Wnt/β-Catenin Pathway Activation Confers Fumonisin B1 Tolerance in Chicken Intestinal Organoid Monolayers by Enhancing Intestinal Stem Cell Function
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Viability Assay
2.2. Isolation and Culture of Chicken Intestinal Organoids
2.3. Hematoxylin-Eosin Staining of Chicken Intestinal Organoids
2.4. Indirect Immunofluorescence
2.5. Development of 2D Chicken Intestinal Organoid Monolayer
2.6. Scanning Electron Microscopy
2.7. EdU Fluorescence Assay
2.8. Transepithelial Electrical Resistance
2.9. RNA Extraction and RT-qPCR
2.10. Statistical Analysis
3. Results
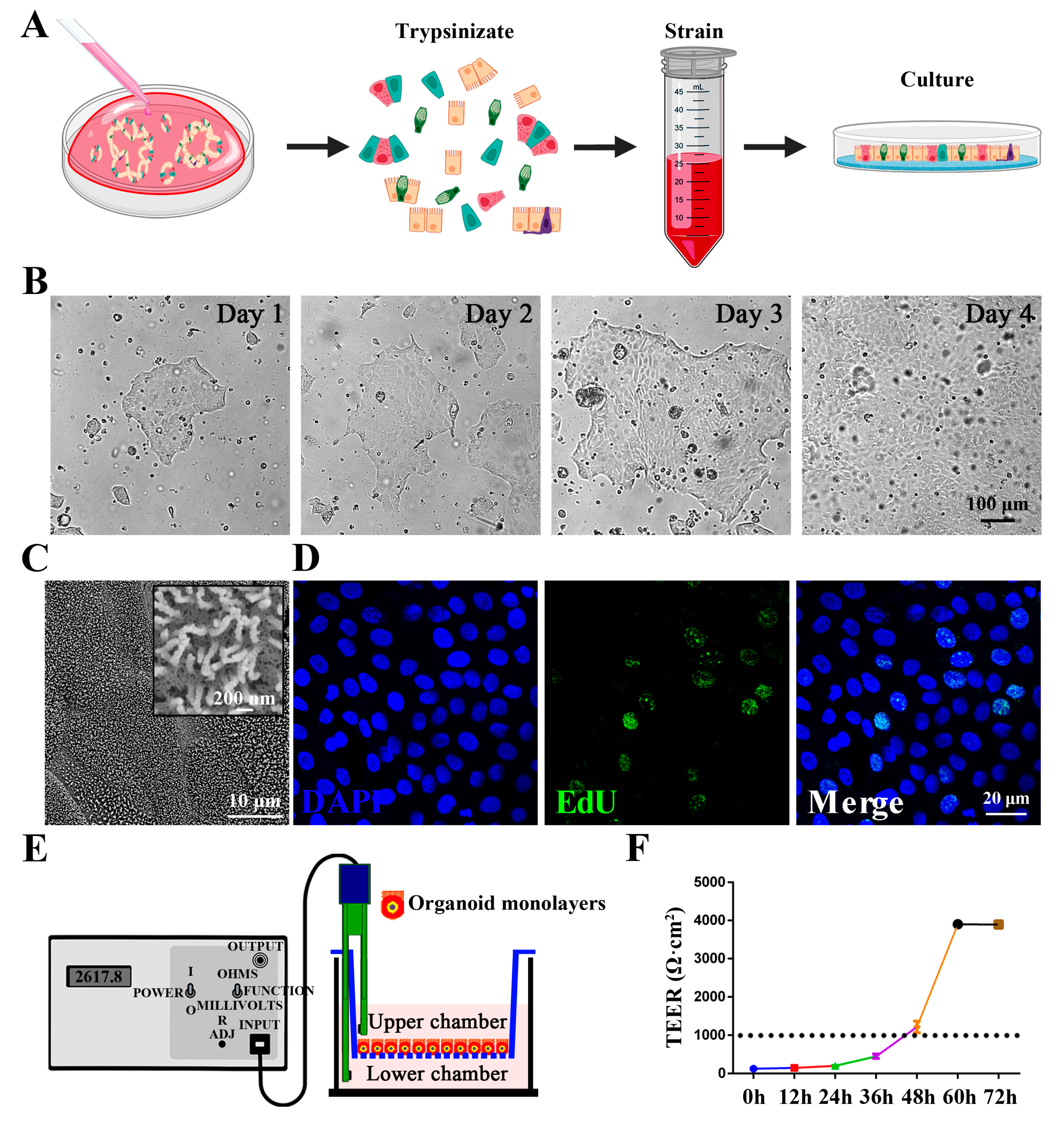
3.1. Isolation and Identification of Chicken 3D Intestinal Organoids
3.2. Establishment and Characterization of Chicken Intestinal Organoid Monolayers
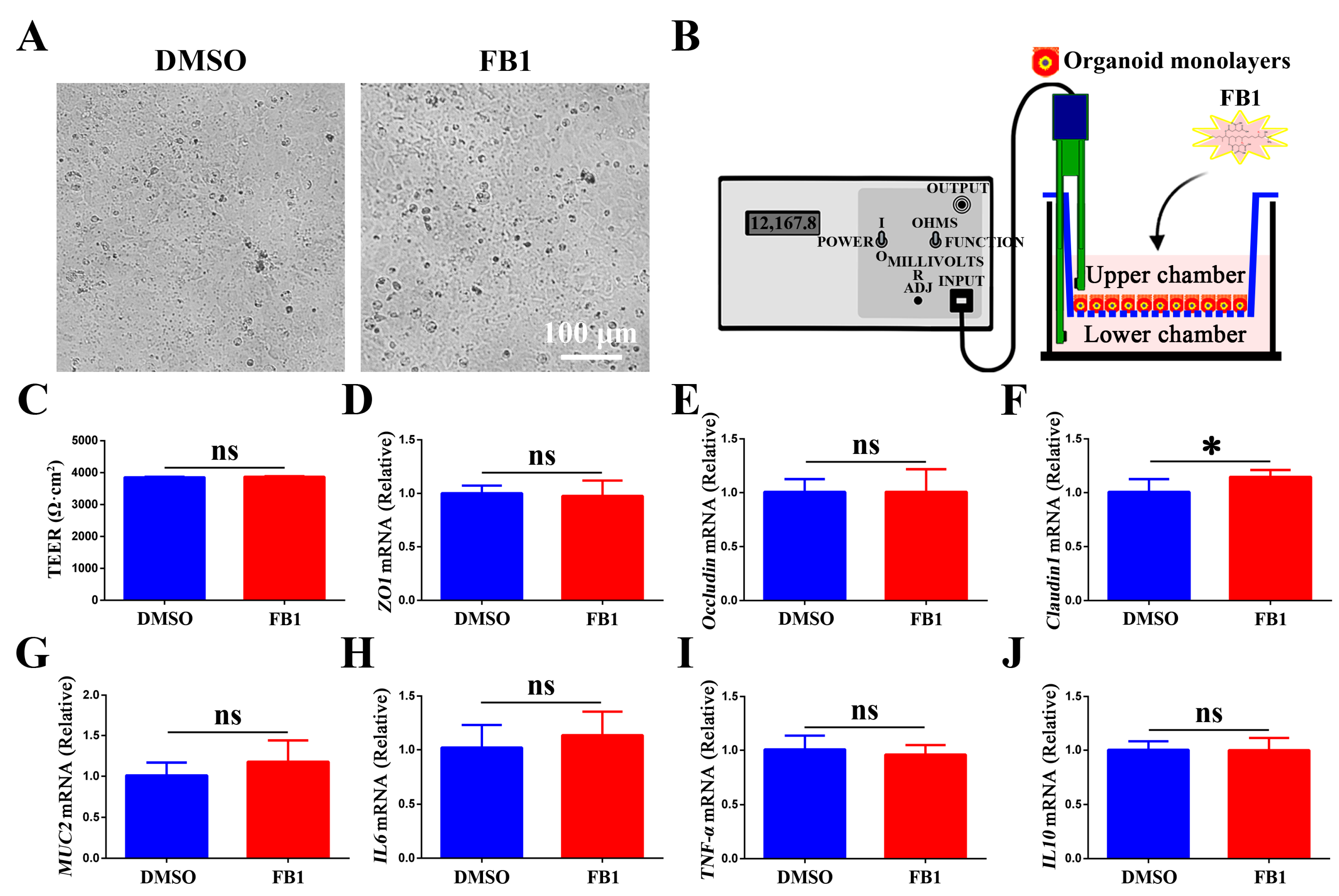
3.3. Effect of FB1 on the Tight Junction Function and Inflammatory Response in Chicken Intestinal Organoid Monolayers
3.4. FB1 Activates the Canonical Wnt/β-Catenin Signaling in Chicken Intestinal Organoid Monolayers
3.5. Wnt/β-Catenin Signaling Promotes FB1-Induced Renewal and Regeneration of Chicken Intestinal Epithelium In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rheeder, J.P.; Marasas, W.F.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F.; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, e05242. [Google Scholar] [CrossRef] [PubMed]
- Keawmanee, P.; Rattanakreetakul, C.; Pongpisutta, R. Microbial Reduction of Fumonisin B1 by the New Isolate Serratia marcescens 329-2. Toxins 2021, 13, 638. [Google Scholar] [CrossRef] [PubMed]
- Lessard, M.; Boudry, G.; Sève, B.; Oswald, I.P.; Lallès, J.-P. Intestinal physiology and peptidase activity in male pigs are modulated by consumption of corn culture extracts containing fumonisins. J. Nutr. 2009, 139, 1303–1307. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Bandyopadhyay, R.; Sulyok, M.; Warth, B.; Krska, R. Fungal and bacterial metabolites in commercial poultry feed from Nigeria. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1288–1299. [Google Scholar]
