Quantitative Proteomics Analysis Reveals XDH Related with Ovarian Oxidative Stress Involved in Broodiness of Geese
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals and Sample Collection
2.3. Morphological Observation of Ovarian Tissues
2.4. Detection of Antioxidant Activity and Reproductive Hormone Levels of Ovaries by ELISA Assay
2.5. Immunohistochemistry
2.6. Quantitative Real-Time PCR
2.7. Western Blotting
2.8. Protein Extraction, Trypsin Digestion, and TMT Proteomic Labeling
2.9. HPLC Fractionation and LC-MS/MS Analyses
2.10. Database Search and Bioinformatics Analysis
2.11. Parallel Reaction Monitoring (PRM) Validation of Goose Ovaries Proteomics Results
2.12. Statistical Analysis and Data Available
3. Results
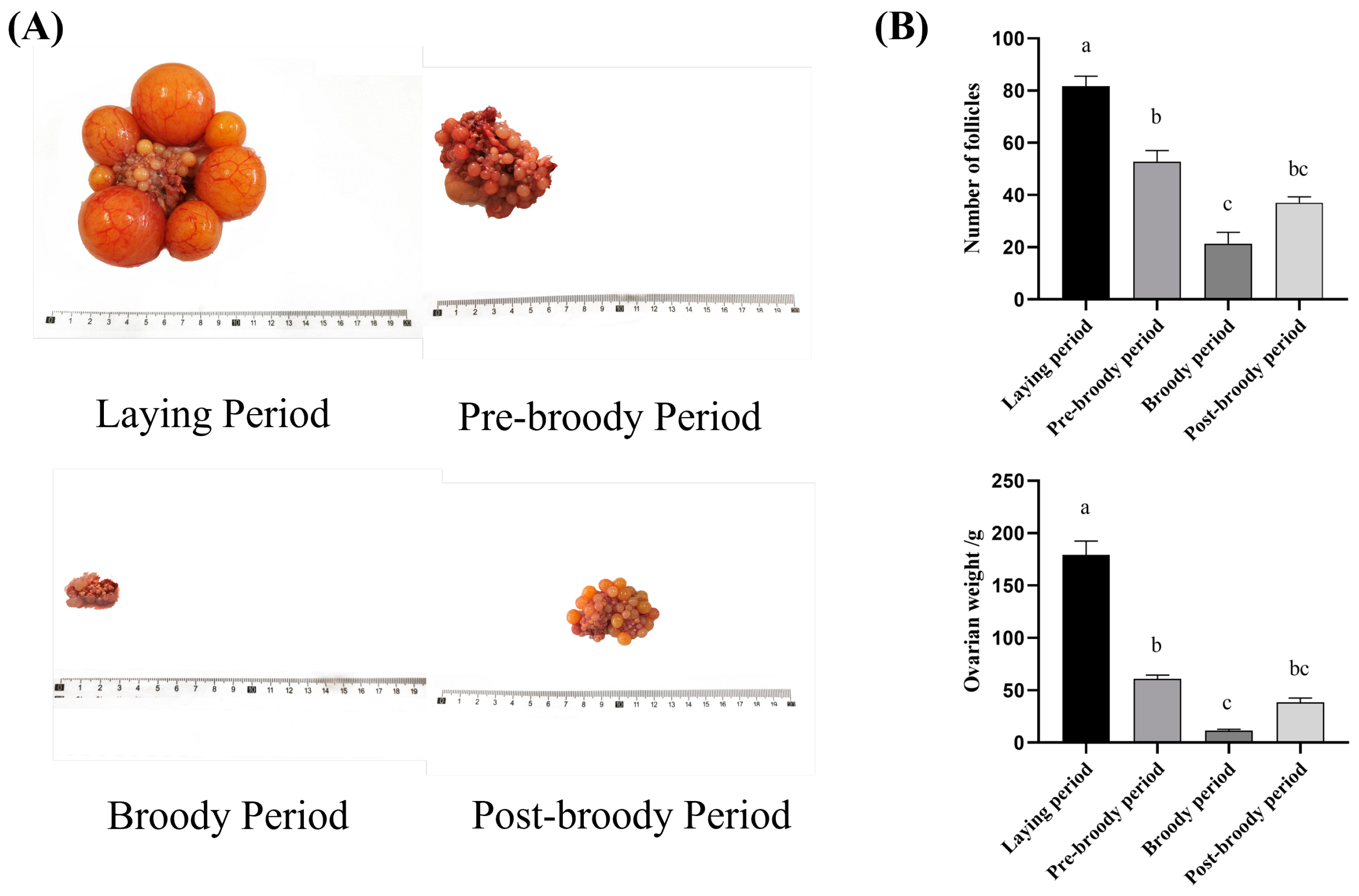
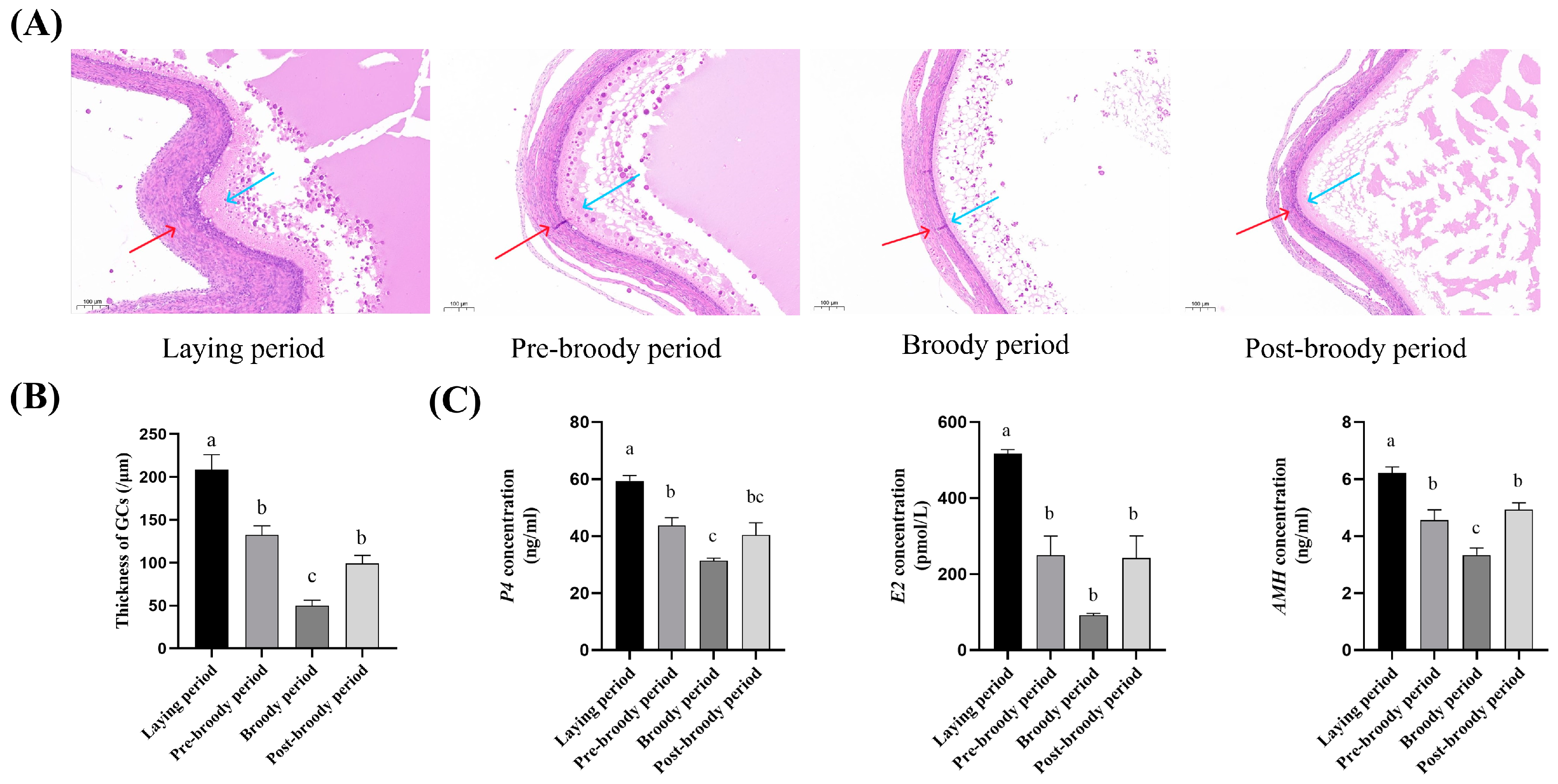
3.1. Morphological and Histological Characteristics of Ovaries in Laying, Pre-Broody, Broody, and Post-Broody Periods
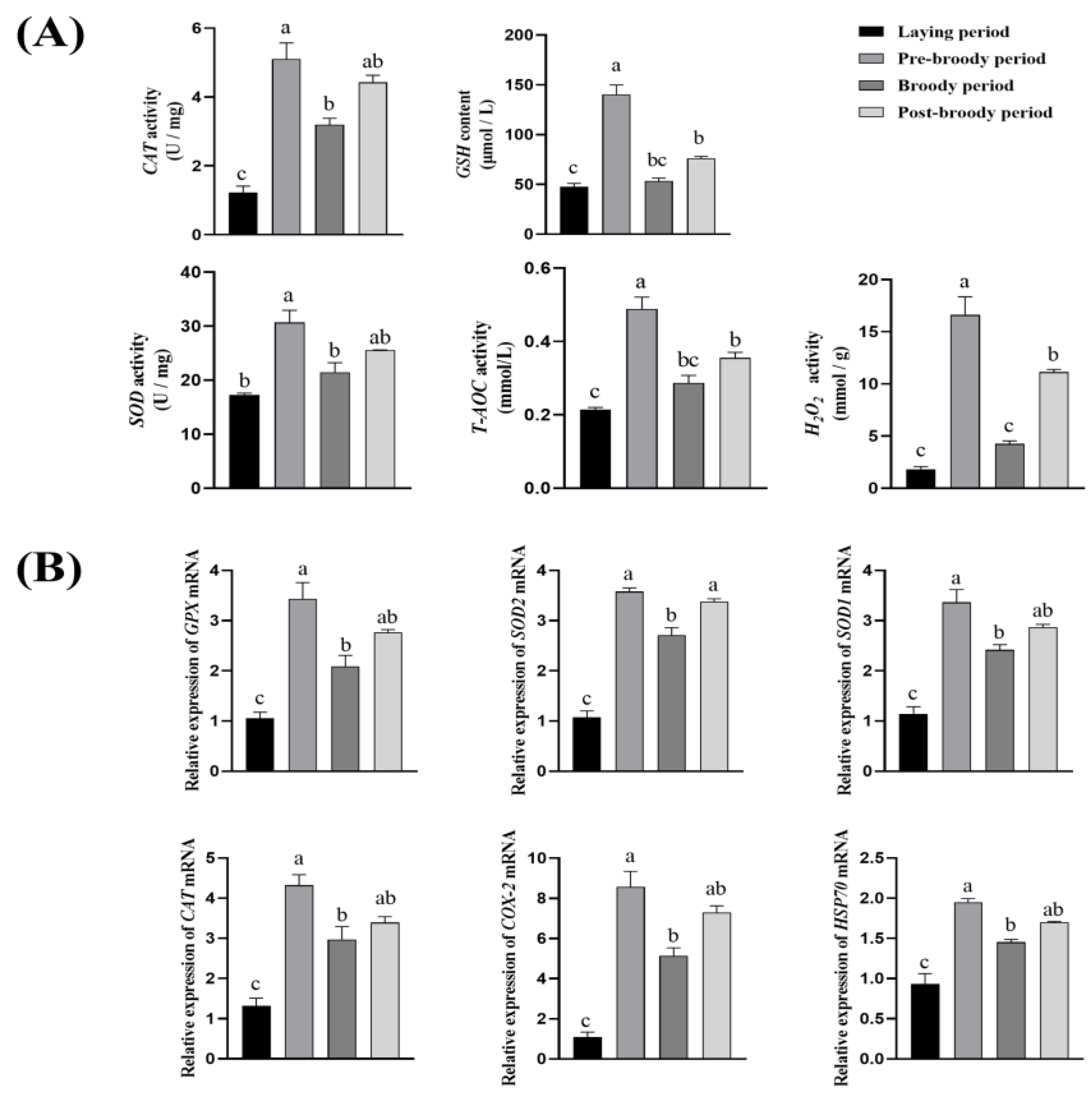
3.2. Antioxidant Activity of Ovaries Among Laying, Pre-Broody, Broody, and Post-Broody Periods
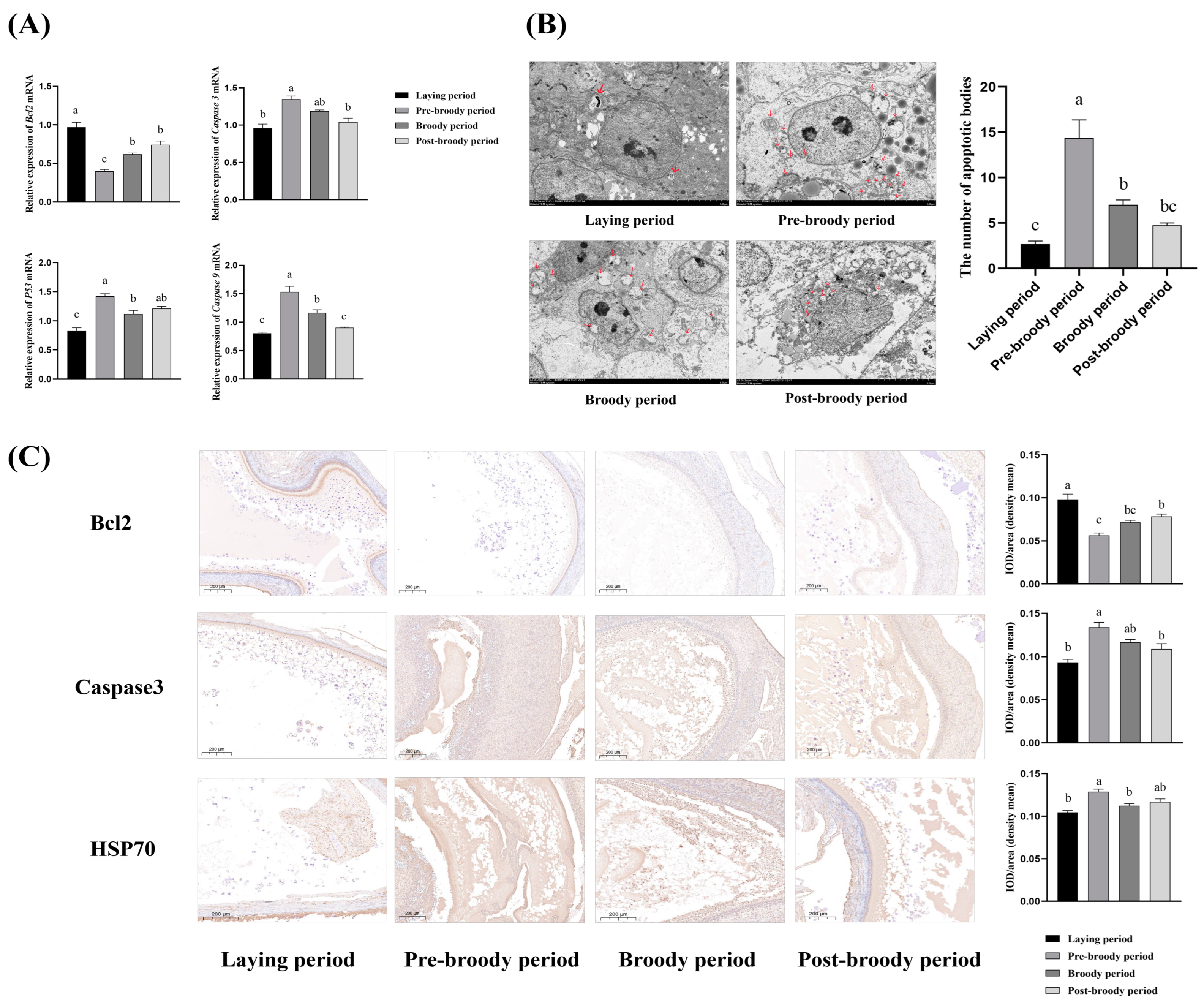
