A Framework for Comprehensive Dairy Calf Health Investigations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Prenatal Factors
3.1. Maternal Nutrition
| Variable | Far-Off Dry Cows | Close-Up Dry Cows |
|---|---|---|
| Dry matter intake (kg/d) | 13–14 | ~13 |
| Metabolizable energy (Mcal/kg) | 1.8–2.0 | 2.1–2.2 |
| Crude protein (% DM) | <16 | <15 |
| Metabolizable protein (MP; g/d) | 1000–1100 | 1200–1400 |
| Methionine (% of MP) | No guideline | 2.6–2.8 |
| Lysine (% of MP) | No guideline | 6.8–7.0 |
| Starch (% DM) | <13 | 16–18 |
| Non-fiber carbohydrate (NFC; % DM) | 20–30 | 30–34 |
| Total fat, optimum (% DM) | 3.5 | 3.5 |
| Calcium (% DM) | 0.5–0.7 | 1.5 |
| Phosphorus (% DM) | 0.3–0.35 | 0.3–0.35 |
| Magnesium (% DM) | 0.2–0.25 | 0.45–0.50 |
| Potassium (% DM) | <2.0 | <1.3 |
| Sodium (% DM) | 0.1–0.2 | 0.1–0.2 |
| Chloride (% DM) | 0.4–0.8 | 0.4–0.8 |
| Sulfur (% DM) | 0.3 | 0.3 |
| Selenium b (mg/kg) | 0.3 | 0.3 |
| Zinc c (mg/kg) | 60–80 | 60–80 |
| Vitamin A (IU) | 75,000 | 75,000 |
| Vitamin D (IU) | 25,000 | 30,000 |
| Vitamin E (IU) | 500 | 1800 |
3.2. Heat Stress
4. Calving Management
5. Perinatal Care
6. Colostrum Management
6.1. Quantification
6.2. Quickness
6.3. Quantity
6.4. Quality
6.5. Cleanliness
6.6. Feeding Method
7. Preweaning Nutrition
7.1. Transition Milk
7.2. Plane of Nutrition
7.3. Milk Sources
7.4. Calf Starter
7.5. Water
7.6. Hygiene Practices
8. Housing and Environment
8.1. Group Size and Stocking Density
8.2. Bedding
8.3. Air Quality and Ventilation
8.4. Drainage
9. Vaccination
10. Calf Health Records Analysis and Feedback
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karszes, J. Dairy Replacement Programs: Costs & Analysis 3rd Quarter 2012; Cornell University: Ithaca, NY, USA, 2014. [Google Scholar]
- Karszes, J. Dairy Replacement Programs: Costs and Analysis Western New York, 1993; Cornell University: Ithaca, NY, USA, 1994; p. 12. [Google Scholar]
- Overton, M.W.; Dhuyvetter, K.C. Symposium review: An abundance of replacement heifers: What is the economic impact of raising more than are needed? J. Dairy Sci. 2020, 103, 3828–3837. [Google Scholar] [CrossRef]
- Soberon, F.; Raffrenato, E.; Everett, R.W.; Van Amburgh, M.E. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012, 95, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Cullens, F.; Brester, J.L. Effect of preweaning disease on the reproductive performance and first-lactation milk production of heifers in a large dairy herd. J. Dairy Sci. 2021, 104, 7008–7017. [Google Scholar] [CrossRef]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Kopral, C.A.; Adams, A.E.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part, V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J. Dairy Sci. 2018, 101, 9229–9244. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.C.; Hurley, D.J.; Reber, A.J. Neonatal immune development in the calf and its impact on vaccine response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 535–556. [Google Scholar] [CrossRef]
- Higgins, D.A.; Stack, M.J.; Richardson, C. Lymphocyte markers in the bovine foetus. Dev. Comp. Immunol. 1983, 7, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Moriel, P.; Piccolo, M.B.; Artioli, L.F.; Marques, R.S.; Poore, M.H.; Cooke, R.F. Short-term energy restriction during late gestation of beef cows decreases postweaning calf humoral immune response to vaccination. J. Anim. Sci. 2016, 94, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.M.; Martin, J.L.; Adams, D.C.; Funston, R.N. Winter grazing system and supplementation during late gestation influence performance of beef cows and steer progeny. J. Anim. Sci. 2009, 87, 1147–1155. [Google Scholar] [CrossRef]
- Stalker, L.A.; Adams, D.C.; Klopfenstein, T.J.; Feuz, D.M.; Funston, R.N. Effects of pre- and postpartum nutrition on reproduction in spring calving cows and calf feedlot performance. J. Anim. Sci. 2006, 84, 2582–2589. [Google Scholar] [CrossRef]
- Johanson, J.M.; Berger, P.J. Birth weight as a predictor of calving ease and perinatal mortality in Holstein cattle. J. Anim. Sci. 2003, 86, 3745–3755. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.C.; Zhang, Z.H.; Zhang, C.Z.; Su, H.W.; Li, S.L. Effect of prepartum maternal energy density on the growth performance, immunity, and antioxidation capability of neonatal calves. J. Anim. Sci. 2012, 95, 4510–4518. [Google Scholar] [CrossRef] [PubMed]
- Molano, R. Your Mature Cows: Your Heifers’ Best Unit of Measure. Available online: https://lactanet.ca/en/mature-cows-heifers-unit-measure/ (accessed on 5 October 2024).