- Grenier, B.; Schwartz-Zimmermann, H.E.; Caha, S.; Moll, W.D.; Schatzmayr, G.; Applegate, T.J. Dose-dependent effects on sphingoid bases and cytokines in chickens fed diets prepared with fusarium verticillioides culture material containing fumonisins. Toxins 2015, 7, 1253–1272. [Google Scholar] [CrossRef]
- Grenier, B.; Applegate, T. Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef]
- Gao, Z.; Luo, K.; Zhu, Q.; Peng, J.; Liu, C.; Wang, X.; Li, S.; Zhang, H. The natural occurrence, toxicity mechanisms and management strategies of Fumonisin B1: A review. Environ. Pollut. 2023, 320, 121065. [Google Scholar] [PubMed]
- Gu, M.J.; Han, S.E.; Hwang, K.; Mayer, E.; Reisinger, N.; Schatzmayr, D.; Park, B.C.; Han, S.H.; Yun, C.H. Hydrolyzed fumonisin B(1) induces less inflammatory responses than fumonisin B(1) in the co-culture model of porcine intestinal epithelial and immune cells. Toxicol. Lett. 2019, 305, 110–116. [Google Scholar]
- Wang, T.; Lei, H.; Zhou, L.; Tang, M.; Liu, Q.; Long, F.; Li, Q.; Su, J. Effect of fumonisin B1 on proliferation and apoptosis of intestinal porcine epithelial cells. Toxins 2022, 14, 471. [Google Scholar] [CrossRef]
- De la Torre, D.I.; Nuñez, L.F.; Astolfi-Ferreira, C.S.; Piantino Ferreira, A.J. Enteric Virus Diversity Examined by Molecular Methods in Brazilian Poultry Flocks. Vet. Sci. 2018, 5, 38. [Google Scholar] [CrossRef]
- Bouhet, S.; Oswald, I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007, 51, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Endo, M.; Kawane, K.; Kadomatsu, T.; Oike, Y. ANGPTL2 expression in the intestinal stem cell niche controls epithelial regeneration and homeostasis. EMBO J. 2017, 36, e201695690. [Google Scholar] [CrossRef]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19. [Google Scholar] [CrossRef]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef]
- Tian, H.; Biehs, B.; Chiu, C.; Siebel, C.W.; Wu, Y.; Costa, M.; de Sauvage, F.J.; Klein, O.D. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep. 2015, 11, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Flier, L.G.V.D.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Zhao, S.; Lv, Y.; Yu, Q. Salmonella infection induced intestinal crypt hyperplasia through Wnt/β-catenin pathway in chicken. Res. Vet. Sci. 2020, 130, 179–183. [Google Scholar] [CrossRef]
