Effects of Dietary Supplementation with Abies sibirica Essential Oil on Growth Performance, Digestive Enzymes, Skin Mucus Immunological Parameters, and Response to Heat Stress in Rainbow Trout
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. SBF Essential Oil Origin and Composition
2.2. Diet Formulation and Feeding Trial
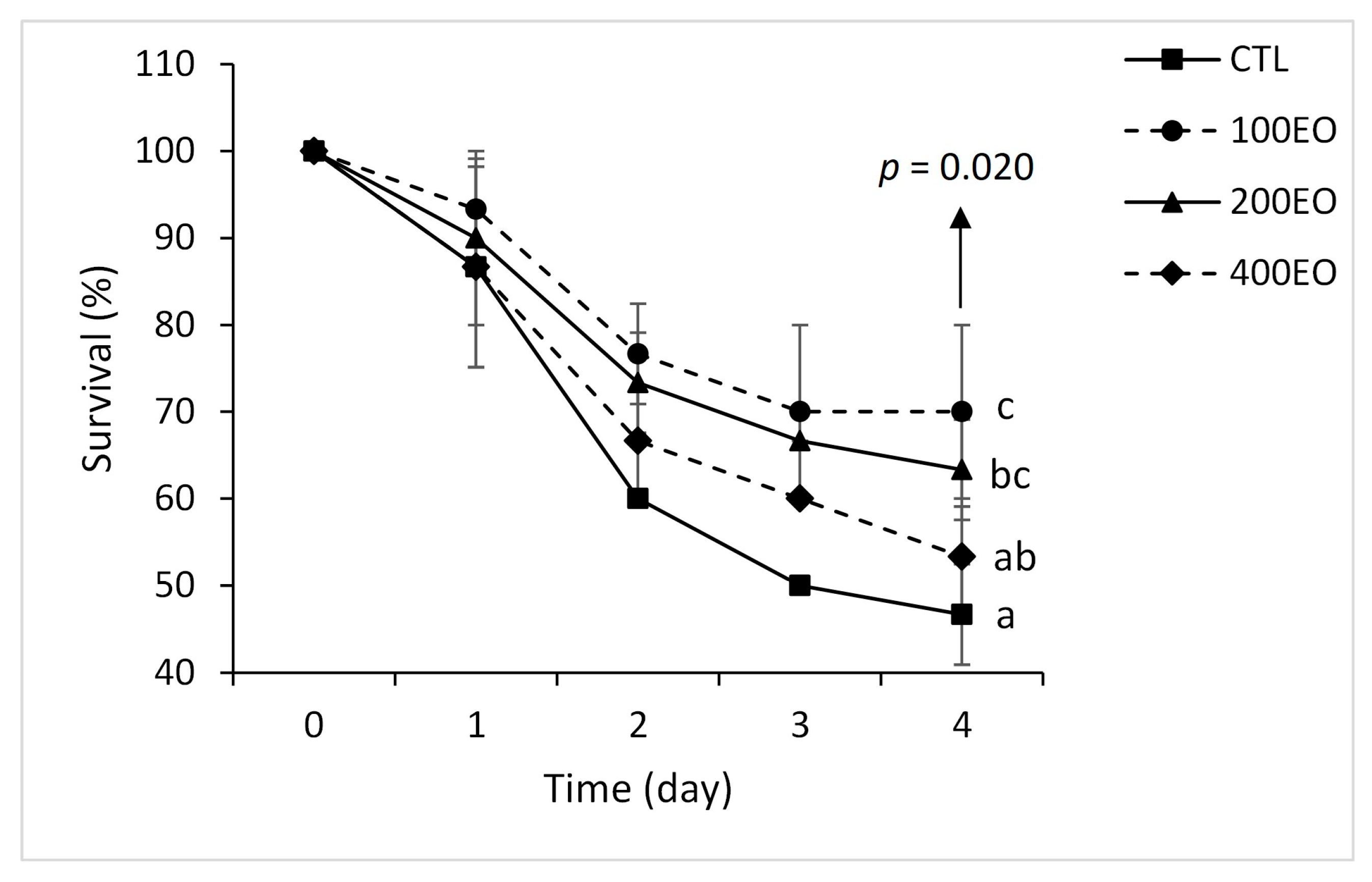
2.3. Heat Stress
2.4. Sampling
2.5. Analysis
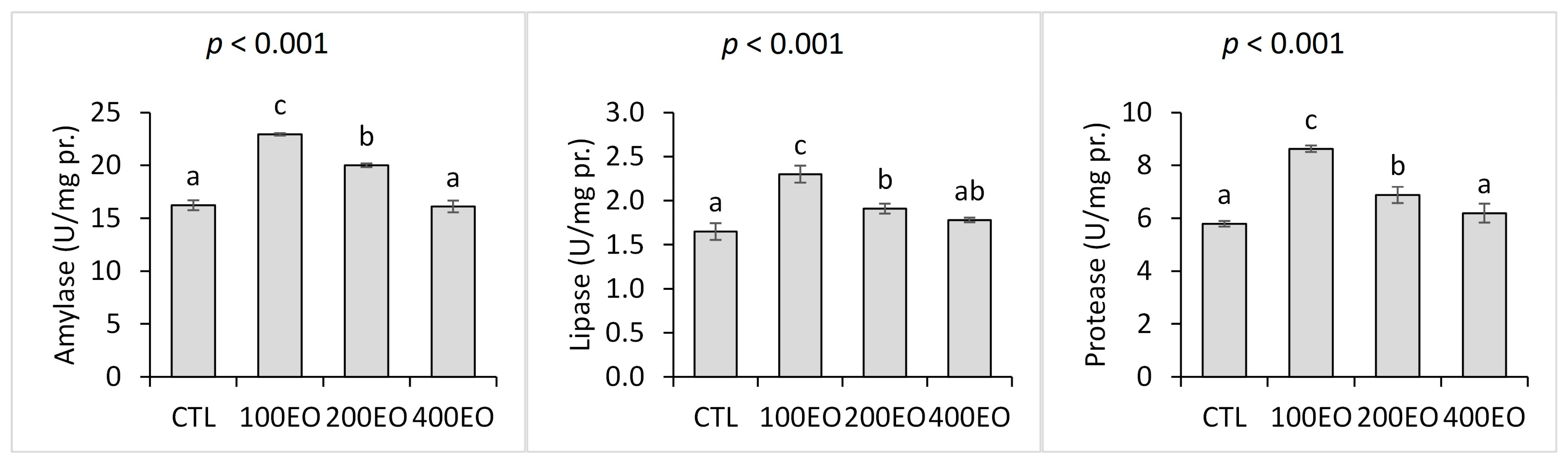
2.5.1. Digestive Enzymes
2.5.2. Skim Mucus and Intestinal Immune-Related Parameters
2.5.3. Plasma Immune-Related Parameters
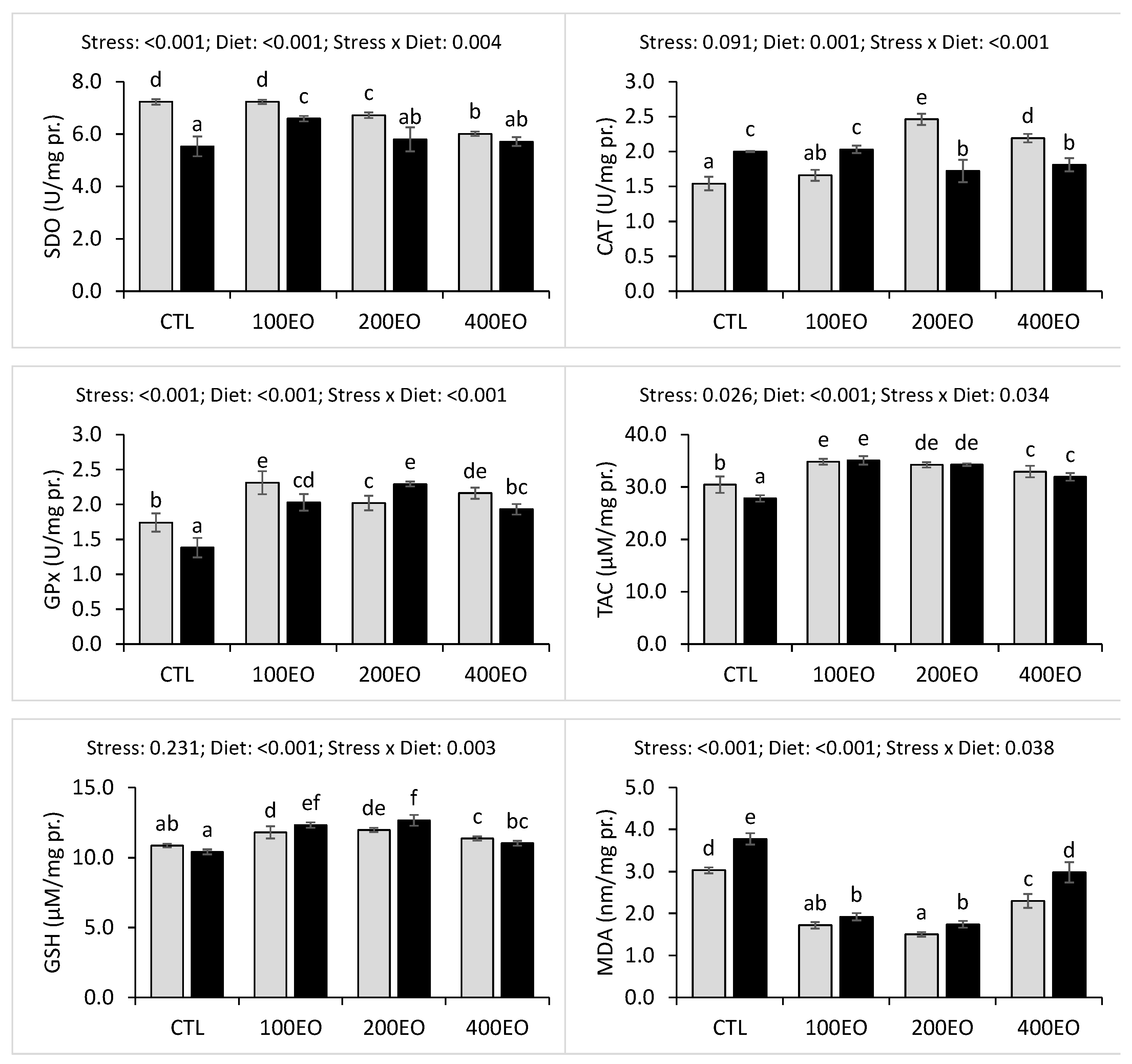
2.5.4. Hepatic Antioxidant-Related Parameters
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Fishery Statistical Collections: Global Aquaculture Production; FAO: Roma, Italy, 2023. [Google Scholar]
- Yousefi, M.; Hoseini, S.M.; Vatnikov, Y.A.; Karamyan, A.; Kulikov, E.V. Dietary thymol supplementation promotes antioxidant responses and thermal stress resistance in rainbow trout, Oncorhynchus mykiss. Animals 2024, 14, 2988. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World. Aquac. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Missaghi, S.; Hondzo, M.; Herb, W. Prediction of lake water temperature, dissolved oxygen, and fish habitat under changing climate. Clim. Change 2017, 141, 747–757. [Google Scholar] [CrossRef]
- Little, A.G.; Loughland, I.; Seebacher, F. What do warming waters mean for fish physiology and fisheries? J. Fish Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Hosseinpour, F.; Vazirzadeh, A.; Farhadi, A.; Sajjadi, S.H. Acclimation to higher temperature and antioxidant supplemented diets improved rainbow trout (Oncorhynchus mykiss) resilience to heatwaves. Sci. Rep. 2024, 14, 11375. [Google Scholar] [CrossRef]
- Yang, C.; Dong, J.; Sun, C.; Li, W.; Tian, Y.; Liu, Z.; Gao, F.; Ye, X. Exposure to heat stress causes downregulation of immune response genes and weakens the disease resistance of Micropterus salmoides. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101011. [Google Scholar] [CrossRef] [PubMed]
- Onomu, A.J.; Okuthe, G.E. The role of functional feed additives in enhancing aquaculture sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Caipang, C.M.A. Phytogenics in aquaculture: A short review of their effects on gut health and microflora in fish. Philipp. J. Fish. 2020, 27, 11–22. [Google Scholar] [CrossRef]
