheva, V.; Logacheva, M.; Perelman, P.L.; Komissarov, A.; Dobrynin, P.; Krasheninnikova, K.; Tamazian, G.; et al. Chromosome-Length Assembly of Baikal seal (Pusa sibirica) Genome Reveals a Historically Large Population Prior to Isolation in Lake Baikal. Genes 2023, 14, 619. [Google Scholar] [CrossRef] [PubMed]
- Quay, W.B. The blood cells of Cetacea with particular reference to the beluga (Delphinapterus leucas Pallas, 1776). Saugetierkd. Mitteil 1954, 2, 49–54. [Google Scholar]
- Williams, C.R., III; Chapman, G.B.; Blake, A.S. Ultrastructural study of the blood cells of the beluga whale, Delphinapterus leucas. J. Morphol. 1991, 209, 97–110. [Google Scholar] [CrossRef]
- Grahl-Nielsen, O.; Halvorsen, A.-K.; Bodoev, N.; Averina, L.; Radnaeva, L.; Pronin, N.; Käkelä, R.; Petrov, E. Fatty acid composition of blubber of the Baikal seal Phoca sibirica and its marine relative, the ringed seal P. hispida. Mar. Ecol. Prog. Ser. 2005, 305, 261–274. [Google Scholar] [CrossRef]
- Lynch, E.C. Peripheral blood smear. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990; Chapter 155. [Google Scholar]
- Weiss, D.J.; Wardrop, K. Drug Induced blood cell disorders. In Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley Blackwell: Ames, IA, USA, 2010; pp. 98–105. [Google Scholar]
- Shirai, K.; Sakai, T. Haematological findings in captive dolphins and whales. Aust. Vet. J. 1997, 75, 512–514. [Google Scholar] [CrossRef]
- McConnell, L.C.; Vaughan, R.W. Some blood values in captive and free-living common seals (Phoca vitulina). Aquat. Mamm. 1983, 10, 9–13. [Google Scholar]
- Kavtsevich, N.N.; Erokhina, I.A.; Svetochev, V.N.; Svetocheva, O.N.; Minzyuk, T.V. Ecological and environmental-physiological researches of pinnipeds of Barents, White and Kara seas in 2015–2019. Oceanology 2020, 7, 198–214. [Google Scholar] [CrossRef]
- Harvey, J.W. The feline blood film: 2. Leukocyte and platelet morphology. J. Feline Med. Surg. 2017, 19, 747–757. [Google Scholar] [CrossRef]
- Cornell, L.H.; Duffield, D.S.; Joseph, B.E.; Stark, B. Hematology and serum chemistry values in the beluga (Delphinapterus leucas). J. Wildl. Dis. 1988, 24, 220–224. [Google Scholar] [CrossRef]
- Bossart, G.D. Immunocytes of the Atlantic Bottlenose Dolphin (Tursiops truncatus) and West Indian Manatee (Trichechus manatus latirostris): Morphologic Characterizations and Correlations Between Healthy and Disease States Under Free-Ranging and Captive Conditions. Ph.D. Dissertation, Florida International University, Miami, FL, USA, 1995. [Google Scholar] [CrossRef]