- Ding, X.; Tang, R.; Zhao, J.; Xu, Y.; Fu, A.; Zhan, X. Lactobacillus reuteri alleviates LPS-induced intestinal mucosal damage by stimulating the expansion of intestinal stem cells via activation of the Wnt/β-catenin signaling pathway in broilers. Poult. Sci. 2024, 103, 104072. [Google Scholar] [CrossRef]
- Lahjouji, T.; Bertaccini, A.; Neves, M.; Puel, S.; Oswald, I.P.; Soler, L. Acute Exposure to Zearalenone Disturbs Intestinal Homeostasis by Modulating the Wnt/β-Catenin Signaling Pathway. Toxins 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.N.; Kim, M.J.; Jung, Y.S.; Lien, E.M.; Jun, S.; Park, J.I. Quiescence Exit of Tert+ Stem Cells by Wnt/β-Catenin Is Indispensable for Intestinal Regeneration. Cell Rep. 2017, 21, 2571–2584. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Fuerer, C.; Ching, W.; Harnish, K.; Logan, C.; Zeng, A.; ten Berge, D.; Kalani, Y. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Blanc, F.; Cherbuy, C.; Egidy, G.; Giuffra, E.; Lacroix-Lamandé, S.; Wiedemann, A. Intestinal organoids in farm animals. Vet. Res. 2021, 52, 33. [Google Scholar] [CrossRef]
- Mitchell, J.; Sutton, K.; Elango, J.N.; Borowska, D.; Perry, F.; Lahaye, L.; Santin, E.; Arsenault, R.J.; Vervelde, L. Chicken intestinal organoids: A novel method to measure the mode of action of feed additives. Front. Immunol. 2024, 15, 1368545. [Google Scholar] [CrossRef]
- Kang, T.H.; Lee, S.I. Establishment of a chicken intestinal organoid culture system to assess deoxynivalenol-induced damage of the intestinal barrier function. J. Anim. Sci. Biotechnol. 2024, 15, 30. [Google Scholar] [CrossRef]
- Nash, T.J.; Morris, K.M.; Mabbott, N.A.; Vervelde, L. Temporal transcriptome profiling of floating apical out chicken enteroids suggest stability and reproducibility. Vet. Res. 2023, 54, 12. [Google Scholar] [CrossRef]
- Li, Y.; Yang, N.; Chen, J.; Huang, X.; Zhang, N.; Yang, S.; Liu, G.; Liu, G. Next-Generation Porcine Intestinal Organoids: An Apical-Out Organoid Model for Swine Enteric Virus Infection and Immune Response Investigations. J. Virol. 2020, 94, e01006–e01020. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Hou, Y.; Huang, Y.; Cai, J.; Wang, G.; Cao, Y.; Chen, Z.; Fang, X.; Bao, W. Porcine Deltacoronavirus Infection Disrupts the Intestinal Mucosal Barrier and Inhibits Intestinal Stem Cell Differentiation to Goblet Cells via the Notch Signaling Pathway. J. Virol. 2023, 97, e0068923. [Google Scholar] [CrossRef]