- Elumalai, P.; Kurian, A.; Lakshmi, S.; Faggio, C.; Esteban, M.A.; Ringø, E. Herbal immunomodulators in aquaculture. Rev. Fish. Sci. Aquac. 2021, 29, 33–57. [Google Scholar] [CrossRef]
- Kamble, M.T.; Yostawonkul, J.; Medhe, S.V.; Chavan, B.R.; Kumar, A.; Palekar, G.R.; Daunde, V.Y.; Tayade, S.H.; Gabriel, N.N.; Ataguba, G.A.; et al. Innovative feed additives for sustainable aquaculture: Phytobiotics encapsulated in organic nanoparticles. In Sustainable Feed Ingredients and Additives for Aquaculture Farming: Perspectives from Africa and Asia; Gabriel, N.N., Abasubong, K.P., Erasmus, V.N., Kamble, M.T., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 501–520. [Google Scholar]
- Dokou, S.; Bitchava, K.; Stylianaki, I.; Chantzi, P.; Efstathiou, A.; Vasilopoulou, K.; Tsoumani, M.; Gouva, E.; Michailidis, G.; Kumar, P. Nano-oregano essential oil improves rainbow trout’s (Oncorhynchus mykiss) growth performance, oxidative status, fatty acid profile of fillet, affects gene expression and supports skin and intestinal histomorphometry. Ann. Anim. Sci. 2023, 23, 1177–1189. [Google Scholar] [CrossRef]
- Hajirezaee, S.; Khanjani, M.H.; Ahani, S.; Ghiasvand, Z. Tarragon (Artemisia dracunculus) essential oil at optimized dietary levels prompted growth, immunity, and resistance to enteric red-mouth disease in the rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2024, 2024, 3273850. [Google Scholar] [CrossRef]
- Yousefi, M.; Hoseini, S.M.; Kulikov, E.V.; Seleznev, S.B.; Petrov, A.K.; Babichev, N.V.; Kochneva, M.V.; Davies, S.J. Effects of dietary Hyssop, Hyssopus officinalis, extract on physiological and antioxidant responses of rainbow trout, Oncorhynchus mykiss, juveniles to thermal stress. Front. Vet. Sci. 2022, 9, 1042063. [Google Scholar] [CrossRef]
- Burkova, V.N.; Sergun, V.P.; Ivanov, A.A. Chemical composition and pharmacological activity of aqueous extract of Siberian fir (Abies sibirica L.) (A review). Russ. J. Bioorg. Chem. 2023, 49, 1553–1566. [Google Scholar] [CrossRef]
- Polyakov, N.A.; Dubinskaya, V.A.; Efremov, A.A.; Efremov, E.A. Biological activity of Abies sibirica essential oil and its major constituents for several enzymes in vitro. Pharm. Chem. J. 2014, 48, 456–460. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Winn-Deen, E.S.; David, H.; Sigler, G.; Chavez, R. Development of a direct assay for alpha-amylase. Clin. Chem. 1988, 34, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Tanaka, S.; Ota, Y. Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiol. Biochem. 1998, 18, 59–69. [Google Scholar] [CrossRef]