- Lauber, M.R.; Fricke, P.M. The association between insemination eligibility and reproductive performance of nulliparous heifers on subsequent body weight and milk production of primiparous Holstein cows. JDS Commun. 2023, 4, 428–432. [Google Scholar] [CrossRef]
- Heinrichs, A.J.; Erb, H.N.; Rogers, G.W.; Cooper, J.B.; Jones, C.M. Variability in Holstein heifer heart-girth measurements and comparison of prediction equations for live weight. Prev. Vet. Med. 2007, 78, 333–338. [Google Scholar] [CrossRef]
- Heinrichs, A.J. The Penn State Particle Separator. Extension publication DSE 2013-186; Pennsylvania State University: College Park, PA, USA, 2013. [Google Scholar]
- Maulfair, D.D.; Heinrichs, A.J. Effects of varying forage particle size and fermentable carbohydrates on feed sorting, ruminal fermentation, and milk and component yields of dairy cows. J. Dairy Sci. 2013, 96, 3085–3097. [Google Scholar] [CrossRef]
- Leonardi, C.; Armentano, L.E. Effect of quantity, quality, and length of alfalfa hay on selective consumption by dairy cows. J. Dairy Sci. 2003, 86, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Devries, T.J.; Holtshausen, L.; Oba, M.; Beauchemin, K.A. Effect of parity and stage of lactation on feed sorting behavior of lactating dairy cows. J. Dairy Sci. 2011, 94, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Havekes, C.D.; Duffield, T.F.; Carpenter, A.J.; DeVries, T.J. Moisture content of high-straw dry cow diets affects intake, health, and performance of transition dairy cows. J. Dairy Sci. 2020, 103, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Havekes, C.D.; Duffield, T.F.; Carpenter, A.J.; DeVries, T.J. Effects of wheat straw chop length in high-straw dry cow diets on intake, health, and performance of dairy cows across the transition period. J. Dairy Sci. 2020, 103, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.R.; van Amburgh, M.E.; Chase, L.E. Nutrient recommendations for dry and lactating Holstein cows. In Proceedings of the Advanced Dairy Nutrition and Management Shortcourse, Cornell University, Ithaca, NY, USA, 3–6 June 2024. [Google Scholar]
- Dahl, G.E.; Tao, S.; Monteiro, A.P.A. Effects of late-gestation heat stress on immunity and performance of calves. J. Dairy Sci. 2016, 99, 3193–3198. [Google Scholar] [CrossRef]
- Tao, S.; Monteiro, A.P.; Thompson, I.M.; Hayen, M.J.; Dahl, G.E. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 2012, 95, 7128–7136. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.T.; Dahl, G.E. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 2016, 99, 8443–8450. [Google Scholar] [CrossRef] [PubMed]
- Toledo, I.M.; Fabris, T.F.; Tao, S.; Dahl, G.E. When do dry cows get heat stressed? Correlations of rectal temperature, respiration rate, and performance. JDS Commun. 2020, 1, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Bar, D.; Kaim, M.; Flamenbaum, I.; Hanochi, B.; Toaff-Rosenstein, R. Technical note: Accelerometer-based recording of heavy breathing in lactating and dry cows as an automated measure of heat load. J. Dairy Sci. 2019, 102, 3480–3486. [Google Scholar] [CrossRef]
- Becker, C.A.; Stone, A.E. Graduate Student Literature Review: Heat abatement strategies used to reduce negative effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 9667–9675. [Google Scholar] [CrossRef]
- Correa-Calderon, A.; Armstrong, D.; Ray, D.; DeNise, S.; Enns, M.; Howison, C. Thermoregulatory responses of Holstein and Brown Swiss heat-stressed dairy cows to two different cooling systems. Int. J. Biometeorol. 2004, 48, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Schutz, K.E.; Tucker, C.B. Dairy cows use and prefer feed bunks fitted with sprinklers. J. Dairy Sci. 2013, 96, 5035–5045. [Google Scholar] [CrossRef]
- Flamenbaum, I.; Wolfenson, D.; Mamen, M.; Berman, A. Cooling dairy cattle by a combination of sprinkling and forced ventilation and its implementation in the shelter system. J. Dairy Sci. 1986, 69, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Reuscher, K.J.; Cook, N.B.; da Silva, T.E.; Mondaca, M.R.; Lutcherhand, K.M.; Van Os, J.M.C. Effect of different air speeds at cow resting height in freestalls on heat stress responses and resting behavior in lactating cows in Wisconsin. J. Dairy Sci. 2023, 106, 9552–9567. [Google Scholar] [CrossRef]
- Mondaca, M.R. Ventilation Systems for Adult Dairy Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 139–156. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; von Keyserlingk, M.; Weary, D. Hot weather increases competition between dairy cows at the drinker. J. Dairy Sci. 2020, 103, 3447–3458. [Google Scholar] [CrossRef]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Mee, J.F. Newborn dairy calf management. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 1. [Google Scholar] [CrossRef]
- Cuttance, E.; Laven, R. Estimation of perinatal mortality in dairy calves: A review. Vet. J. 2019, 252, 105356. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.E.; Garry, F.B.; Tomlinson, S.M.; Garber, L.P. Impacts of dystocia on health and survival of dairy calves. J. Dairy Sci. 2007, 90, 1751–1760. [Google Scholar] [CrossRef]
- Carrier, J.; Godden, S.; Fetrow, J.; Stewart, S.; Rapnicki, P. Predictors of stillbirth for cows moved to calving pens when calving is imminent. In Proceedings of the Thirty-Ninth Annual Conference, American Association of Bovine Practitioners, Saint Paul, MN, USA, 21–23 September 2006; pp. 158–159. [Google Scholar]
- NASEM. Nutrient Requirements of Dairy Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2021; p. 502. [Google Scholar]
- Chassagne, M.; Barnouin, J.; Chacornac, J.P. Risk factors for stillbirth in Holstein heifers under field conditions in France: A prospective survey. Theriogenology 1999, 51, 1477–1488. [Google Scholar] [CrossRef]
- Piwczynski, D.; Nogalski, Z.; Sitkowska, B. Statistical modeling of calving ease and stillbirths in dairy cattle using the classification tree technique. Livest. Sci. 2013, 154, 19–27. [Google Scholar] [CrossRef]