- Goto, Y.; Kiyono, H. Epithelial barrier: An interface for the cross-communication between gut flora and immune system. Immunol. Rev. 2012, 245, 147–163. [Google Scholar] [CrossRef]
- Pan, M.; Barua, N.; Ip, M. Mucin-degrading gut commensals isolated from healthy faecal donor suppress intestinal epithelial inflammation and regulate tight junction barrier function. Front. Immunol. 2022, 13, 1021094. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Pan, H.; Zhang, Y.; Zhang, Y.; Zhu, Q.; Hong, Y.; Huang, Z.; Li, Y.; Feng, X.; Fang, Y. GelNB molecular coating as a biophysical barrier to isolate intestinal irritating metabolites and regulate intestinal microbial homeostasis in the treatment of inflammatory bowel disease. Bioact. Mater. 2023, 19, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Vandussen, K.L.; Sawey, E.T.; Wade, A.W.; Kasper, C.; Rakshit, S.; Bhatt, R.G.; Stoeck, A.; Maillard, I.; Crawford, H.C. ADAM10 Regulates Notch Function in Intestinal Stem Cells of Mice. Gastroenterology 2014, 147, 822–834.e813. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Lessard, M.; Oswald, I.P.; David, J.C. Consumption of fumonisin B1 for 9 days induces stress proteins along the gastrointestinal tract of pigs. Toxicon 2010, 55, 244–249. [Google Scholar] [CrossRef]
- Antonissen, G.; Croubels, S.; Pasmans, F.; Ducatelle, R.; Eeckhaut, V.; Devreese, M.; Verlinden, M.; Haesebrouck, F.; Eeckhout, M.; De Saeger, S.; et al. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet. Res. 2015, 46, 98. [Google Scholar] [CrossRef]
- Dang, H.A.; Zsolnai, A.; Kovács, M.; Bors, I.; Szabó-Fodor, J. In vitro Interaction between Fumonisin B1 and the Intestinal Microflora of Pigs. Pol. J. Microbiol. 2017, 66, 245–250. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Lessard, M.; Boudry, G.L. Intestinal barrier function is modulated by short-term exposure to fumonisin B1 in Ussing chambers. Vet. Res. Commun. 2009, 33, 1039–1043. [Google Scholar] [CrossRef]