- Iversen, S.L.; Jørgensen, M.H. Azocasein assay for alkaline protease in complex fermentation broth. Biotechnol. Tech. 1995, 9, 573–576. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; Stolen, J.S., Ed.; SOS Publication: Fair haven, NJ, USA, 1990; pp. 101–103. [Google Scholar]
- Esmaeili, M.; Kenari, A.A.; Rombenso, A.N. Effects of fish meal replacement with meat and bone meal using garlic (Allium sativum) powder on growth, feeding, digestive enzymes and apparent digestibility of nutrients and fatty acids in juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Aquacult. Nutr. 2017, 23, 1225–1234. [Google Scholar] [CrossRef]
- Siwicki, A.; Anderson, D. Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum. In Fish Disease Diagnosis and Prevention Methods; Siwicki, A., Anderson, D., Waluga, J., Eds.; Wydawnictwo Instytutu Rybactwa Srodladowego: Olsztyn, Poland, 1993; pp. 105–112. [Google Scholar]
- Yano, T. Assays of hemolytic complement activity. In Techniques in Fish Immunology; Stolen, J.S., Ed.; SOS Publication: Fair haven, NJ, USA, 1992; pp. 131–141. [Google Scholar]
- Marklund, S.L. Pyrogallol autoxidation. In Handbook Methods For Oxygen Radical Research; Greenwald, R., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 243–248. [Google Scholar]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Hu, M.-L. Measurement of protein thiol groups and glutathione in plasma. In Methods Enzymol; Academic Press: Cambridge, MA, USA, 1994; pp. 380–385. [Google Scholar]
- Sattar, A.A.; Matin, A.A.; Hadwan, M.H.; Hadwan, A.M.; Mohammed, R.M. Rapid and effective protocol to measure glutathione peroxidase activity. Bull. Natl. Res. Cent. 2024, 48, 100. [Google Scholar] [CrossRef]
- Lim, C.S.H.; Lim, S.L. Ferric reducing capacity versus ferric reducing antioxidant power for measuring total antioxidant capacity. Lab. Med. 2013, 44, 51–55. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods Enzymol; Fleischer, S., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 1978; pp. 302–310. [Google Scholar]