- Berry, D.P.; Ring, S.C. Short communication: Animal-level factors associated with whether a dairy female is mated to a dairy or beef bull. J. Dairy Sci. 2020, 103, 8343–8349. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, K.L.; Jensen, M.B.; Heegaard, P.M.; von Keyserlingk, M.A. Effect of moving dairy cows at different stages of labor on behavior during parturition. J. Dairy Sci. 2013, 96, 1638–1646. [Google Scholar] [CrossRef]
- Carrier, J. Behavioural and Metabolic Observations of Dairy Cows in the Transition Period; University of Minnesota: Minneapolis, MN, USA, 2007. [Google Scholar]
- Schuenemann, G.M.; Nieto, I.; Bas, S.; Galvao, K.N.; Workman, J. Assessment of calving progress and reference times for obstetric intervention during dystocia in Holstein dairy cows. J. Dairy Sci. 2011, 94, 5494–5501. [Google Scholar] [CrossRef] [PubMed]
- USDA. Dairy 2007, Heifer Calf Health and Management Practices on U.S. Dairy Operations, 2007; Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services, National Animal Health Monitoring System: Fort Collins, CO, USA, 2010. [Google Scholar]
- Mills, K.E.; Koralesky, K.E.; Weary, D.M.; von Keyserlingk, M.A.G. Dairy farmer advising in relation to the development of standard operating procedures. J. Dairy Sci. 2020, 103, 11524–11534. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.F. Managing the Calf at Calving Time. Am. Assoc. Bov. Pract. Conf. Proc. 2008, 46–53. [Google Scholar] [CrossRef]
- Bellows, R.; Lammoglia, M. Effects of severity of dystocia on cold tolerance and serum concentrations of glucose and cortisol in neonatal beef calves. Theriogenology 2000, 53, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Vermorel, M.; Dardillat, C.; Vernet, J.; Saido, D.C.; Demigne, C. Energy metabolism and thermoregulation in the newborn calf. Ann. Rech. Vét. 1983, 4, 382–389. [Google Scholar]
- Davis, C.L.; Drackley, J.K. Colostrum. In The Development, Nutrition, and Management of the Young Calf, 1st ed.; Iowa State University: Ames, IA, USA, 1998. [Google Scholar]
- Copeland, A.T.; Kreuder, A.J.; Dewell, G.; Dewell, R.; Wiley, C.; Yuan, L.; Mochel, J.P.; Smith, J.S. Randomized comparison between a forced air system and warm water bath for resuscitation of neonatal hypothermic calves with or without oral ad-ministration of caffeine. J. Vet. Int. Med. 2024, 38, 1941–1950. [Google Scholar] [CrossRef]
- Murray, C.F.; Haley, D.B.; Duffield, T.F.; Pearl, D.L.; Deelen, S.M.; Leslie, K.E. A Field study to evaluate the effects of meloxicam NSAID therapy and calving assistance on newborn calf vigor, improvement of health and growth in pre-weaned Holstein calves. Bov. Pract. 2015, 49, 1–12. [Google Scholar] [CrossRef]
- Renaud, D.; Duffield, T.; LeBlanc, S.; Ferguson, S.; Haley, D.; Kelton, D. Risk factors associated with mortality at a milk-fed veal calf facility: A prospective cohort study. . J. Dairy Sci. 2018, 101, 2659–2668. [Google Scholar] [CrossRef]
- Camp, M.; Winder, C.; Gomez, D.; Duffield, T.; Savor, N.; Renaud, D. Evaluating the effectiveness of a single application of 7% iodine tincture umbilical dip as a prevention of infection of the external umbilical structures in dairy calves. J. Dairy Sci. 2022, 105, 6083–6093. [Google Scholar] [CrossRef]
- Faradonbeh, Y.K.; Faradonbeh, M.K. Evaluate the risk factors umbilical cord bacterial infection in calves in Shahrekord city. J. Entomol. Zool. Stud. 2016, 4, 162–166. [Google Scholar]
- Perrot, F.; Joulié, A.; Herry, V.; Masset, N.; Lemaire, G.; Barral, A.; Raboisson, D.; Roy, C.; Herman, N. Failure of Passive Immunity Transfer Is Not a Risk Factor for Omphalitis in Beef Calves. Vet. Sci. 2023, 10, 544. [Google Scholar] [CrossRef]
- Wooding, F.B. Current topic: The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 1992, 13, 101–113. [Google Scholar] [CrossRef]
- Renaud, D.L.; Duffield, T.F.; LeBlanc, S.J.; Kelton, D.F. Short communication: Validation of methods for practically evaluating failed passive transfer of immunity in calves arriving at a veal facility. J. Dairy Sci. 2018, 101, 9516–9520. [Google Scholar] [CrossRef]
- Wilm, J.; Costa, J.H.C.; Neave, H.W.; Weary, D.M.; von Keyserlingk, M.A.G. Technical note: Serum total protein and immunoglobulin G concentrations in neonatal dairy calves over the first 10 days of age. J. Dairy Sci. 2018, 101, 6430–6436. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.J.; Steele, M.A.; Nagorske, M.; Sargent, R.; Renaud, D.L. Hot topic: Accuracy of refractometry as an indirect method to measure failed transfer of passive immunity in dairy calves fed colostrum replacer and maternal colostrum. J. Dairy Sci. 2021, 104, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.C. Failure of passive transfer of colostral immunoglobulins and neonatal disease in calves: A review. In Proceedings of the 4th International Symposium on Neonatal Diarrhea, Veterinary Infectious Disease Organization (VIDa), Saskatoon, SK, Canada, 1–5 October 1983. [Google Scholar]
- Buczinski, S.; Gicquel, E.; Fecteau, G.; Takwoingi, Y.; Chigerwe, M.; Vandeweerd, J.M. Systematic Review and Meta-Analysis of Diagnostic Accuracy of Serum Refractometry and Brix Refractometry for the Diagnosis of Inadequate Transfer of Passive Immunity in Calves. J. Vet. Intern. Med. 2018, 32, 474–483. [Google Scholar] [CrossRef]
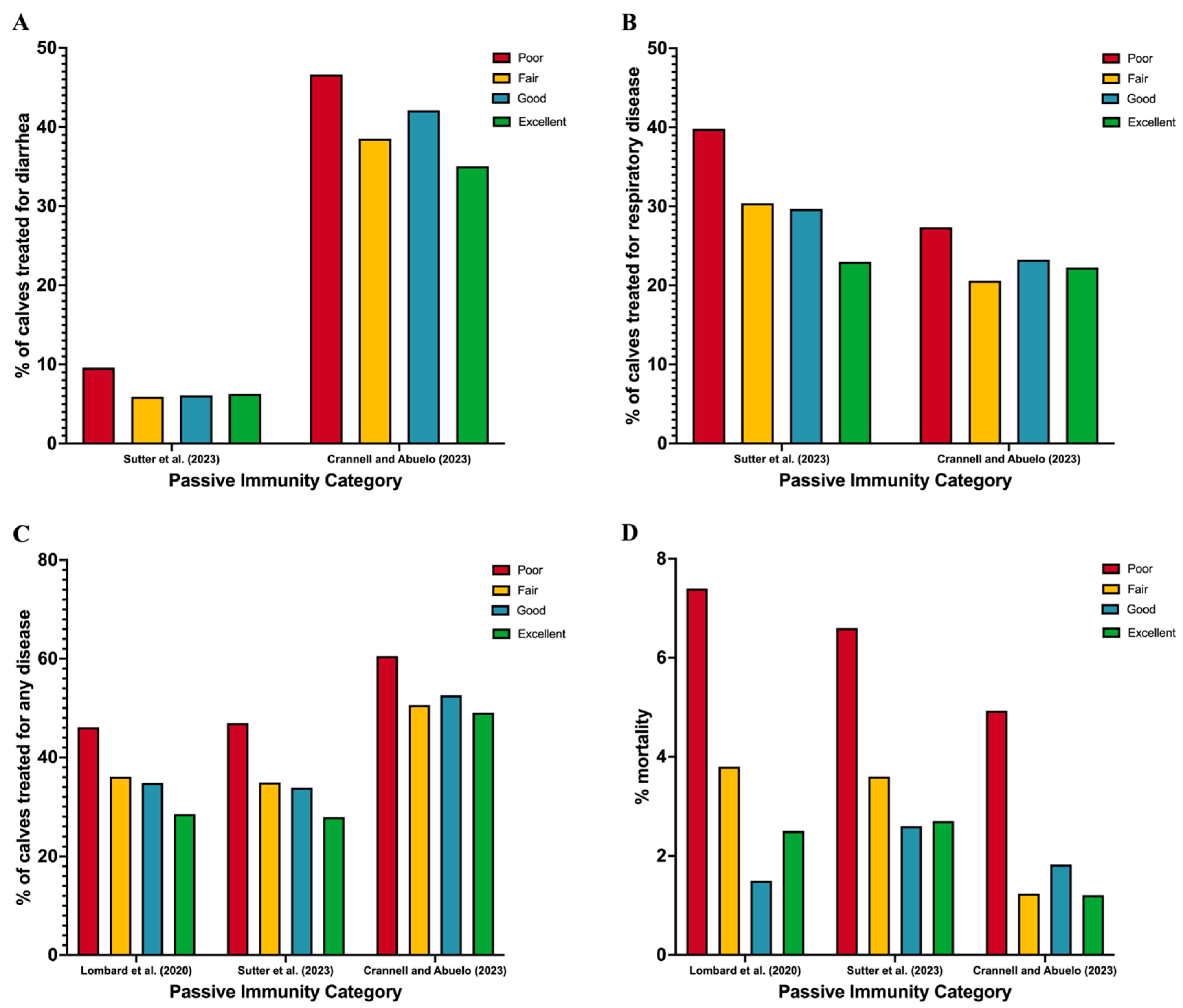
- Lombard, J.; Urie, N.; Garry, F.; Godden, S.; Quigley, J.; Earleywine, T.; McGuirk, S.; Moore, D.; Branan, M.; Chamorro, M.; et al. Consensus recommendations on calf- and herd-level passive immunity in dairy calves in the United States. J. Dairy Sci. 2020, 103, 7611–7624. [Google Scholar] [CrossRef] [PubMed]
- Sutter, F.; Venjakob, P.L.; Heuwieser, W.; Borchardt, S. Association between transfer of passive immunity, health, and performance of female dairy calves from birth to weaning. J. Dairy Sci. 2023, 106, 7043–7055. [Google Scholar] [CrossRef] [PubMed]
- Crannell, P.; Abuelo, A. Comparison of calf morbidity, mortality, and future performance across categories of passive immunity: A retrospective cohort study in a dairy herd. J. Dairy Sci. 2023, 106, 2729–2738. [Google Scholar] [CrossRef]