3.3. Apoptosis Level of Ovaries During Laying, Pre-Broody Broody, Broody, and Post-Broody Periods
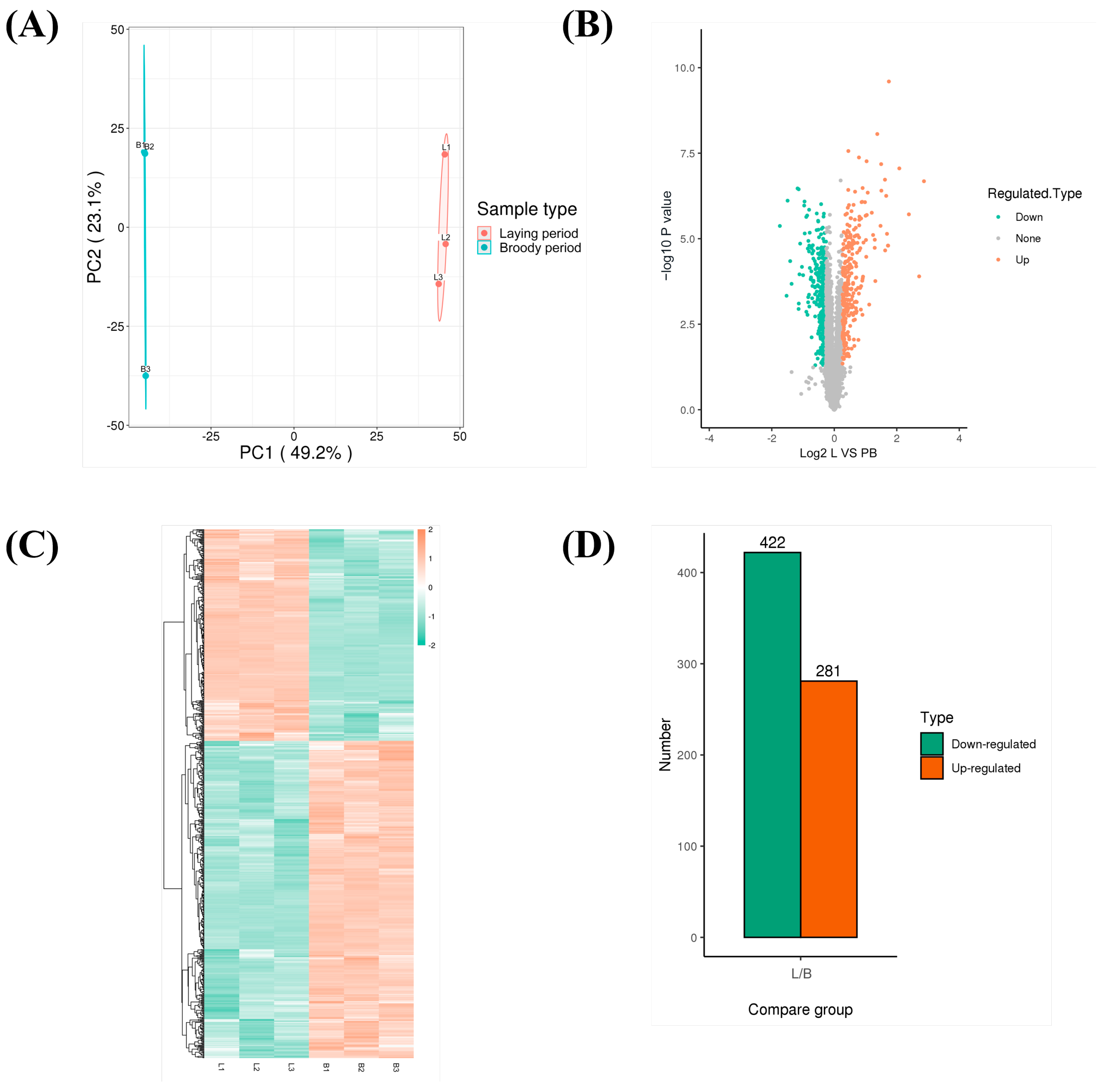
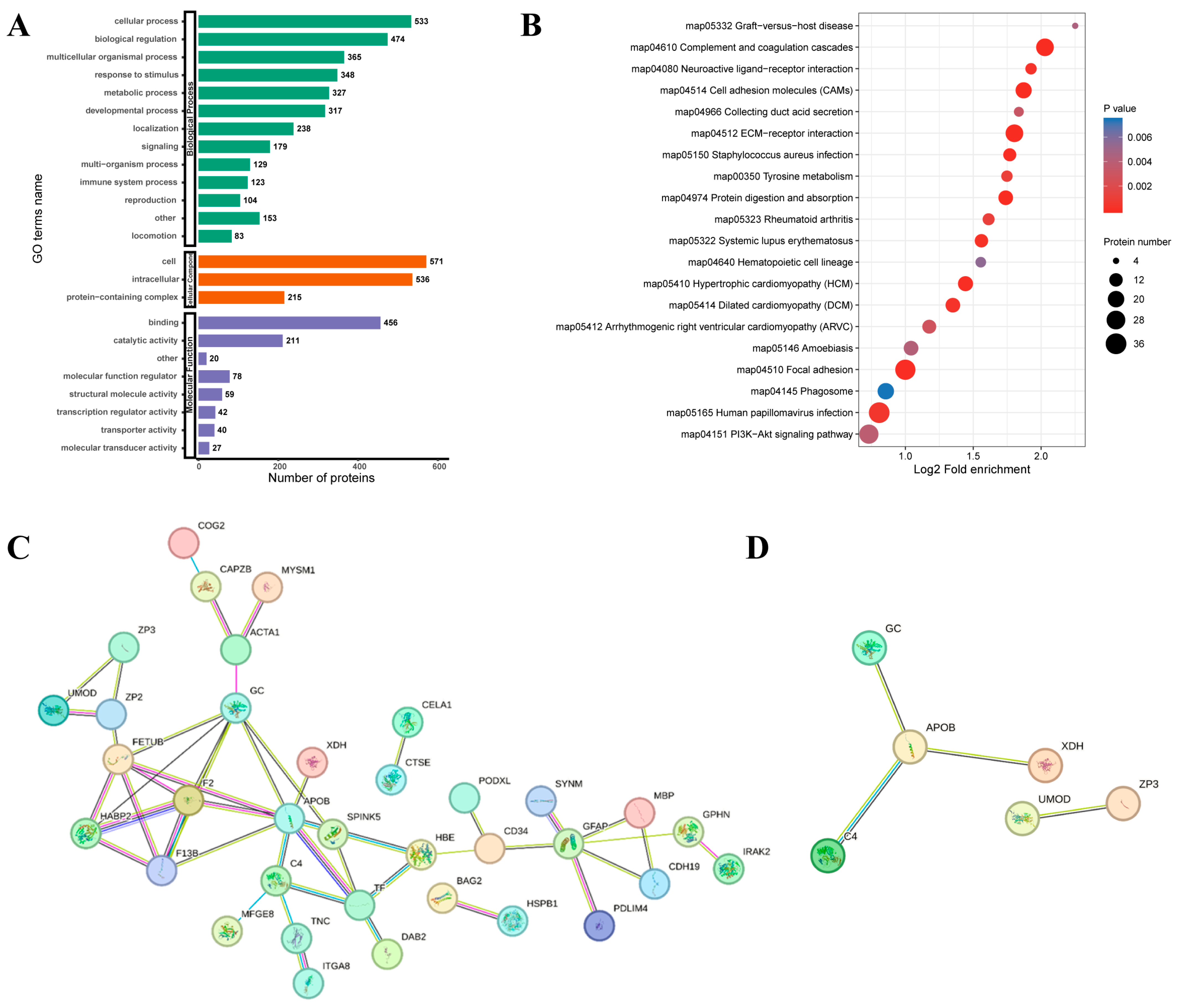
3.4. Quantitative Proteomics Analysis of Ovaries Between Laying and Pre-Broody Periods
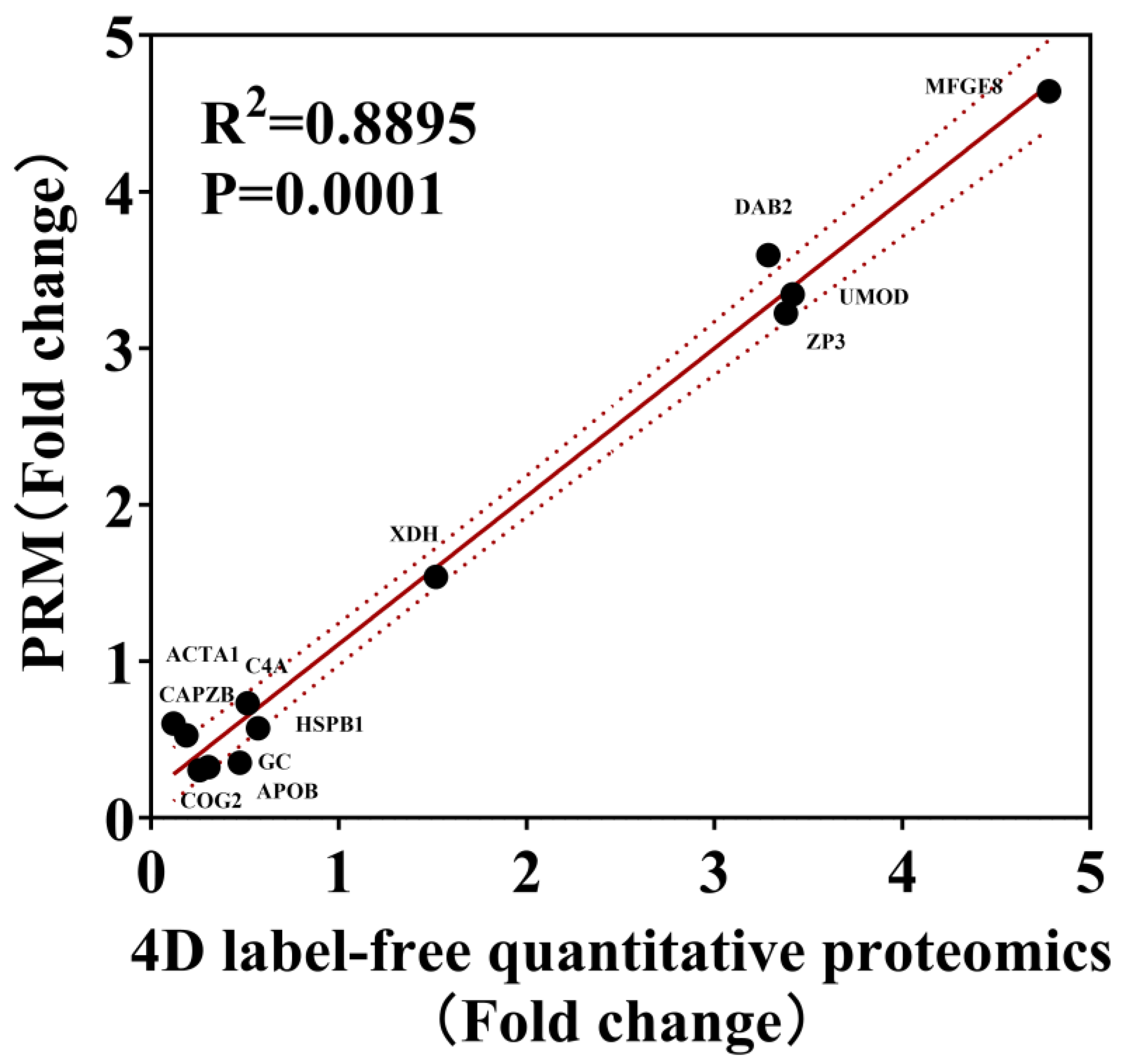
3.5. Parallel Reaction Monitoring (PRM) Analysis of DEPs Between Laying and Broody Periods
3.6. XDH Promotes Ovarian Oxidative Stress in Mediating Broodiness of Geese
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The State of the World’s Animal Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2007; pp. 252–256. Available online: https://openknowledge.fao.org/handle/20.500.14283/a1250e (accessed on 22 November 2024).