- Park, J.; Kang, S.-J. The ontogenesis and heterogeneity of basophils. Discov. Immunol. 2024, 3, keae003. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.; Briggs, C.; McFadden, S.; Zini, G.; Burthem, J.; Rozenberg, G.; Proytcheva, M.; Machin, S.J. ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int. J. Lab. Hematol. 2015, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Wickham, L.L.; Elsner, R.; White, F.C.; Cornell, L.H. Blood viscosity in phocid seals: Possible adaptations to diving. J. Comp. Physiol. B 1989, 159, 153–158. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | Baikal Seal (This Study, Mean ± SD) | Harbor Seal [27] | Spotted Seal (Our Unpublished Data) (Mean ± SD) | Beluga Whale [28] (Mean ± SD) | Pacific Bottlenose Dolphins (Our Data) (Mean ± SD) | Irrawaddy River Dolphin [29] (Min–Max) |
|---|---|---|---|---|---|---|
| Metarubricytes (normoblasts) | ND * | ND | ND | 9.9 ± 0.8 | ND | ND |
| Reticulocytes | 8.8 ± 0.8 | 9.3 ± 0.6 | 7.7 ± 0.6 | 10.3 ± 0.8 | 7.6 ± 0.5 | ND |
| Red blood cells | 8.2 ± 0.6 | 7.2 ± 0.7 | 7.6 ± 0.3 | 8.6 ± 0.7 | 6.8 ± 0.9 | 6–7 |
| Neutrophils | 12.8 ± 1.0 | 12.6 ± 0.8 | 12.3 ± 0.8 | 14.1 ± 1.1 | 13.1 ± 1.2 | 10–12 |
| Eosinophils | 14.5 ± 0.8 | 14.3 ± 0.8 | 13.3 ± 0.8 | 14.0 ± 1.2 | 12.3 ± 1.0 | 8–10 |
| Eosinophil granules | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | ND | 0.5 ± 0.07 | ND |
| Basophils | 14.9 ± 1.5 | 13.9 ± 1.1 | 15.2 ± 1.1 | 13.0 ± 1.9 | 12.7 ± 0.2 | ND |
| Basophil granules | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.9 ± 0.3 | ND | 0.5 ± 0.08 | ND |
| Monocytes | 15.0 ± 1.6 | 14.8 ± 1.2 | 15.5 ± 1.2 | 15.0 ± 2.2 | 13.9 ± 1.6 | 14–16 |
| Lymphocytes | 9.6 ± 1.3 | 9.0 ± 0.5 small; 11.3 ± 1.1 large | 9.1 ± 1.4 | 10.8 ± 1.3 | 11.1 ± 1.6 | 8–10 |
| Platelets | 2.1 ± 0.6 | 2.6 ± 0.8 | 2.2 ± 0.7 | 3.5 ± 1.7 | 3.0 ± 0.7 | 1–2 |
| Variable | Rank Sum Male | Rank Sum Female | U | Z | p-Value | Z Adjusted | p-Value | Valid N Male | Valid N Female |
|---|---|---|---|---|---|---|---|---|---|
| RBCs * | 8271 | 8382 | 2600 | −4.08 | 0.000 | −4.07 | 0.000 | 106 | 76 |
| HGB * | 6416 | 7446 | 2138 | −4.11 | 0.000 | −4.11 | 0.000 | 92 | 74 |
| HCT * | 6496 | 7532 | 2031 | −4.52 | 0.000 | −4.51 | 0.000 | 94 | 73 |
| MCV | 4307 | 3319 | 1679 | −0.80 | 0.422 | −0.80 | 0.422 | 72 | 51 |
| MCH | 4543 | 3207 | 1842 | −0.10 | 0.923 | −0.09 | 0.923 | 73 | 51 |
| MCHC | 4784 | 2966 | 1640 | 1.12 | 0.262 | 1.12 | 0.261 | 73 | 51 |
| Ret | 3999 | 3023 | 1443 | −1.24 | 0.215 | −1.26 | 0.208 | 71 | 47 |
| ESR | 2963 | 953 | 628 | 1.47 | 0.141 | 1.58 | 0.115 | 63 | 25 |
| WBC | 10,485 | 5986 | 3136 | 2.41 | 0.016 | 2.42 | 0.015 | 106 | 75 |
| Neut band | 3540 | 2238 | 1125 | −1.21 | 0.227 | −1.29 | 0.195 | 69 | 38 |
| Neut segm | 6839 | 5251 | 2550 | 1.59 | 0.113 | 1.59 | 0.112 | 82 | 73 |
| EOS | 6635 | 5147 | 2519 | 1.45 | 0.147 | 1.45 | 0.147 | 81 | 72 |
| BAS | 2023 | 1464 | 798 | 0.44 | 0.659 | 0.45 | 0.654 | 47 | 36 |
| Mon | 8618 | 6782 | 3622 | 0.51 | 0.611 | 0.51 | 0.611 | 96 | 79 |
| Lymph | 6301 | 5635 | 2898 | −0.19 | 0.845 | −0.19 | 0.845 | 82 | 72 |
| PLTs * | 7698 | 3477 | 1586 | 4.24 | 0.000 | 4.24 | 0.000 | 88 | 61 |
| Variable | Pooled N | H | p-Value |
|---|---|---|---|
| RBCs | 220 | 107 | 0.0000 |
| HGB | 204 | 304 | 0.0004 |
| HCT | 205 | 61 | 0.0000 |