- Fischer, A.J.; Song, Y.; He, Z.; Haines, D.M.; Guan, L.L.; Steele, M.A. Effect of delaying colostrum feeding on passive transfer and intestinal bacterial colonization in neonatal male Holstein calves. J. Dairy Sci. 2018, 101, 3099–3109. [Google Scholar] [CrossRef]
- Morin, D.E.; Nelson, S.V.; Reid, E.D.; Nagy, D.W.; Dahl, G.E.; Constable, P.D. Effect of colostral volume, interval between calving and first milking, and photoperiod on colostral IgG concentrations in dairy cows. J. Am. Vet. Med. Assoc. 2010, 237, 420–428. [Google Scholar] [CrossRef]
- Conneely, M.; Berry, D.P.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Effect of feeding colostrum at different volumes and subsequent number of transition milk feeds on the serum immunoglobulin G concentration and health status of dairy calves. J. Dairy Sci. 2014, 97, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.P.; Dubuc, J.; Freycon, P.; Buczinski, S. A calf-level study on colostrum management practices associated with adequate transfer of passive immunity in Quebec dairy herds. J. Dairy Sci. 2021, 104, 4904–4913. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.S.; Pletts, S.; Pyo, J.; Haines, D.; Guan, L.L.; Steele, M. Feeding colostrum or a 1:1 colostrum:whole milk mixture for 3 days after birth increases serum immunoglobulin G and apparent immunoglobulin G persistency in Holstein bulls. J. Dairy Sci. 2020, 103, 11833–11843. [Google Scholar] [CrossRef] [PubMed]
- Renaud, D.L.; Waalderbos, K.M.; Beavers, L.; Duffield, T.F.; Leslie, K.E.; Windeyer, M.C. Risk factors associated with failed transfer of passive immunity in male and female dairy calves: A 2008 retrospective cross-sectional study. J. Dairy Sci. 2020, 103, 3521–3528. [Google Scholar] [CrossRef]
- Abuelo, A.; Cullens, F.; Hanes, A.; Brester, J.L. Impact of 2 Versus 1 Colostrum Meals on Failure of Transfer of Passive Immunity, Pre-Weaning Morbidity and Mortality, and Performance of Dairy Calves in a Large Dairy Herd. Animals 2021, 11, 12. [Google Scholar] [CrossRef]
- Lopez, A.J.; Yohe, T.T.; Echeverry-Munera, J.; Nagorske, M.; Renaud, D.L.; Steele, M.A. Effects of a low- or high-frequency colostrum feeding protocol on immunoglobulin G absorption in newborn calves. J. Dairy Sci. 2022, 105, 6318–6326. [Google Scholar] [CrossRef]
- Blum, J.W.; Hammon, H. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Livest. Prod. Sci. 2000, 66, 151–159. [Google Scholar] [CrossRef]
- McGuirk, S.M.; Collins, M. Managing the production, storage, and delivery of colostrum. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 593–603. [Google Scholar] [CrossRef]
- Shivley, C.B.; Lombard, J.E.; Urie, N.J.; Haines, D.M.; Sargent, R.; Kopral, C.A.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part II. Factors associated with colostrum quality and passive transfer status of dairy heifer calves. J. Dairy Sci. 2018, 101, 9185–9198. [Google Scholar] [CrossRef]
- Bielmann, V.; Gillan, J.; Perkins, N.R.; Skidmore, A.L.; Godden, S.; Leslie, K.E. An evaluation of Brix refractometry instruments for measurement of colostrum quality in dairy cattle. J. Dairy Sci. 2010, 93, 3713–3721. [Google Scholar] [CrossRef]
- Bartier, A.L.; Windeyer, M.C.; Doepel, L. Evaluation of on-farm tools for colostrum quality measurement. J. Dairy Sci. 2015, 98, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Leal Yepes, F.A.; Overton, T.R.; Lock, A.L.; Lamb, S.V.; Wakshlag, J.J.; Nydam, D.V. Effect of dry period dietary energy level in dairy cattle on volume, concentrations of immunoglobulin G, insulin, and fatty acid composition of colostrum. J. Dairy Sci. 2016, 99, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, T.A.; Overton, T.R.; Mann, S. Epidemiology of bovine colostrum production in New York Holstein herds: Prepartum nutrition and metabolic indicators. J. Dairy Sci. 2023, 106, 4896–4905. [Google Scholar] [CrossRef]
- Lopez, A.J.; Echeverry-Munera, J.; McCarthy, H.; Welboren, A.C.; Pineda, A.; Nagorske, M.; Renaud, D.L.; Steele, M.A. Effects of enriching IgG concentration in low- and medium-quality colostrum with colostrum replacer on IgG absorption in newborn Holstein calves. J. Dairy Sci. 2023, 106, 3680–3691. [Google Scholar] [CrossRef] [PubMed]
- Gelsinger, S.L.; Jones, C.M.; Heinrichs, A.J. Effect of colostrum heat treatment and bacterial population on immunoglobulin G absorption and health of neonatal calves. J. Dairy Sci. 2015, 98, 4640–4645. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Berry, D.P.; Murphy, J.P.; Lorenz, I.; Kennedy, E. The effect of colostrum storage conditions on dairy heifer calf serum immunoglobulin G concentration and preweaning health and growth rate. J. Dairy Sci. 2017, 100, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Godden, S.; Bey, R.; Rapnicki, P.; Fetrow, J.; Farnsworth, R.; Scanlon, M.; Arnold, Y.; Clow, L.; Mueller, K.; et al. Preventing bacterial contamination and proliferation during the harvest, storage, and feeding of fresh bovine colostrum. J. Dairy Sci. 2005, 88, 2571–2578. [Google Scholar] [CrossRef]
- McMartin, S.; Godden, S.; Metzger, L.; Feirtag, J.; Bey, R.; Stabel, J.; Goyal, S.; Fetrow, J.; Wells, S.; Chester-Jones, H. Heat treatment of bovine colostrum. I: Effects of temperature on viscosity and immunoglobulin G level. J. Dairy Sci. 2006, 89, 2110–2118. [Google Scholar] [CrossRef]
- Desjardins-Morrissette, M.; van Niekerk, J.K.; Haines, D.; Sugino, T.; Oba, M.; Steele, M.A. The effect of tube versus bottle feeding colostrum on immunoglobulin G absorption, abomasal emptying, and plasma hormone concentrations in newborn calves. J. Dairy Sci. 2018, 101, 4168–4179. [Google Scholar] [CrossRef]
- Besser, T.E.; Gay, C.C.; Pritchett, L. Comparison of three methods of feeding colostrum to dairy calves. J. Am. Vet. Med. Assoc. 1991, 198, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Godden, S. Colostrum management for dairy calves. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.; Pletts, S.; Romao, J.; Inabu, Y.; He, Z.; Haines, D.; Sugino, T.; Guan, L.; Steele, M. The effects of extended colostrum feeding on gastrointestinal tract growth of the neonatal dairy calf. J. Dairy Sci. 2018, 96, 170–171. [Google Scholar] [CrossRef]