- Hafsan, H.; Saleh, M.M.; Zabibah, R.S.; Obaid, R.F.; Jabbar, H.S.; Mustafa, Y.F.; Sultan, M.Q.; Gabr, G.A.; Ramírez-Coronel, A.A.; Khodadadi, M.; et al. Dietary thymol improved growth, body composition, digestive enzyme activities, hematology, immunity, antioxidant defense, and resistance to Streptococcus iniae in the rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr. 2022, 2022, 3288139. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Mirghaed, A.T.; Iri, Y.; Ghelichpour, M. Effects of dietary cineole administration on growth performance, hematological and biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 766–772. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquacult. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Salinas, I.; Ding, Y.; Fernández-Montero, Á.; Sunyer, J.O. Mucosal immunity in fish. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 387–443. [Google Scholar]
- Khanzadeh, M.; Hoseinifar, S.H.; Zargari, A.; Van Doan, H. Effects of dietary supplements Sargassum ilicifolium and Spirulina platensis on growth parameters, immunity and gene expression in juvenile Asian seabass (Lates calcarifer). J. Agric. Food Res. 2025, 19, 101689. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Sringarm, K.; Jaturasitha, S.; Yuangsoi, B.; Dawood, M.A.; Esteban, M.Á.; Ringø, E.; Faggio, C. Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish Immunol. 2019, 93, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, Y.; Huang, J.; Li, Y.; Wu, S.; Zhao, L.; Sun, T.; Kang, Y.; Liu, Z. Dietary supplementation of Chinese herbal medicines enhances the immune response and resistance of rainbow trout (Oncorhynchus mykiss) to infectious hematopoietic necrosis virus. Front. Vet. Sci. 2024, 11, 1341920. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.-A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xiao, Y.; Xiao, Z.; Liu, T.; Li, J.; Li, P.; Han, F. Lysozymes in fish. J. Agric. Food Chem. 2021, 69, 15039–15051. [Google Scholar] [CrossRef] [PubMed]
- Conforto, E.; Vílchez-Gómez, L.; Parrinello, D.; Parisi, M.G.; Esteban, M.Á.; Cammarata, M.; Guardiola, F.A. Role of mucosal immune response and histopathological study in European eel (Anguilla anguilla L.) intraperitoneal challenged by Vibrio anguillarum or Tenacibaculum soleae. Fish Shellfish Immunol. 2021, 114, 330–339. [Google Scholar] [CrossRef]