- Yang, W.; Chen, X.; Liu, Z.; Zhao, Y.; Chen, Y.; Geng, Z. Integrated transcriptome and proteome revealed that the declined expression of cell cycle-related genes associated with follicular atresia in geese. BMC Genom. 2023, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.Q.; Ran, J.S.; Li, J.J.; Ren, P.; Zhang, X.X.; Liu, Y.P. Genetic regulation of broodiness in poultry. Yi Chuan Hered. 2019, 41, 391–403. [Google Scholar] [CrossRef]
- Bello, S.F.; Adeola, A.C.; Nie, Q. The study of candidate genes in the improvement of egg production in ducks—A review. Poult. Sci. 2022, 101, 101850. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiu, Y.; Wang, Q.; Gao, M.; Cao, Z.; Luan, X. ADPN Regulates Oxidative Stress-Induced Follicular Atresia in Geese by Modulating Granulosa Cell Apoptosis and Autophagy. Int. J. Mol. Sci. 2024, 25, 5400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.Y.; An, C.; Weng, K.Q.; Cao, Z.F.; Xu, Q.; Chen, G.H. Effect of active immunization with recombinant-derived goose INH-alpha, AMH, and PRL fusion protein on broodiness onset and egg production in geese (Anser cygnoides). Poult. Sci. 2021, 100, 101452. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lou, Y.; He, K.; Yang, S.; Yu, W.; Han, L.; Zhao, A. Goose broodiness is involved in granulosa cell autophagy and homeostatic imbalance of follicular hormones. Poult. Sci. 2016, 95, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Kovács, J.; Forgó, V.; Péczely, P. The fine structure of the follicular cells in growing and atretic ovarian follicles of the domestic goose. Cell Tissue Res. 1992, 267, 561–569. [Google Scholar] [CrossRef]
- Romanov, M. Genetics of broodiness in poultry—A review. Asian Australas. J. Anim. Sci. 2001, 14, 1647–1654. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef]
- Pritsos, C.A. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem.-Biol. Interact. 2000, 129, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age 1997, 20, 127–140. [Google Scholar] [CrossRef]
- Ohtsubo, T.; Rovira, I.I.; Starost, M.F.; Liu, C.; Finkel, T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ. Res. 2004, 95, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Vorbach, C.; Harrison, R.; Capecchi, M.R. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003, 24, 512–517. [Google Scholar] [CrossRef]
- Margolin, Y.; Behrman, H.R. Xanthine oxidase and dehydrogenase activities in rat ovarian tissues. Am. J. Physiol. 1992, 262 Pt 1, E173–E178. [Google Scholar] [CrossRef] [PubMed]
- Meneshian, A.; Bulkley, G.B. The physiology of endothelial xanthine oxidase: From urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 2002, 9, 161–175. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, Y.; Chen, S.T.; Kang, J.W.; Pan, J.M.; Liu, X.Z.; Li, S.Y.; Yan, G.J.; Liu, A.X.; Huang, Q.T.; et al. In situ Synthesized Monosodium Urate Crystal Enhances Endometrium Decidualization via Sterile Inflammation During Pregnancy. Front. Cell Dev. Biol. 2021, 9, 702590. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Chaube, S.K. Human Chorionic Gonadotropin Mediated Generation of Reactive Oxygen Species Is Sufficient to Induce Meiotic Exit but Not Apoptosis in Rat Oocytes. BioRes. Open Access 2017, 6, 110–122. [Google Scholar] [CrossRef]
- Abaza, M.S.; Afzal, M.; Al-Attiyah, R.J.; Guleri, R. Methylferulate from Tamarix aucheriana inhibits growth and enhances chemosensitivity of human colorectal cancer cells: Possible mechanism of action. BMC Complement. Altern. Med. 2016, 16, 384. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Ma, J.; Xiong, Y.; Xiong, X.; Lan, D.; Li, J.; Yin, S. Factors Influencing the Maturation and Developmental Competence of Yak (Bos grunniens) Oocytes In Vitro. Genes 2023, 14, 1882. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Gao, B.W.; Wang, J.; Wang, X.W.; Ren, Q.L.; Chen, J.F.; Ma, Q.; Xing, B.S. Chronic Exposure to Diquat Causes Reproductive Toxicity in Female Mice. PLoS ONE 2016, 11, e0147075. [Google Scholar] [CrossRef]
- Wang, J.; Jia, R.; Gong, H.; Celi, P.; Zhuo, Y.; Ding, X.; Bai, S.; Zeng, Q.; Yin, H.; Xu, S.; et al. The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions. Antioxidants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Knight, J.W. Mechanisms of oxidative stress in porcine oocytes and the role of antioxidants. Reprod. Fertil. Dev. 2008, 20, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.X.; Duan, X.; Cui, X.S.; Kim, N.H.; Xiong, B.; Sun, S.C. Melamine Induces Oxidative Stress in Mouse Ovary. PLoS ONE 2015, 10, e0142564. [Google Scholar] [CrossRef]