- Uyama, T.; Renaud, D.L.; Morrison, E.I.; McClure, J.T.; LeBlanc, S.J.; Winder, C.B.; de Jong, E.; McCubbin, K.D.; Barkema, H.W.; Dufour, S.; et al. Associations of calf management practices with antimicrobial use in Canadian dairy calves. J. Dairy Sci. 2022, 105, 9084–9097. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, B.; Cullens, F.; VandeHaar, M.J.; Nielsen, M.W. Short communication: Effects of transition milk and milk replacer supplemented with colostrum replacer on growth and health of dairy calves. J. Dairy Sci. 2020, 103, 12104–12108. [Google Scholar] [CrossRef] [PubMed]
- Kargar, S.; Bahadori-Moghaddam, M.; Ghoreishi, S.M.; Akhlaghi, A.; Kanani, M.; Pazoki, A.; Ghaffari, M.H. Extended transition milk feeding for 3 weeks improves growth performance and reduces the susceptibility to diarrhea in newborn female Holstein calves. Animal 2021, 15, 100151. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.; Hare, K.; Pletts, S.; Inabu, Y.; Haines, D.; Sugino, T.; Guan, L.L.; Steele, M. Feeding colostrum or a 1:1 colostrum:milk mixture for 3 days postnatal increases small intestinal development and minimally influences plasma glucagon-like peptide-2 and serum insulin-like growth factor-1 concentrations in Holstein bull calves. J. Dairy Sci. 2020, 103, 4236–4251. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Cernicchiaro, N.; Haines, D.M. Evaluation of the effects of colostrum replacer supplementation of the milk replacer ration on the occurrence of disease, antibiotic therapy, and performance of pre-weaned dairy calves. J. Dairy Sci. 2017, 100, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Berge, A.C.; Moore, D.A.; Besser, T.E.; Sischo, W.M. Targeting therapy to minimize antimicrobial use in preweaned calves: Effects on health, growth, and treatment costs. J. Dairy Sci. 2009, 92, 4707–4714. [Google Scholar] [CrossRef]
- Ballou, M.A. Immune responses of Holstein and Jersey calves during the preweaning and immediate postweaned periods when fed varying planes of milk replacer. J. Dairy Sci. 2012, 95, 7319–7330. [Google Scholar] [CrossRef] [PubMed]
- Sharon, K.P.; Liang, Y.; Sanchez, N.C.B.; Carroll, J.A.; Broadway, P.R.; Davis, E.M.; Ballou, M.A. Pre-weaning plane of nutrition and Mannheimia haemolytica dose influence inflammatory responses to a bovine herpesvirus-1 and Mannheimia haemolytica challenge in post-weaning Holstein calves. J. Dairy Sci. 2019, 102, 9082–9096. [Google Scholar] [CrossRef]
- Dubrovsky, S.A.; Van Eenennaam, A.L.; Karle, B.M.; Rossitto, P.V.; Lehenbauer, T.W.; Aly, S.S. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7306–7319. [Google Scholar] [CrossRef]
- Ollivett, T.L.; Nydam, D.V.; Linden, T.C.; Bowman, D.D.; Van Amburgh, M.E. Effect of nutritional plane on health and performance in dairy calves after experimental infection with Cryptosporidium parvum. J. Am. Vet. Med. Assoc. 2012, 241, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, K.; Costa, J.H.C.; Neave, H.W.; von Keyserlingk, M.A.G.; Weary, D.M. The effect of milk allowance on behavior and weight gains in dairy calves. J. Dairy Sci. 2017, 100, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Rosadiuk, J.P.; Bruinje, T.C.; Moslemipur, F.; Fischer-Tlustos, A.J.; Renaud, D.L.; Ambrose, D.J.; Steele, M.A. Differing planes of pre- and postweaning phase nutrition in Holstein heifers: I. Effects on feed intake, growth efficiency, and metabolic and development indicators. J. Dairy Sci. 2021, 104, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Conneely, M.; Berry, D.P.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Effects of milk feeding volume and frequency on body weight and health of dairy heifer calves. Livest. Sci. 2014, 161, 90–94. [Google Scholar] [CrossRef]
- Chapman, C.E.; Erickson, P.S.; Quigley, J.D.; Hill, T.M.; Bateman, H.G., 2nd; Suarez-Mena, F.X.; Schlotterbeck, R.L. Effect of milk replacer program on calf performance and digestion of nutrients with age of the dairy calf. J. Dairy Sci. 2016, 99, 2740–2747. [Google Scholar] [CrossRef]
- Medrano-Galarza, C.; LeBlanc, S.J.; Jones-Bitton, A.; DeVries, T.J.; Rushen, J.; Marie de Passille, A.; Endres, M.I.; Haley, D.B. Associations between management practices and within-pen prevalence of calf diarrhea and respiratory disease on dairy farms using automated milk feeders. J. Dairy Sci. 2018, 101, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.A.; Machado, F.S.; Campos, M.M.; Lopes, D.R.G.; Costa, S.F.; Mantovani, H.C.; Lopes, F.C.F.; Marcondes, M.I.; Pereira, L.G.R.; Tomich, T.R.; et al. The effects of increasing amounts of milk replacer powder added to whole milk on passage rate, nutrient digestibility, ruminal development, and body composition in dairy calves. J. Dairy Sci. 2016, 99, 8746–8758. [Google Scholar] [CrossRef]
- Floren, H.K.; Sischo, W.M.; Crudo, C.; Moore, D.A. Technical note: Use of a digital and an optical Brix refractometer to estimate total solids in milk replacer solutions for calves. J. Dairy Sci. 2016, 99, 7517–7522. [Google Scholar] [CrossRef] [PubMed]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Kopral, C.A.; Adams, A.E.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part, I. Descriptive characteristics of preweaned heifer raising practices. J. Dairy Sci. 2018, 101, 9168–9184. [Google Scholar] [CrossRef]
- Wilson, D.J.; Pempek, J.A.; Cheng, T.Y.; Habing, G.; Proudfoot, K.L.; Winder, C.B.; Renaud, D.L. A survey of male and female dairy calf care practices and opportunities for change. J. Dairy Sci. 2023, 106, 703–717. [Google Scholar] [CrossRef]
