Metabolomic Analysis of Environmental Biomarkers Reveals Markers of Mate Preference in Female Giant Pandas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Estrus Identification by Behavioral Observation
2.3. Estrus Identification by ELISA
2.4. Collection and Classification of Environmental Biomarkers Derived from Anogenital Gland Secretions (AGS)
2.5. LC-MS/MS
2.6. Statistics Analysis
3. Results
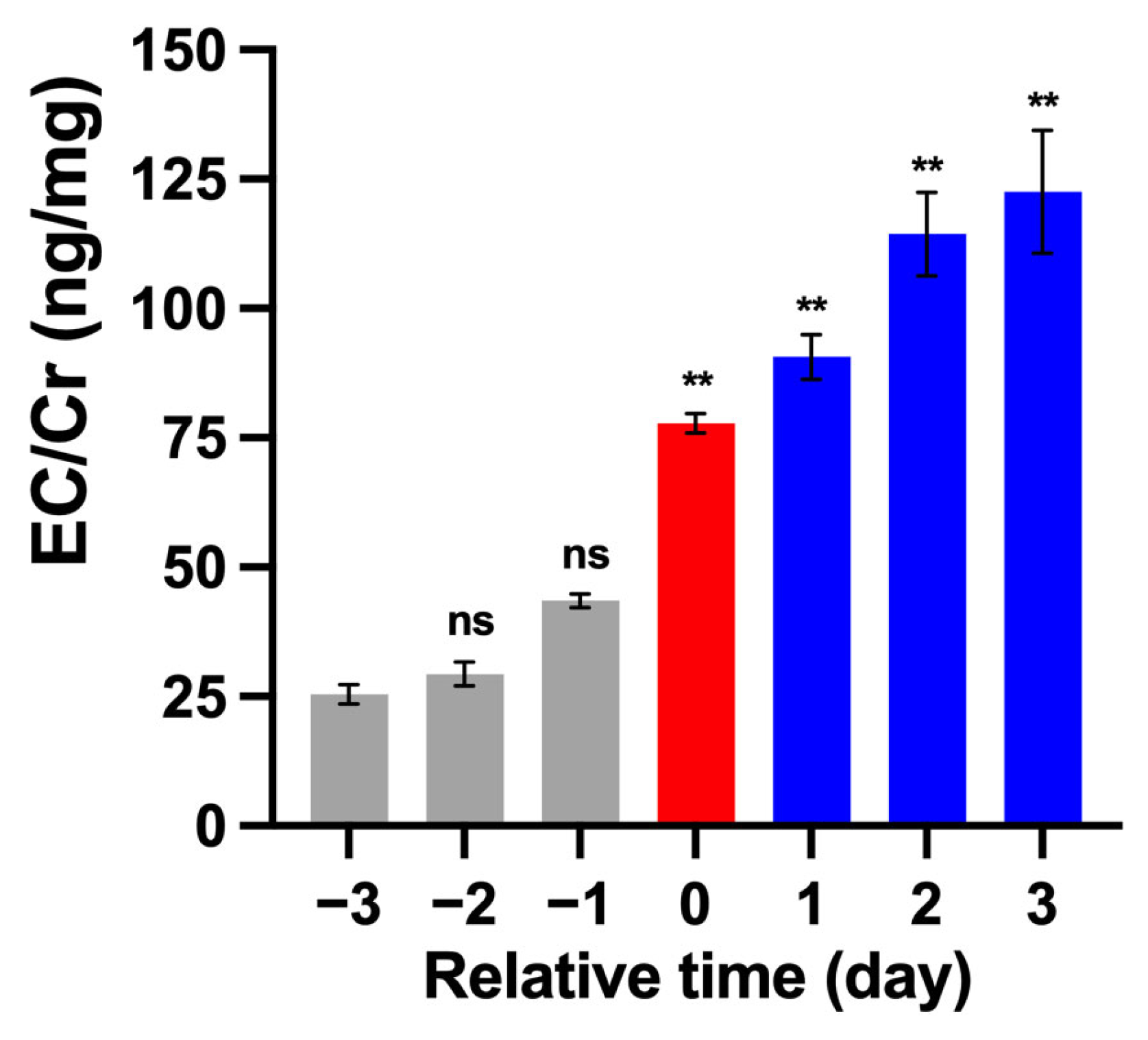
3.1. Estrus-Associated Hormone Levels in the Urine of 19 Female Giant Pandas
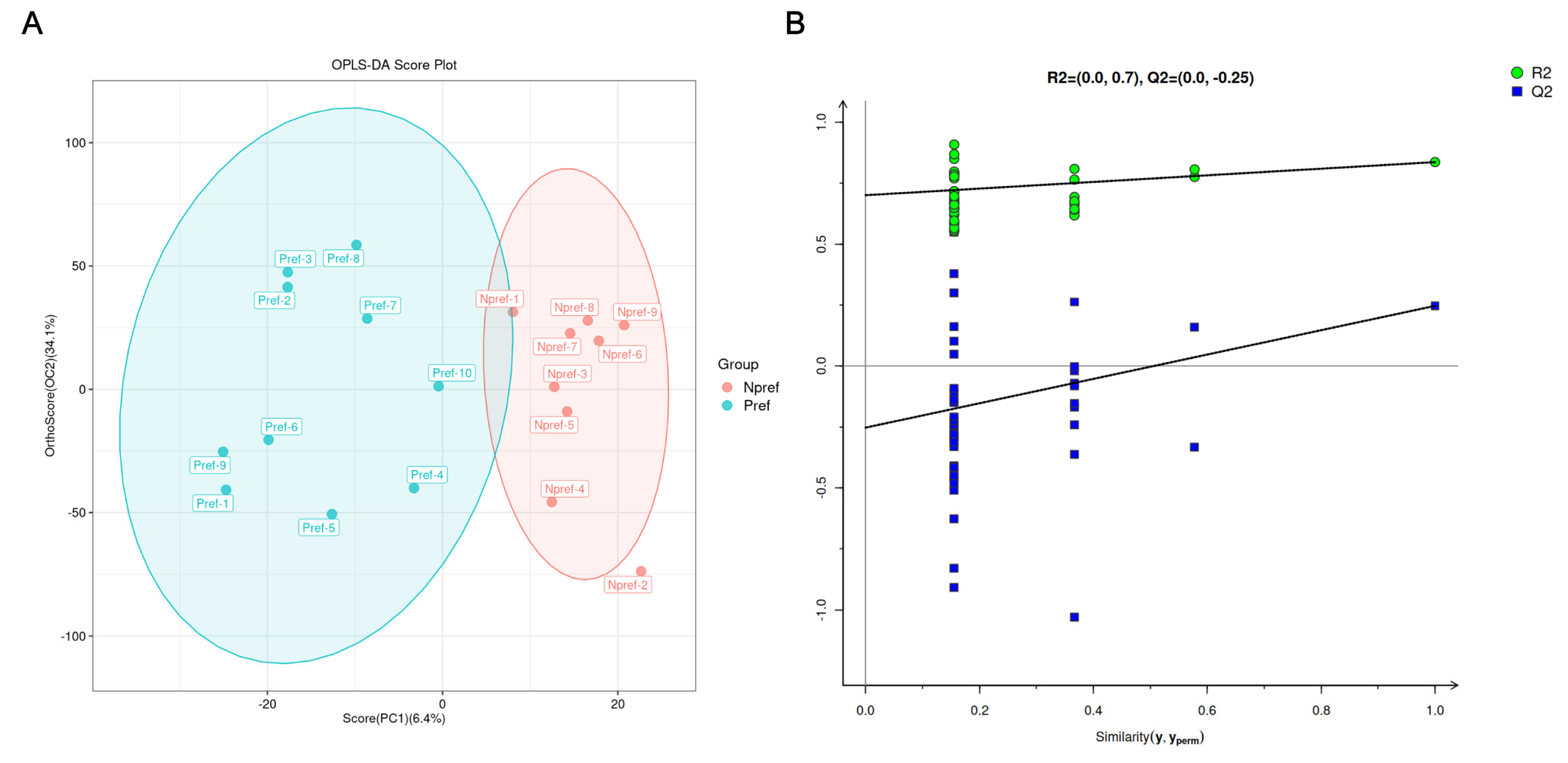
3.2. Global Features of Metabolic Profiles of Two Groups Environmental Biomarkers from Female Giant Pandas
3.3. Differential Metabolites Screening Between the Pref and Npref Group
3.4. Pathway Enrichment Analysis of Differential Metabolites Between the Pref and Npref Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, F.; Hu, Y.; Yan, L.; Nie, Y.; Wu, Q.; Zhang, Z. Giant pandas are not an evolutionary cul-de-sac: Evidence from multidisciplinary research. Mol. Biol. Evol. 2015, 32, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Qi, D.; Swaisgood, R.R.; Wang, L.; Jin, Y.; Wu, Q.; Wei, F.; Nie, Y. Symbiotic bacteria mediate volatile chemical signal synthesis in a large solitary mammal species. ISME J 2021, 15, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Pan, W.; Xie, Z. The giant panda as a social, biological and conservation phenomenon. Giant Pandas Biol. Vet. Med. Manag. 2009, 2, 16. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, D.; Sun, L.; Wei, R.; Zhang, G.; Sun, R. Anogenital gland secretions code for sex and age in the giant panda, Ailuropoda melanoleuca. Can. J. Zool. 2004, 82, 1596–1604. [Google Scholar] [CrossRef]
- Bouts, T.; Taylor, P.; Li, D.; Gasthuys, F.; Quievy, A.; Schauvliege, S. Anesthesia in Captive Giant Pandas (Ailuropoda melanoleuca) with Medetomidine-Ketamine. J. Zoo. Wildl. Med. 2024, 54, 796–800. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Li, D.; Zhang, G.; Wei, R.; Huang, Z.; Zhou, Y.; Zhou, Q.; Liu, Y.; Wildt, D.E.; et al. Relationship of the estrogen surge and multiple mates to cub paternity in the giant panda (Ailuropoda melanoleuca): Implications for optimal timing of copulation or artificial insemination. Biol. Reprod. 2012, 87, 112. [Google Scholar] [CrossRef]