- Sandrine, B.; Edith, H.; Nicolas, L.; Asmaa, F.; Stéphanie, M.; Marianna, R.; Pierre, G.; Elena, M.; Oswald, I.P. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar]
- Bouhet, S.; Dorze, E.L.; Peres, S.; Fairbrother, J.M.; Oswald, I.P. Mycotoxin fumonisin B1 selectively down-regulates the basal IL-8 expression in pig intestine: In vivo and in vitro studies. Food Chem. Toxicol. 2006, 44, 1768–1773. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Dobrowolski, P.; Donaldson, J.; Świetlicka, I.; Puzio, I.; Kamiński, D.; Wiącek, D.; Kushnir, V.; Brezvyn, O. Changes in the intestinal histomorphometry, the expression of intestinal tight junction proteins, and the bone structure and liver of pre-laying hens following oral administration of fumonisins for 21 days. Toxins 2021, 13, 375. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.H.; Keum, M.C.; Han, E.; An, B.K.; Chang, H.H.; Choi, Y.H.; Moon, B.H.; Lee, K.W. Effects of fumonisin B1 and mycotoxin binders on growth performance, tibia characteristics, gut physiology, and stress indicators in broiler chickens raised in different stocking densities. Poult. Sci. 2018, 97, 845–854. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.; Martínez-Larra?aga, M.-R.; Anadón, A.; Martínez, M.-A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef]
- Gratz, S.; Wu, Q.K.; El-Nezami, H.; Juvonen, R.O.; Mykkanen, H.; Turner, P.C. Lactobacillus rhamnosus Strain GG Reduces Aflatoxin B1 Transport, Metabolism, and Toxicity in Caco-2 Cells. Appl. Environ. Microbiol. 2007, 73, 3958. [Google Scholar] [CrossRef] [PubMed]
- Rosario, G.S.; Latorre, J.D.; Bielke, L.R.; Kuttappan, V.A.; Wolfenden, A.D.; Xochitl, H.V.; Ruben, M.G.; Vicente, J.L.; Annie, D.; David, C. Leaky Gut and Mycotoxins: Aflatoxin B1 Does Not Increase Gut Permeability in Broiler Chickens. Front. Vet. Sci. 2016, 3, 10. [Google Scholar]