- Amado, L.; Berends, H.; Leal, L.N.; Wilms, J.; Van Laar, H.; Gerrits, W.J.J.; Martín-Tereso, J. Effect of energy source in calf milk replacer on performance, digestibility, and gut permeability in rearing calves. J. Dairy Sci. 2019, 102, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Wilms, J.N.; Ghaffari, M.H.; Steele, M.A.; Sauerwein, H.; Martin-Tereso, J.; Leal, L.N. Macronutrient profile in milk replacer or a whole milk powder modulates growth performance, feeding behavior, and blood metabolites in ad libitum-fed calves. J. Dairy Sci. 2022, 105, 6670–6692. [Google Scholar] [CrossRef] [PubMed]
- Lodge, G.A.; Lister, E.E. Effects of Increasing Energy Value of a Whole Milk Diet for Calves.1. Nutrient Digestibility and Nitrogen Retention. Can. J. Anim. Sci. 1973, 53, 307–316. [Google Scholar] [CrossRef]
- Diaz, M.C.; Van Amburgh, M.E.; Smith, J.M.; Kelsey, J.M.; Hutten, E.L. Composition of growth of Holstein calves fed milk replacer from birth to 105-kilogram body weight. J. Dairy Sci. 2001, 84, 830–842. [Google Scholar] [CrossRef]
- Ran, L.; Wu, X.; Shen, X.; Zhang, K.; Ren, F.; Huang, K. Effects of selenium form on blood and milk selenium concentrations, milk component and milk fatty acid composition in dairy cows. J. Sci. Food Agric. 2010, 90, 2214–2219. [Google Scholar] [CrossRef]
- Wilms, J.N.; van der Nat, V.; Ghaffari, M.H.; Steele, M.A.; Sauerwein, H.; Martin-Tereso, J.; Leal, L.N. Fat composition of milk replacer influences growth performance, feeding behavior, and plasma fatty acid profile in ad libitum-fed calves. J. Dairy Sci. 2024, 107, 2797–2817. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Toullec, R.; Lalles, J.P. Intestinal digestion of dietary and endogenous proteins along the small intestine of calves fed soybean or potato. J. Dairy Sci. 2001, 79, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, T.; Hultquist, K.; Wu, J.; Casper, D.P. Feeding an amino acid formulated milk replacer for Holstein calves. J. Dairy Sci. 2020, 98, 11. [Google Scholar] [CrossRef] [PubMed]
- Terosky, T.L.; Heinrichs, A.J.; Wilson, L.L. A comparison of milk protein sources in diets of calves up to eight weeks of age. J. Dairy Sci. 1997, 80, 2977–2983. [Google Scholar] [CrossRef]
- Montagne, L.; Crevieu-Gabriel, I.; Toullec, R.; Lalles, J.P. Influence of dietary protein level and source on the course of protein digestion along the small intestine of the veal calf. J. Dairy Sci. 2003, 86, 934–943. [Google Scholar] [CrossRef]
- Berends, H.; van Laar, H.; Leal, L.N.; Gerrits, W.J.J.; Martin-Tereso, J. Effects of exchanging lactose for fat in milk replacer on ad libitum feed intake and growth performance in dairy calves. J. Dairy Sci. 2020, 103, 4275–4287. [Google Scholar] [CrossRef] [PubMed]
- Penati, M.; Sala, G.; Biscarini, F.; Boccardo, A.; Bronzo, V.; Castiglioni, B.; Cremonesi, P.; Moroni, P.; Pravettoni, D.; Addis, M.F. Feeding Pre-weaned Calves With Waste Milk Containing Antibiotic Residues Is Related to a Higher Incidence of Diarrhea and Alterations in the Fecal Microbiota. Front. Vet. Sci. 2021, 8, 650150. [Google Scholar] [CrossRef] [PubMed]
- Aust, V.; Knappstein, K.; Kunz, H.J.; Kaspar, H.; Wallmann, J.; Kaske, M. Feeding untreated and pasteurized waste milk and bulk milk to calves: Effects on calf performance, health status and antibiotic resistance of faecal bacteria. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Yohe, T.T.; Schramm, H.; Parsons, C.L.M.; Tucker, H.L.M.; Enger, B.D.; Hardy, N.R.; Daniels, K.M. Form of calf diet and the rumen. I: Impact on growth and development. J. Dairy Sci. 2019, 102, 8486–8501. [Google Scholar] [CrossRef]
- Hill, T.M.; Quigley, J.D.; Bateman, H.G., 2nd; Suarez-Mena, F.X.; Dennis, T.S.; Schlotterbeck, R.L. Effect of milk replacer program on calf performance and digestion of nutrients in dairy calves to 4 months of age. J. Dairy Sci. 2016, 99, 8103–8110. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.D.; Hill, T.M.; Dennis, T.S.; Suarez-Mena, F.X.; Schlotterbeck, R.L. Effects of feeding milk replacer at 2 rates with pelleted, low-starch or texturized, high-starch starters on calf performance and digestion. J. Dairy Sci. 2018, 101, 5937–5948. [Google Scholar] [CrossRef]
- Castells, L.; Bach, A.; Araujo, G.; Montoro, C.; Terre, M. Effect of different forage sources on performance and feeding behavior of Holstein calves. J. Dairy Sci. 2012, 95, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.K.; Jones, C.M.; Heinrichs, A.J. Effect of converting weaned dairy calves from a component-fed diet to a total mixed ration on growth and nutrient digestibility. J. Dairy Sci. 2020, 103, 6190–6199. [Google Scholar] [CrossRef]
- Quigley, J.D. Invited review: An evaluation of EFSA opinion on calf welfare from a nutritional and management perspective. J. Dairy Sci. 2024, 107, 7483–7503. [Google Scholar] [CrossRef]
- Welk, A.; Neave, H.W.; Spitzer, H.B.; von Keyserlingk, M.A.G.; Weary, D.M. Effects of intake-based weaning and forage type on feeding behavior and growth of dairy calves fed by automated feeders. J. Dairy Sci. 2022, 105, 9119–9136. [Google Scholar] [CrossRef] [PubMed]
- Kertz, A.F.; Reutzel, L.F.; Mahoney, J.H. Ad libitum water intake by neonatal calves and its relationship to calf starter intake, weight gain, feces score, and season. J. Dairy Sci. 1984, 67, 2964–2969. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, H.; Kramer, A.J.; Appuhamy, J. Drinking water intake of newborn dairy calves and its effects on feed intake, growth performance, health status, and nutrient digestibility. J. Dairy Sci. 2019, 102, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.Y.; LaPierre, P.A.; Brost, K.N.; Drackley, J.K. Intake and growth in transported Holstein calves classified as diarrheic or healthy within the first 21 days after arrival in a retrospective observational study. J. Dairy Sci. 2019, 102, 10997–11008. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, A.; Tuomisto, L.; Kauppinen, R. Effect of drinking water temperature on water intake and performance of dairy calves. J. Dairy Sci. 2011, 94, 2475–2480. [Google Scholar] [CrossRef]
- Quigley, J.D. Predicting Water Intake in Young Calves. Available online: https://www.calfnotes.com/pdffiles/CN068.pdf (accessed on 19 October 2024).