- Gurler, H.; Malama, E.; Heppelmann, M.; Calisici, O.; Leiding, C.; Kastelic, J.P.; Bollwein, H. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology 2016, 86, 562–571. [Google Scholar] [CrossRef]
- Kersey, D.C.; Wildt, D.E.; Brown, J.L.; Snyder, R.J.; Huang, Y.; Monfort, S.L. Endocrine milieu of perioestrus in the giant panda (Ailuropoda melanoleuca), as determined by non-invasive hormone measures. Reprod. Fertil. Dev. 2010, 22, 901–912. [Google Scholar] [CrossRef]
- Li, D.; Wintle, N.J.P.; Zhang, G.; Wang, C.; Luo, B.; Martin-Wintle, M.S.; Owen, M.A.; Swaisgood, R.R. Analyzing the past to understand the future: Natural mating yields better reproductive rates than artificial insemination in the giant panda. Biol. Conserv. 2017, 216, 10–17. [Google Scholar] [CrossRef]
- Martin-Wintle, M.S.; Shepherdson, D.; Zhang, G.; Zhang, H.; Li, D.; Zhou, X.; Li, R.; Swaisgood, R.R. Free mate choice enhances conservation breeding in the endangered giant panda. Nat. Commun. 2015, 6, 10125. [Google Scholar] [CrossRef]
- Martin, M.S. The Role of Mate Preference and Personality on Reproductive Performance in an Ex-Situ Conservation Breeding Program for the Giant Panda (Ailuropoda melanoleuca). Ph.D. Thesis, Portland State University, Portland, OR, USA, 2014. [Google Scholar]
- Peng, J.J.; Jiang, Z.G.; Qin, G.Y.; Huang, Q.C.; Li, Y.X.; Jiao, Z.; Zhang, F.Q.; Fang, H.S.; Zhang, J.G.; Lu, Y.P.; et al. Mate choice in giant panda (Ailuropoda melanoleuca). Belg. J. Zool. 2009, 139, 87–92. [Google Scholar]
- Wilson, A.E. Chemical Signaling by Giant Pandas to Communicate Sexual Receptivity. Ph.D. Thesis, Mississippi State University, Starkville, MS, USA, 2017. [Google Scholar]
- Nie, Y.; Swaisgood, R.R.; Zhang, Z.; Hu, Y.; Ma, Y.; Wei, F. Giant panda scent-marking strategies in the wild: Role of season, sex and marking surface. Anim. Behav. 2012, 84, 39–44. [Google Scholar] [CrossRef]
- Ma, R.; Zheng, W.; Guo, J.; Hou, R.; Huang, H.; Xue, F.; Zhou, Y.; Wu, W.; Huang, C.; Gu, J.; et al. Symbiotic microbiota and odor ensure mating in time for giant pandas. Front. Microbiol. 2022, 13, 1015513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Nie, Y.; Hu, Y.; Swaisgood, R.R.; Zhang, Y.; Liu, D.; Wei, F. Seasonal and reproductive variation in chemical constituents of scent signals in wild giant pandas. Sci. China Life Sci. 2019, 62, 648–660. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, B.; Yang, B.; Li, D.; Wei, R.; Xie, C.; Yu, J.; Meng, X.; Cheng, J.; He, M.; et al. Case Report: A case study on the relationship between obesity and estrus in female captive panda. Front. Vet. Sci. 2025, 12, 1552754. [Google Scholar] [CrossRef]
- Martin-Wintle, M.S.; Kersey, D.C.; Wintle, N.J.P.; Aitken-Palmer, C.; Owen, M.A.; Swaisgood, R.R. Comprehensive Breeding Techniques for the Giant Panda. Adv. Exp. Med. Biol. 2019, 1200, 275–308. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Yuan, F.; Cao, D.; Zhang, Z. The Science Underlying Giant Panda Conservation Translocations. Animals 2023, 13, 3332. [Google Scholar] [CrossRef]
- Wang, J.; Yin, J.; Peng, D.; Zhang, X.; Shi, Z.; Li, W.; Shi, Y.; Sun, M.; Jiang, N.; Cheng, B.; et al. 4-Nitrophenol at environmentally relevant concentrations mediates reproductive toxicity in Caenorhabditis elegans via metabolic disorders-induced estrogen signaling pathway. J. Environ. Sci. 2025, 147, 244–258. [Google Scholar] [CrossRef]
- Raza, M.R.A.; Rajput, A.S.; Sasidharan, J.K.; Tomar, A.K.S.; Pandey, H.O.; Singh, M.; Patra, M.K. Effect of prostaglandin treatment on the estrus behaviour, follicular and luteal morphometry and serum hormone profile in sub-estrus buffaloes during non-breeding season. Reprod. Domest. Anim. 2024, 59, e14617. [Google Scholar] [CrossRef]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Duffy, D.M. Novel contraceptive targets to inhibit ovulation: The prostaglandin E2 pathway. Hum. Reprod. Update 2015, 21, 652–670. [Google Scholar] [CrossRef]
- Yu, D.; Wan, H.; Tong, C.; Guang, L.; Chen, G.; Su, J.; Zhang, L.; Wang, Y.; Xiao, Z.; Zhai, J.; et al. A multi-tissue metabolome atlas of primate pregnancy. Cell 2024, 187, 764–781.e714. [Google Scholar] [CrossRef]
- Stanczyk, F.Z. Metabolism of endogenous and exogenous estrogens in women. J. Steroid Biochem. Mol. Biol. 2024, 242, 106539. [Google Scholar] [CrossRef]