- Lallès, J.-P. Biology, environmental and nutritional modulation of skin mucus alkaline phosphatase in fish: A review. Fish Shellfish Immunol. 2019, 89, 179–186. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.C. Stress and immunity in fish. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 609–655. [Google Scholar]
- Barreto, M.O.; Planellas, S.R.; Yang, Y.; Phillips, C.; Descovich, K. Emerging indicators of fish welfare in aquaculture. Rev. Aquacult. 2022, 14, 343–361. [Google Scholar] [CrossRef]
- Raissy, M.; Kabootarkhani, M.A.; Sanisales, K.; Mohammadi, M.; Rashidian, G. The synergistic effects of combined use of Mentha longifolia, Thymus carmanicus, and Trachyspermum copticum on growth performance, feed utilization, and expression of key immune genes in rainbow trout (Oncorhynchus mykiss). Front. Vet. Sci. 2022, 8, 810261. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Mendivil, C.O. Dietary fish, fish nutrients, and immune function: A review. Front. Nutr. 2021, 7, 617652. [Google Scholar] [CrossRef]
- Bedekar, M.K.; KV, R. Overview of fish immune system. In Fish Immune System and Vaccines; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–16. [Google Scholar]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquacult. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Shakouri, M.; Yousefi, S.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Mazandarani, M.; Mozanzadeh, M.T.; Tulino, M.G.; Faggio, C. Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol. 2020, 100, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Hoseini, S.M.; Abtahi, B.; Vatnikov, Y.A.; Kulikov, E.V.; Yurievna, R.N. Effects of dietary methanolic extract of hyssop, Hyssopus officinalis, on growth performance, hepatic antioxidant, humoral and intestinal immunity, and intestinal bacteria of rainbow trout, Oncorhynchus mykiss. Front. Mar. Sci. 2022, 9, 1026651. [Google Scholar] [CrossRef]
- Hu, X.; Ma, W.; Zhang, D.; Tian, Z.; Yang, Y.; Huang, Y.; Hong, Y. Application of natural antioxidants as feed additives in aquaculture: A review. Biology 2025, 14, 87. [Google Scholar] [CrossRef]
- Zou, W.; Huang, X.; Han, F.; Li, Z. Effects of probiotic-fermented chinese herb on immune response and growth performance in common carp (Cyprinus carpio). Fishes 2025, 10, 196. [Google Scholar] [CrossRef]