- Andrews, S.C.; Robinson, A.K.; Rodriguez-Quinones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Wilms, J.; Berends, H.; Martin-Tereso, J. Hypertonic milk replacers increase gastrointestinal permeability in healthy dairy calves. J. Dairy Sci. 2019, 102, 1237–1246. [Google Scholar] [CrossRef]
- Jorgensen, M.W.; Adams-Progar, A.; de Passille, A.M.; Rushen, J.; Godden, S.M.; Chester-Jones, H.; Endres, M.I. Factors associated with dairy calf health in automated feeding systems in the Upper Midwest United States. J. Dairy Sci. 2017, 100, 5675–5686. [Google Scholar] [CrossRef] [PubMed]
- Renaud, D.L.; Kelton, D.F.; LeBlanc, S.J.; Haley, D.B.; Jalbert, A.B.; Duffield, T.F. Validation of commercial luminometry swabs for total bacteria and coliform counts in colostrum-feeding equipment. J. Dairy Sci. 2017, 100, 9459–9465. [Google Scholar] [CrossRef]
- Nordlund, K.V.; Halbach, C.E. Calf Barn Design to Optimize Health and Ease of Management. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.; Liberg, P. The effect of group size on health and growth rate of Swedish dairy calves housed in pens with automatic milk-feeders. Prev. Vet. Med. 2006, 73, 43–53. [Google Scholar] [CrossRef]
- Svensson, C.; Lundborg, K.; Emanuelson, U.; Olsson, S.O. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev. Vet. Med. 2003, 58, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Bolt, S.L.; Boyland, N.K.; Mlynski, D.T.; James, R.; Croft, D.P. Pair Housing of Dairy Calves and Age at Pairing: Effects on Weaning Stress, Health, Production and Social Networks. PLoS ONE 2017, 12, e0166926. [Google Scholar] [CrossRef] [PubMed]
- Buckova, K.; Sarova, R.; Moravcsikova, A.; Spinka, M. The effect of pair housing on dairy calf health, performance, and behavior. J. Dairy Sci. 2021, 104, 10282–10290. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.H.; Meagher, R.K.; von Keyserlingk, M.A.; Weary, D.M. Early pair housing increases solid feed intake and weight gains in dairy calves. J. Dairy Sci. 2015, 98, 6381–6386. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.W.; Adams-Progar, A.; de Passille, A.M.; Rushen, J.; Salfer, J.A.; Endres, M.I. Mortality and health treatment rates of dairy calves in automated milk feeding systems in the Upper Midwest of the United States. J. Dairy Sci. 2017, 100, 9186–9193. [Google Scholar] [CrossRef]
- McGuirk, S.M. Disease management of dairy calves and heifers. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Renaud, D.L.; Kelton, D.F.; LeBlanc, S.J.; Haley, D.B.; Duffield, T.F. Calf management risk factors on dairy farms associated with male calf mortality on veal farms. J. Dairy Sci. 2018, 101, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Panivivat, R.; Kegley, E.B.; Pennington, J.A.; Kellogg, D.W.; Krumpelman, S.L. Growth performance and health of dairy calves bedded with different types of materials. J. Dairy Sci. 2004, 87, 3736–3745. [Google Scholar] [CrossRef]
- Lago, A.; McGuirk, S.M.; Bennett, T.B.; Cook, N.B.; Nordlund, K.V. Calf respiratory disease and pen microenvironments in naturally ventilated calf barns in winter. J. Dairy Sci. 2006, 89, 4014–4025. [Google Scholar] [CrossRef] [PubMed]
- van Leenen, K.; Jouret, J.; Demeyer, P.; Vermeir, P.; Leenknecht, D.; Van Driessche, L.; De Cremer, L.; Masmeijer, C.; Boyen, F.; Deprez, P.; et al. Particulate matter and airborne endotoxin concentration in calf barns and their association with lung consolidation, inflammation, and infection. J. Dairy Sci. 2021, 104, 5932–5947. [Google Scholar] [CrossRef] [PubMed]
- van Leenen, K.; Jouret, J.; Demeyer, P.; Van Driessche, L.; De Cremer, L.; Masmeijer, C.; Boyen, F.; Deprez, P.; Pardon, B. Associations of barn air quality parameters with ultrasonographic lung lesions, airway inflammation and infection in group-housed calves. Prev. Vet. Med. 2020, 181, 105056. [Google Scholar] [CrossRef] [PubMed]
- Halbach, C.; Robertson, J. Ensuring optimal ventilation of calf buildings. Practice 2021, 43, 571–578. [Google Scholar] [CrossRef]
- Wathes, C.M.; Jones, C.D.; Webster, A.J. Ventilation, air hygiene and animal health. Vet. Rec. 1983, 113, 554–559. [Google Scholar] [PubMed]
- Dado-Senn, B.; Ouellet, V.; Lantigua, V.; Van Os, J.; Laporta, J. Methods for detecting heat stress in hutch-housed dairy calves in a continental climate. J. Dairy Sci. 2023, 106, 1039–1050. [Google Scholar] [CrossRef]
- Dado-Senn, B.; Vega Acosta, L.; Torres Rivera, M.; Field, S.L.; Marrero, M.G.; Davidson, B.D.; Tao, S.; Fabris, T.F.; Ortiz-Colon, G.; Dahl, G.E.; et al. Pre- and postnatal heat stress abatement affects dairy calf thermoregulation and performance. J. Dairy Sci. 2020, 103, 4822–4837. [Google Scholar] [CrossRef]
- Hill, T.M.; Bateman, H.G., 2nd; Aldrich, J.M.; Schlotterbeck, R.L. Comparisons of housing, bedding, and cooling options for dairy calves. J. Dairy Sci. 2011, 94, 2138–2146. [Google Scholar] [CrossRef]