- Dopavogui, L.; Polizzi, A.; Fougerat, A.; Gourbeyre, P.; Terciolo, C.; Klement, W.; Pinton, P.; Laffite, J.; Cossalter, A.M.; Bailly, J.D.; et al. Tissular Genomic Responses to Oral FB1 Exposure in Pigs. Toxins 2022, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Duff, J.; Bardi, I.; Zabielska, M.; Atanur, S.S.; Jabbour, R.J.; Simon, A.; Tomas, A.; Smolenski, R.T.; Harding, S.E.; et al. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat. Commun. 2019, 10, 2168. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Huo, S.; Du, J.; Zhang, J.; Song, M.; Cui, Y.; Li, Y. Correction to “T-2 Toxin Induces Kidney Fibrosis via the mtROS-NLRP3-Wnt/β-Catenin Axis”. J. Agric. Food Chem. 2024, 72, 3. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Z.; Sheppard, A. Both canonical and noncanonical Wnt signalling may be required for detoxification following ETP class mycotoxin exposure. Toxicol. Lett. 2017, 271, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Georgess, D.; Huso, T.; Cope, L.; Belton, A.; Chang, Y.-T.; Kuang, W.; Gu, Q.; Zhang, X.; Senger, S. HMGA1 amplifies Wnt signalling and expands the intestinal stem cell compartment and Paneth cell niche. Nat. Commun. 2017, 8, 15008. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2023, 24, 45–62. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Cao, Y.; Shan, Y.; Zhang, X.; Xia, L.; Wang, H.; Wu, S.; Bao, W. Wnt/β-Catenin Pathway Activation Confers Fumonisin B1 Tolerance in Chicken Intestinal Organoid Monolayers by Enhancing Intestinal Stem Cell Function. Animals 2025, 15, 2850. https://doi.org/10.3390/ani15192850
Zhang S, Cao Y, Shan Y, Zhang X, Xia L, Wang H, Wu S, Bao W. Wnt/β-Catenin Pathway Activation Confers Fumonisin B1 Tolerance in Chicken Intestinal Organoid Monolayers by Enhancing Intestinal Stem Cell Function. Animals. 2025; 15(19):2850. https://doi.org/10.3390/ani15192850
Chicago/Turabian StyleZhang, Shuai, Yanan Cao, Yiyi Shan, Xueli Zhang, Liangxing Xia, Haifei Wang, Shenglong Wu, and Wenbin Bao. 2025. "Wnt/β-Catenin Pathway Activation Confers Fumonisin B1 Tolerance in Chicken Intestinal Organoid Monolayers by Enhancing Intestinal Stem Cell Function" Animals 15, no. 19: 2850. https://doi.org/10.3390/ani15192850
APA StyleZhang, S., Cao, Y., Shan, Y., Zhang, X., Xia, L., Wang, H., Wu, S., & Bao, W. (2025). Wnt/β-Catenin Pathway Activation Confers Fumonisin B1 Tolerance in Chicken Intestinal Organoid Monolayers by Enhancing Intestinal Stem Cell Function. Animals, 15(19), 2850. https://doi.org/10.3390/ani15192850