- de Macedo, A.R.S.; de Oliveira, J.F.A.; Sommerfeld, S.; Notário, F.O.; Martins, M.M.; Bastos, L.M.; Bezerra, B.G.P.; Lisboa, L.D.S.; Rocha, H.A.O.; Araujo, R.M.; et al. Unlocking the power of Libidibia ferrea extracts: Antimicrobial, antioxidant, and protective properties for potential use in poultry production. Poult. Sci. 2024, 103, 103668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Huo, Z.; Chen, Y.; Liu, J.; Zhao, Z.; Meng, F.; Su, Q.; Bao, W.; Zhang, L.; et al. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef]
- Ding, X.; Cai, C.; Jia, R.; Bai, S.; Zeng, Q.; Mao, X.; Xu, S.; Zhang, K.; Wang, J. Dietary resveratrol improved production performance, egg quality, and intestinal health of laying hens under oxidative stress. Poult. Sci. 2022, 101, 101886. [Google Scholar] [CrossRef]
- Ye, P.; Li, M.; Liao, W.; Ge, K.; Jin, S.; Zhang, C.; Chen, X.; Geng, Z. Hypothalamic transcriptome analysis reveals the neuroendocrine mechanisms in controlling broodiness of Muscovy duck (Cairina moschata). PLoS ONE 2019, 14, e0207050. [Google Scholar] [CrossRef]
- Zhao, S.; Gu, T.; Weng, K.; Zhang, Y.; Cao, Z.; Zhang, Y.; Zhao, W.; Chen, G.; Xu, Q. Phosphoproteome Reveals Extracellular Regulated Protein Kinase Phosphorylation Mediated by Mitogen-Activated Protein Kinase Kinase-Regulating Granulosa Cell Apoptosis in Broody Geese. Int. J. Mol. Sci. 2023, 24, 2278. [Google Scholar] [CrossRef]
- Hou, L.; Gu, T.; Weng, K.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effects of Oxidative Stress on the Autophagy and Apoptosis of Granulosa Cells in Broody Geese. Int. J. Mol. Sci. 2023, 24, 2154. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, Y.Z.; Gu, T.T.; Cao, Z.F.; Zhao, W.M.; Qin, H.R.; Xu, Q.; Chen, G.H. Comparison of the broody behavior characteristics of different breeds of geese. Poult. Sci. 2019, 98, 5226–5233. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Song, L.; Weng, K.; Bao, Q.; Wu, J.; Zhang, Y.; Xu, Q.; Zhang, Y. TMT-based quantitative proteomic analysis unveils uterine fluid difference in hens producing normal and pimpled eggs. Poult. Sci. 2023, 102, 103081. [Google Scholar] [CrossRef]
- Lou, Y.; Yu, W.; Han, L.; Yang, S.; Wang, Y.; Ren, T.; Yu, J.; Zhao, A. ROS activates autophagy in follicular granulosa cells via mTOR pathway to regulate broodiness in goose. Anim. Reprod. Sci. 2017, 185, 97–103. [Google Scholar] [CrossRef]
- Jiang, D.; Guo, Y.; Niu, C.; Long, S.; Jiang, Y.; Wang, Z.; Wang, X.; Sun, Q.; Ling, W.; An, X.; et al. Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins. Int. J. Mol. Sci. 2023, 24, 5793. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, L.; Chen, W.; Zhang, Y.; Hua, R.; Wang, W.; Zhang, T.; Wu, H. The protective effects of pretreatment with resveratrol in cyclophosphamide-induced rat ovarian granulosa cell injury: In vitro study. Reprod. Toxicol. 2020, 95, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yan, M.; Chen, J.; Li, H.; Zhang, Y. MicroRNA-129-1-3p attenuates autophagy-dependent cell death by targeting MCU in granulosa cells of laying hens under H2O2-induced oxidative stress. Poult. Sci. 2023, 102, 103006. [Google Scholar] [CrossRef]
- Fletcher, Q.E.; Selman, C.; Boutin, S.; McAdam, A.G.; Woods, S.B.; Seo, A.Y.; Leeuwenburgh, C.; Speakman, J.R.; Humphries, M.M. Oxidative damage increases with reproductive energy expenditure and is reduced by food-supplementation. Evolution 2013, 67, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, N.B.; Monaghan, P. Does reproduction cause oxidative stress? An open question. Trends Ecol. Evol. 2013, 28, 347–350. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Thakkar, H.; Tyan, F.; Gim, S.; Robinson, H.; Lee, C.; Pandey, S.K.; Nwokorie, C.; Onwudiwe, N.; Srivastava, R.K. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene 2001, 20, 6073–6083. [Google Scholar] [CrossRef] [PubMed]
- Pawson, T.; Nash, P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000, 14, 1027–1047. [Google Scholar] [CrossRef]