| MCV | 161 | 126 | 0.0000 |
| MCH | 162 | 107 | 0.0000 |
| MCHC | 162 | 80 | 0.0000 |
| Ret | 156 | 25 | 0.0088 |
| ESR | 126 | 46 | 0.0000 |
| WBC | 219 | 77 | 0.0000 |
| Neut band | 145 | 49 | 0.0000 |
| Neut segm | 193 | 78 | 0.0000 |
| EOS | 191 | 27 | 0.0040 |
| BAS | 121 | 39 | 0.0000 |
| Mon | 213 | 44 | 0.0000 |
| Lymph | 192 | 46 | 0.0000 |
| PLTs | 187 | 34 | 0.0003 |
| Males (n = 5, samples = 30) | Females (n = 4, samples = 25) | ||
|---|---|---|---|
| RBCs, ×1012/L | Mean ± st.dev * | 4.8 ± 0.3 | 5.0 ± 0.2 |
| Range ** | 3.4–5.9 | 3.7–6.3 | |
| HGB, g/L | Mean ± st.dev | 249.3 ± 7.0 | 264.4 ± 8.9 |
| Range | 192.0–304.0 | 199.0–306.0 | |
| HCT, % | Mean ± st.dev | 54.8 ± 1.5 | 58.0 ± 2.5 |
| Range | 46.5–83.0 | 40.8–69.7 | |
| MCV, fl | Mean ± st.dev | 111.4 ± 2.9 | 112.5 ± 3.9 |
| Range | 99.1–139.7 | 102.4–133.6 | |
| MCH, pg | Mean ± st.dev | 51.3 ± 2.2 | 51.0 ± 1.3 |
| Range | 43.7–60.6 | 44.7–58.5 | |
| MCHC, g/L | Mean ± st.dev | 46.1 ± 0.7 | 45.4 ± 0.7 |
| Range | 34.7–54.9 | 39.0–52.0 | |
| Ret, % | Mean ± st.dev | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Range | 0.1–1.1 | 0.2–1.0 | |
| ESR, mm/h | Mean ± st.dev | 0.7 ± 0.4 | 0.4 ± 0.1 |
| Range | 0.0–9.0 | 0.0–1.5 | |
| WBC, ×109/L | Mean ± st.dev | 6.6 ± 1.2 | 5.9 ± 0.4 |
| Range | 4.0–12.1 | 3.0–8.8 | |
| Neut band, 109/L | Mean ± st.dev | 0.09 ± 0.1 | 0.1 ± 0.05 |
| Range | 0.0–1.4 | 0.0–0.7 | |
| Neut seg, 109/L | Mean ± st.dev | 4.4 ± 1.2 | 3.6 ± 0.2 |
| Range | 1.9–10.1 | 0.0–6.1 | |
| Eos, 109/L | Mean ± st.dev | 0.1 ± 0.04 | 0.1 ± 0.08 |
| Range | 0.0–0.5 | 0.0–0.7 | |
| Bas, 109/L | Mean ± st.dev | 0.07 ± 0.02 | 0.05 ± 0.03 |
| Range | 0.0–0.3 | 0.0–0.3 | |
| Mon, 109/L | Mean ± st.dev | 0.2 ± 0.1 | 0.2 ± 0.05 |
| Range | 0.01–0.8 | 0.0–1.0 | |
| Lymph, 109/L | Mean ± st.dev | 1.4 ± 0.1 | 1.3 ± 0.3 |
| Range | 0.6–2.8 | 0.0–3.0 | |
| PLTs, 109/L | Mean ± st.dev | 210.1 ± 25.4 | 171.8 ± 7.6 |
| Range | 116.0–398.0 | 101.0–290.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esipova, P.; Suvorova, I.; Yachmen, V.; Pushchin, I. Blood Morphology and Hematology of Adult Baikal Seals (Pusa sibirica Gmelin, 1788) Under Professional Care. Animals 2025, 15, 217. https://doi.org/10.3390/ani15020217
Esipova P, Suvorova I, Yachmen V, Pushchin I. Blood Morphology and Hematology of Adult Baikal Seals (Pusa sibirica Gmelin, 1788) Under Professional Care. Animals. 2025; 15(2):217. https://doi.org/10.3390/ani15020217
Chicago/Turabian StyleEsipova, Polina, Irina Suvorova, Veronika Yachmen, and Igor Pushchin. 2025. "Blood Morphology and Hematology of Adult Baikal Seals (Pusa sibirica Gmelin, 1788) Under Professional Care" Animals 15, no. 2: 217. https://doi.org/10.3390/ani15020217
APA StyleEsipova, P., Suvorova, I., Yachmen, V., & Pushchin, I. (2025). Blood Morphology and Hematology of Adult Baikal Seals (Pusa sibirica Gmelin, 1788) Under Professional Care. Animals, 15(2), 217. https://doi.org/10.3390/ani15020217