- Kelly, W.G.; De Leon, O.; Rizkallah, T.H. The role of 19-hydroxy-delta4-androstene-3,17-dione in the conversion of circulating delta4-androstene-3, 17-dione to estrone. J. Clin. Endocrinol. Metab. 1976, 43, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Chimote, B.N.; Chimote, N.M. Dehydroepiandrosterone (DHEA) and Its Sulfate (DHEA-S) in Mammalian Reproduction: Known Roles and Novel Paradigms. Vitam. Horm. 2018, 108, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Kersey, D.C.; Wildt, D.E.; Brown, J.L.; Snyder, R.J.; Huang, Y.; Monfort, S.L. Rising fecal glucocorticoid concentrations track reproductive activity in the female giant panda (Ailuropoda melanoleuca). Gen. Comp. Endocrinol. 2011, 173, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Graham, L.H.; Wielebnowski, N.; Swanson, W.F.; Wildt, D.E.; Howard, J.G. Understanding the basic reproductive biology of wild felids by monitoring of faecal steroids. J. Reprod. Fertil. Suppl. 2001, 57, 71–82. [Google Scholar]
- Kinoshita, K.; Inada, S.; Seki, K.; Sasaki, A.; Hama, N.; Kusunoki, H. Long-term monitoring of fecal steroid hormones in female snow leopards (Panthera uncia) during pregnancy or pseudopregnancy. PLoS ONE 2011, 6, e19314. [Google Scholar] [CrossRef][Green Version]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J. Nutr. 2007, 137, 1586S–1590S; discussion 1597S–1598S. [Google Scholar] [CrossRef]
- Flydal, M.I.; Martinez, A. Phenylalanine hydroxylase: Function, structure, and regulation. IUBMB Life 2013, 65, 341–349. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Ke, J.; Huang, X.; Wang, M.; Li, M.; Lan, J.; Wu, K.; Wang, L. Metabolomic Analysis of Environmental Biomarkers Reveals Markers of Mate Preference in Female Giant Pandas. Animals 2025, 15, 2873. https://doi.org/10.3390/ani15192873
Feng Y, Ke J, Huang X, Wang M, Li M, Lan J, Wu K, Wang L. Metabolomic Analysis of Environmental Biomarkers Reveals Markers of Mate Preference in Female Giant Pandas. Animals. 2025; 15(19):2873. https://doi.org/10.3390/ani15192873
Chicago/Turabian StyleFeng, Yongyou, Jing Ke, Xiangming Huang, Maohua Wang, Mingxi Li, Jingchao Lan, Kongju Wu, and Linjie Wang. 2025. "Metabolomic Analysis of Environmental Biomarkers Reveals Markers of Mate Preference in Female Giant Pandas" Animals 15, no. 19: 2873. https://doi.org/10.3390/ani15192873
APA StyleFeng, Y., Ke, J., Huang, X., Wang, M., Li, M., Lan, J., Wu, K., & Wang, L. (2025). Metabolomic Analysis of Environmental Biomarkers Reveals Markers of Mate Preference in Female Giant Pandas. Animals, 15(19), 2873. https://doi.org/10.3390/ani15192873