- Tizard, I. Veterinary Immunology, 9th ed.; Elsevier: St. Louis, MO, USA, 2013. [Google Scholar]
- Ollivett, T.; Leslie, K.; Duffield, T.; Nydam, D.; Hewson, J.; Caswell, J.; Dunn, P.; Kelton, D. Field trial to evaluate the effect of an intranasal respiratory vaccine protocol on calf health, ultrasonographic lung consolidation, and growth in Holstein dairy calves. J. Dairy Sci. 2018, 101, 8159–8168. [Google Scholar] [CrossRef] [PubMed]
- Jourquin, S.; Lowie, T.; Debruyne, F.; Chantillon, L.; Clinquart, J.; Pas, M.; Boone, R.; Hoflack, G.; Vertenten, G.; Sustronck, B.; et al. Effect of on-arrival bovine respiratory disease vaccination on ultrasound-confirmed pneumonia and production parameters in male dairy calves: A randomized clinical trial. J. Dairy Sci. 2023, 106, 9260–9275. [Google Scholar] [CrossRef] [PubMed]
- McGuirk, S.; Peek, S. Timely diagnosis of dairy calf respiratory disease using a standardized scoring system. Anim. Health Res. Rev. 2014, 15, 145–147. [Google Scholar] [CrossRef]
- Love, W.; Lehenbauer, T.; Kass, P.; Van Eenennaam, A.; Aly, S. Development of a novel clinical scoring systemfor on-farmdiagnosis of bovine respiratory disease in pre-weaned dairy calves. PeerJ 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.; Owen, F.; Albright, J.; Appleman, R.; Lamb, R.; Muller, L. Guidelines toward more uniformity in measuring and reporting calf experimental-data. J. Dairy Sci. 1977, 60, 989–991. [Google Scholar] [CrossRef]
- Atkinson, O. To survey Current Practices and Performance and to Determine the Success Factors for Rearing Replacement Heifers in Wales. Welsh Dairy Youngstock Project, Full Report. Available online: https://dairyveterinaryconsultancy.co.uk/download/the-welsh-dairy-youngstock-project-full- (accessed on 19 October 2024).
- Edwards, K.Y.; LeBlanc, S.J.; DeVries, T.J.; Steele, M.A.; Costa, J.H.C.; Renaud, D.L. Barriers to recording calf health data on dairy farms in Ontario. JDS Commun. 2024, 5, 42–46. [Google Scholar] [CrossRef]
- Palczynski, L.J.; Bleach, E.C.L.; Brennan, M.L.; Robinson, P.A. Youngstock Management as "The Key for Everything"? Perceived Value of Calves and the Role of Calf Performance Monitoring and Advice on Dairy Farms. Front. Anim. Sci. 2022, 3, 18. [Google Scholar] [CrossRef]
- Wilson, D.J.; Saraceni, J.; Roche, S.M.; Pempek, J.A.; Habing, G.; Proudfoot, K.L.; Renaud, D.L. How can better calf care be realized on dairy farms? A qualitative interview study of veterinarians and farmers. J. Dairy Sci. 2024, 107, 1706. [Google Scholar] [CrossRef]
- Dairy Calf and Heifer Association. Gold Standards. Available online: https://calfandheifer.org/member/ (accessed on 11 November 2024).
- Ollivett, T. Calf Health Module-#WeanClean. Available online: https://thedairylandinitiative.vetmed.wisc.edu/home/calf-health-module/ (accessed on 1 January 2015).
| TPI Category | Serum IgG (g/L) | Equivalent STP (g/dL) | Equivalent Brix % | Target % of Calves |
|---|---|---|---|---|
| Excellent | ≥25.0 | ≥6.2 | ≥9.4 | >40 |
| Good | 18.0–24.9 | 5.8–6.1 | 8.9–9.3 | ~30 |
| Fair | 10.0–17.9 | 5.1–5.7 | 8.1–8.8 | ~20 |
| Poor | <10.0 | <5.1 | <8.1 | <10 |
| Bodyweight (kg) | Target ADG b (g/d) | ME c (Mcal/d) | MP d (g/d) | CP e (% of DMI) |
|---|---|---|---|---|
| 50 | 600 | 3.98 | 193 | 23.6 |
| 900 | 5.13 | 267 | 25.2 | |
| 75 | 900 | 6.10 | 284 | 22.6 |
| 1200 | 7.40 | 360 | 23.6 | |
| 100 | 900 | 6.93 | 300 | 21.0 |
| 1200 | 8.31 | 379 | 22.0 | |
| 125 | 900 | 7.70 | 316 | 19.9 |
| 1200 | 9.14 | 397 | 21.0 |
| Ambient Temperature (°C) | Ambient Temperature (°F) | Percentage Increase in Metabolizable Energy |
|---|---|---|
| 30 | 86 | +9% |
| 20 | 68 | 0% |
| 10 | 50 | +19% |
| 0 | 32 | +38% |
| −10 | 14 | +56% |
| −20 | −4 | +75% |
| −30 | −22 | +94% |
| Key Performance Indicator | Target |
|---|---|
| Transfer of passive immunity a | ≥40% excellent ~30% good ~20% fair <10% poor |
| Neonatal calf diarrhea morbidity rate b | <15% |
| BRD morbidity rate b | <10% |
| Mortality rate b | <3% |
| Lung consolidation c (≥3 cm2) at weaning | <15% |
| Average daily gain b | ≥800 g/d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, K.Y.; Renaud, D.L. A Framework for Comprehensive Dairy Calf Health Investigations. Animals 2025, 15, 181. https://doi.org/10.3390/ani15020181
Edwards KY, Renaud DL. A Framework for Comprehensive Dairy Calf Health Investigations. Animals. 2025; 15(2):181. https://doi.org/10.3390/ani15020181
Chicago/Turabian StyleEdwards, Kristen Y., and David L. Renaud. 2025. "A Framework for Comprehensive Dairy Calf Health Investigations" Animals 15, no. 2: 181. https://doi.org/10.3390/ani15020181
APA StyleEdwards, K. Y., & Renaud, D. L. (2025). A Framework for Comprehensive Dairy Calf Health Investigations. Animals, 15(2), 181. https://doi.org/10.3390/ani15020181