- Swamy, J.M.; Naik, M.G.; Rathore, S.S.; Srinivasa, K.H.; Monica, K.S. Dietary supplementation of Nile tilapia (Oreochromis niloticus) diets with bay laurel (Laurus nobilis): Alleviation of oxidative stress and amelioration of immune response, serum biochemistry, and resistance against Aeromonas hydrophila. Fish Physiol. Biochem. 2023; in press. [Google Scholar]
- Zhou, X.; Wang, Y.; Yu, J.; Li, J.; Wu, Q.; Bao, S.; Jiang, L.; Liu, B. Effects of dietary fermented Chinese herbal medicines on growth performance, digestive enzyme activity, liver antioxidant capacity, and intestinal inflammatory gene expression of juvenile largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 25, 101269. [Google Scholar] [CrossRef]
- Srivastava, R.; Choudhury, P.K.; Dev, S.K.; Rathore, V. Molecular simulation studies of alpha pinene, an alkene in search for oxidative stress targeted therapeutic paradigms for the treatment of Parkinson’s disease: A computational approach and its in-vitro antioxidant validation. Lett. Drug Des. Discov. 2021, 18, 1117–1135. [Google Scholar] [CrossRef]
- Balachandran, A.; Choi, S.B.; Beata, M.-M.; Małgorzata, J.; Froemming, G.R.; Lavilla, C.A., Jr.; Billacura, M.P.; Siyumbwa, S.N.; Okechukwu, P.N. Antioxidant, wound healing potential and in silico assessment of naringin, eicosane and octacosane. Molecules 2023, 28, 1043. [Google Scholar] [CrossRef]
- Rahimi, K.; Zalaghi, M.; Shehnizad, E.G.; Salari, G.; Baghdezfoli, F.; Ebrahimifar, A. The effects of alpha-pinene on inflammatory responses and oxidative stress in the formalin test. Brain Res. Bull. 2023, 203, 110774. [Google Scholar] [CrossRef]
- Onanuga, A.O.; Okpala, E.O. Chemical compositions and antioxidant activity of volatile oils from Morinda citrifolia and Beta vulgaris leaves from Nigeria. Biol. Med. Nat. Prod. Chem. 2022, 11, 161–167. [Google Scholar] [CrossRef]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Li, B.; Ding, L.; Wei, X.; Wang, P.; Chen, Z.; Han, S.; Huang, T.; Wang, B.; et al. Physiological responses to heat stress in the liver of rainbow trout (Oncorhynchus mykiss) revealed by UPLC-QTOF-MS metabolomics and biochemical assays. Ecotoxicol. Environ. Saf. 2022, 242, 113949. [Google Scholar] [CrossRef] [PubMed]

| Detected Compounds | Area% | RT (min) |
|---|---|---|
| α-Pinene | 10.36 | 5.042 |
| Camphene | 2.19 | 5.327 |
| Δ-3-Carene | 2.13 | 6.624 |
| l-Bornyl acetate | 8.85 | 12.99 |
| trans-Caryophyllene | 0.19 | 15.984 |