- Peng, W.-S.; Gao, M.; Yao, X.-F.; Tong, Y.-Y.; Zhang, H.-H.; He, X. Magnolol supplementation alleviates diquat-induced oxidative stress via PI3K–Akt in broiler chickens. Anim. Sci. J. 2023, 94, e13891. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P.; Virot, S.; Chaufour, S.; Firdaus, W.; Kretz-Remy, C.; Diaz-Latoud, C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid. Redox Signal. 2005, 7, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Tao, Y.; Xiao, X. Small heat shock protein alphaB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem. Biophys. Res. Commun. 2007, 354, 109–114. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, L.; Zeng, Z.; Liu, X.; Li, T.; He, X.; Ma, Y.; Meng, W.; Zeng, H.; Long, Y.; et al. MFG-E8 stabilized by deubiquitinase USP14 suppresses cigarette smoke-induced ferroptosis in bronchial epithelial cells. Cell Death Dis. 2023, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Yang, C.; Chai, S.-M.; Guo, H.; Seim, I.; Yang, G. Evolutionary impacts of purine metabolism genes on mammalian oxidative stress adaptation. Zool. Res. 2022, 43, 241–254. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress—A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.J. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Li, L.; Yan, H.C.; Zhang, T.; Zhang, P.; Sun, Z.Y.; De Felici, M.; Reiter, R.J.; Shen, W. Identification of oxidative stress-related Xdh gene as a di(2-ethylhexyl)phthalate (DEHP) target and the use of melatonin to alleviate the DEHP-induced impairments in newborn mouse ovaries. J. Pineal Res. 2019, 67, e12577. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Tian, S.; Du, R.; Lin, T.; Xiao, X.; Wang, R.; Chen, R.; Geng, H.; Subramanian, S.; et al. C1QBP regulates apoptosis of renal cell carcinoma via modulating xanthine dehydrogenase (XDH) mediated ROS generation. Int. J. Med. Sci. 2022, 19, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Sun, Q.; Jiang, Y.; Zhou, X.; Kang, L.; Wang, Z.; Wang, X.; An, X.; Ji, C.; Ling, W.; et al. Effects of exogenous spermidine on autophagy and antioxidant capacity in ovaries and granulosa cells of Sichuan white geese. J. Anim. Sci. 2023, 101, skad301. [Google Scholar] [CrossRef] [PubMed]

| Item | Content (%) | Item | Content (%) |
|---|---|---|---|
| Corn | 35 | AME (MJ/kg) | 10.82 |
| Soybean meal | 15 | CP (%) | 15.15 |
| Alfalfa meal | 5 | Crude fiber (%) | 5.24 |
| Rice hull | 10 | Calcium (%) | 1.17 |
| Barley | 10 | Total phosphorus (%) | 0.64 |
| Wheat | 15 | ||
| Bran | 5 | ||
| Vitamin and trace mineral mix | 5 |
| Gene | Nucleotide Sequence (5′~3′) | Length/bp | Tm/°C |
|---|---|---|---|
| Bcl-2 | F: ATGACCGAGTACCTGAACCG R: GCTCCCACCAGAACCAAAC | 155 | 60 |
| Caspase-3 | F: CTGGTATTGAGGCAGACAGTGG R: CAGCACCCTACACAGAGACTGAA | 158 | 60 |
| Caspase-9 | F: TTCCAGGCTCTGTCGGGTAA R: GTCCAGCGTTTCCACATACCA | 150 | 60 |
| p53 | F: ACCGGTGAGGGGACGAGAA R: GAGCGGCGGCGAGTTGGAG | 190 | 60 |
| β-actin | F: GAGAAATTGTCCGTGACATCA R: CCTGAACCTCTCATTGCCA | 152 | 60 |
| GPX | F: GCAAGGGGTACAAGCCCAACT R: GATGATGTACTGCGGGTTGGTC | 149 | 60 |
| SOD-1 | F: CACCTGCTGTAACCATTCTTAGT R: GGCTCCTCATCTTCCAAACC | 137 | 60 |
| SOD-2 | F: CCTGACCTGCCGTATGACTATG R: ACCTGAGCTGTAACATCACCTTTT | 161 | 60 |
| CAT | F: ATACAGTTCGTGACCCTCG R: CCAGAAGTCCCATACCAT | 188 | 60 |
| COX-2 | F: CGATGAACCAGACCTCACCC R: TCTGGGGTGGGCACTATGTA | 111 | 60 |
| Hsp70 | F: GGGAGACAAGTCCGAGAATGTGC R: GTTTGGTGGGAATGGTGGTGTTAC | 128 | 60 |
| XDH | F: GTAGGGAACACGGAAGTGGG | 126 | 60 |
| R: GCCAAAGGTGACCCCTGTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Zhang, Y.; Jiang, Y.; Gu, W.; Zhao, S.; Vongsangnak, W.; Zhang, Y.; Xu, Q.; Zhang, Y. Quantitative Proteomics Analysis Reveals XDH Related with Ovarian Oxidative Stress Involved in Broodiness of Geese. Animals 2025, 15, 182. https://doi.org/10.3390/ani15020182
Zhou N, Zhang Y, Jiang Y, Gu W, Zhao S, Vongsangnak W, Zhang Y, Xu Q, Zhang Y. Quantitative Proteomics Analysis Reveals XDH Related with Ovarian Oxidative Stress Involved in Broodiness of Geese. Animals. 2025; 15(2):182. https://doi.org/10.3390/ani15020182
Chicago/Turabian StyleZhou, Ning, Yaoyao Zhang, Youluan Jiang, Wang Gu, Shuai Zhao, Wanwipa Vongsangnak, Yang Zhang, Qi Xu, and Yu Zhang. 2025. "Quantitative Proteomics Analysis Reveals XDH Related with Ovarian Oxidative Stress Involved in Broodiness of Geese" Animals 15, no. 2: 182. https://doi.org/10.3390/ani15020182
APA StyleZhou, N., Zhang, Y., Jiang, Y., Gu, W., Zhao, S., Vongsangnak, W., Zhang, Y., Xu, Q., & Zhang, Y. (2025). Quantitative Proteomics Analysis Reveals XDH Related with Ovarian Oxidative Stress Involved in Broodiness of Geese. Animals, 15(2), 182. https://doi.org/10.3390/ani15020182