| α-Humulene | 0.12 | 16.695 |
| β-Bisabolene | 0.07 | 17.806 |
| 14-β-H-Pregna | 39.68 | 27.233 |
| Methyldiethylborane | 8.04 | 29.459 |
| 2-Octadecoxyethanol | 12.36 | 30.243 |
| Hexacosane | 2.25 | 34.928 |
| Octacosane | 3.06 | 37.320 |
| Eicosane | 10.05 | 37.331 |
| 2-(4-Methylphenyl)indolizine | 0.3 | 44.802 |
| Total | 99.67 |
| Ingredients | g/kg | Proximate Composition | |
|---|---|---|---|
| Fishmeal 1 | 210 | Crude protein | 425 |
| Poultry slaughterhouse by product | 300 | Crude lipid | 148 |
| Soybean meal | 160 | Moisture | 91.20 |
| Wheat meal | 200 | Ash | 75.8 |
| Sunflower oil | 15 | Crude fiber | 23.5 |
| Soybean oil | 20 | Gross energy (kcal/g) 4 | 4759 |
| Corn meal | 80 | ||
| Methionine | 3 | ||
| Lysine | 2 | ||
| Vitamin premix 2 | 5 | ||
| Mineral premix 3 | 5 |
| CTL | 100EO | 200EO | 400EO | p-Value | |
|---|---|---|---|---|---|
| Initial weight (g) | 4.09 ± 0.11 | 4.15 ± 0.07 | 4.10 ± 0.19 | 4.17 ± 0.05 | 0.784 |
| Final weight (g) | 18.3 ± 0.42 a | 23.1 ± 1.21 c | 21.5 ± 0.54 b | 20.1 ± 0.91 b | 0.001 |
| Weight gain (%) | 250 ± 21.3 a | 459 ± 21.5 c | 427 ± 26.5 bc | 384 ± 24.2 ab | 0.002 |
| Specific growth rate (%/d) | 2.50 ± 0.08 a | 2.86 ± 0.06 c | 2.76 ± 0.08 bc | 2.62 ± 0.09 ab | 0.003 |
| Feed conversion ratio | 1.10 ± 0.03 b | 0.89 ± 0.06 a | 0.97 ± 0.04 a | 1.10 ± 0.08 b | 0.004 |
| Survival (%) | 100 | 100 | 100 | 100 |
| CTL | 100EO | 200EO | 400EO | Stress | Diet | Stress × Diet | |
|---|---|---|---|---|---|---|---|
| Cortisol (ng/mL) | 81.4 ± 5.40 | 50.7 ± 2.06 | 68.5 ± 6.20 | 71.5 ± 3.68 | 0.001 | <0.001 | 0.916 |
| 98.6 ± 2.76 | 64.3 ± 10.4 | 81.6 ± 12.4 | 89.4 ± 6.93 | Before < After | CTLc; 100EO a; 200EO b; 400EO b | ||
| Glucose (mg/dL) | 51.3 ± 6.81 a | 47.0 ± 4.58 a | 48.0 ± 7.81 a | 45.3 ± 5.51 a | <0.001 | 0.132 | 0.008 |
| 135 ± 10.5 d | 103 ± 8.00 b | 107 ± 13.3 bc | 121 ± 15.7 cd |
| Treatments | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|
| Stress | CTL | 100EO | 200EO | 400EO | Stress | Diet | Stress × Diet | |
| Plasma | ||||||||
| Lysozyme (U/mL) | Before | 33.7 ± 6.61 a | 50.1 ± 0.90 c | 47.2 ± 1.00 bc | 44.2 ± 0.95 b | 0.111 | 0.771 | <0.001 |
| After | 30.0 ± 1.82 a | 48.1 ± 0.56 bc | 44.6 ± 1.06 b | 43.9 ± 0.31 b | ||||
| ACH50 (U/mL) | Before | 140 ± 0.97 b | 149 ± 1.80 e | 144 ± 1.58 cd | 145 ± 0.86 d | <0.001 | <0.001 | <0.001 |
| After | 124 ± 2.00 a | 142 ± 3.79 bcd | 143 ± 0.65 bcd | 141 ± 1.68 bc | ||||
| Total Ig (mg/mL) | Before | 20.9 ± 0.28 d | 26.6 ± 1.11 g | 24.3 ± 0.31 f | 23.0 ± 0.23 e | <0.001 | <0.001 | 0.041 |
| After | 15.4 ± 0.60 a | 20.3 ± 0.60 d | 18.9 ± 0.15 c | 17.6 ± 0.14 b | ||||
| Intestine | ||||||||
| Lysozyme (U/mg pr.) | Before | 8.48 ± 0.19 d | 9.95 ± 0.06 f | 10.4 ± 0.27 g | 9.40 ± 0.16 e | <0.001 | <0.001 | <0.001 |
| After | 7.15 ± 0.05 a | 9.96 ± 0.09 f | 8.13 ± 0.22 c | 7.61 ± 0.32 b | ||||
| Total Ig (mg/g ww.) | Before | 19.9 ± 0.64 d | 21.5 ± 0.33 e | 23.3 ± 0.49 f | 20.0 ± 0.61 d | <0.001 | <0.001 | <0.001 |
| After | 16.7 ± 0.18 a | 18.2 ± 0.36 c | 17.6 ± 0.09 bc | 17.1 ± 0.24 ab | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi, M.; Adineh, H.; Vatnikov, Y.A.; Kulikov, E.V.; Petrukhina, O.A.; Sotnikova, E.D.; Telezhenkova, A.I.; Hoseini, S.M. Effects of Dietary Supplementation with Abies sibirica Essential Oil on Growth Performance, Digestive Enzymes, Skin Mucus Immunological Parameters, and Response to Heat Stress in Rainbow Trout. Animals 2025, 15, 2911. https://doi.org/10.3390/ani15192911
Yousefi M, Adineh H, Vatnikov YA, Kulikov EV, Petrukhina OA, Sotnikova ED, Telezhenkova AI, Hoseini SM. Effects of Dietary Supplementation with Abies sibirica Essential Oil on Growth Performance, Digestive Enzymes, Skin Mucus Immunological Parameters, and Response to Heat Stress in Rainbow Trout. Animals. 2025; 15(19):2911. https://doi.org/10.3390/ani15192911
Chicago/Turabian StyleYousefi, Morteza, Hossein Adineh, Yury Anatolyevich Vatnikov, Evgeny Vladimirovich Kulikov, Olesya Anatolyevna Petrukhina, Elena Dmitriyevna Sotnikova, Alena Igorevna Telezhenkova, and Seyyed Morteza Hoseini. 2025. "Effects of Dietary Supplementation with Abies sibirica Essential Oil on Growth Performance, Digestive Enzymes, Skin Mucus Immunological Parameters, and Response to Heat Stress in Rainbow Trout" Animals 15, no. 19: 2911. https://doi.org/10.3390/ani15192911
APA StyleYousefi, M., Adineh, H., Vatnikov, Y. A., Kulikov, E. V., Petrukhina, O. A., Sotnikova, E. D., Telezhenkova, A. I., & Hoseini, S. M. (2025). Effects of Dietary Supplementation with Abies sibirica Essential Oil on Growth Performance, Digestive Enzymes, Skin Mucus Immunological Parameters, and Response to Heat Stress in Rainbow Trout. Animals, 15(19), 2911. https://doi.org/10.3390/ani15192911






