Habitat Shifts in the Pacific Saury (Cololabis saira) Population in the High Seas of the North Pacific Under Medium-to-Long-Term Climate Scenarios Based on Vessel Position Data and Ensemble Species Distribution Models
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.1.1. Vessel Position Data
2.1.2. Fishing Activity Identification
2.1.3. Presence Data Spatial Thinning
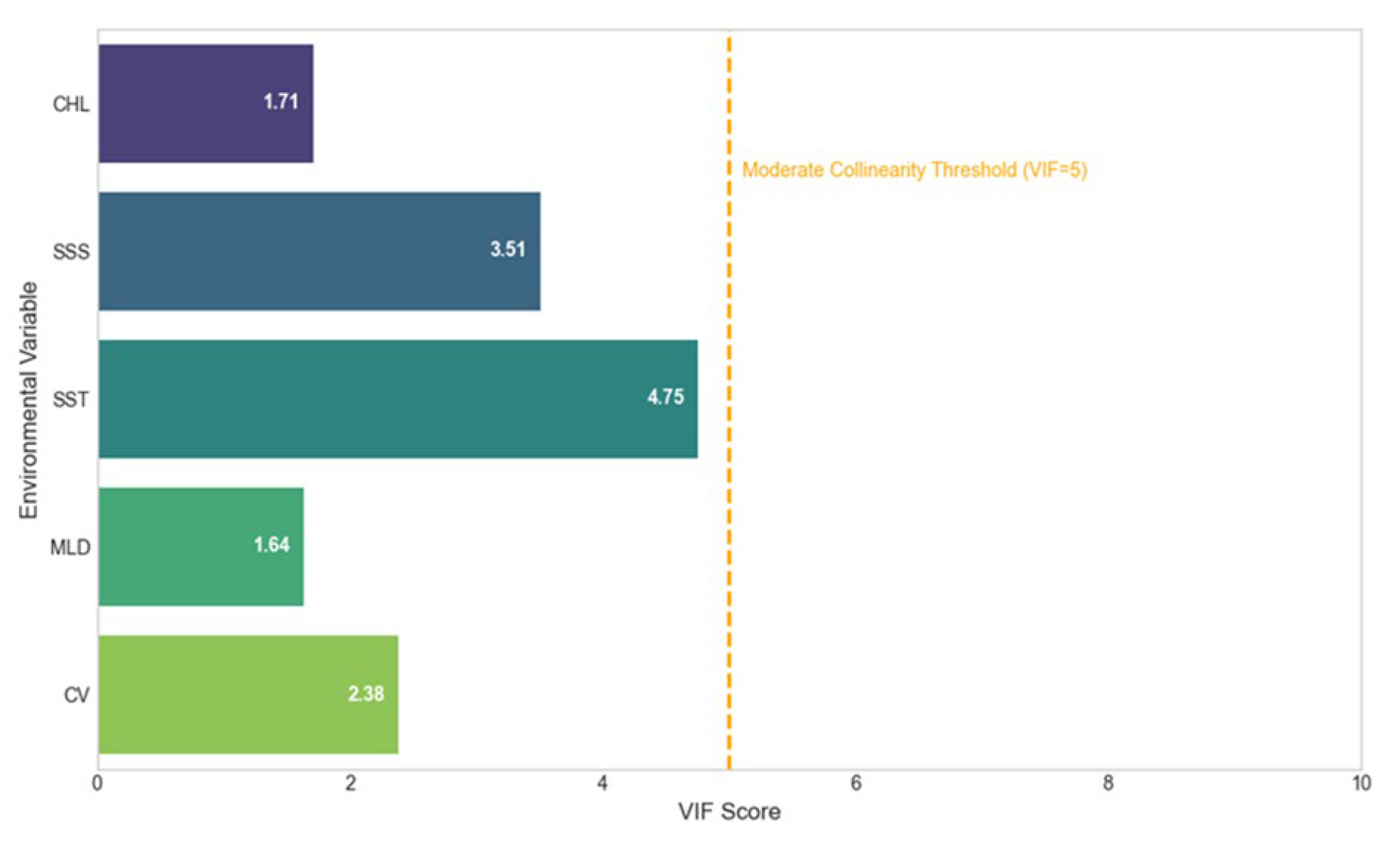
2.1.4. Environmental Variables
2.1.5. Harmonization of Environmental Data
2.2. Ensemble Habitat Suitability Model
2.2.1. Integrated Species Distribution Model Framework
2.2.2. Ensemble Model Construction and Selection
3. Results
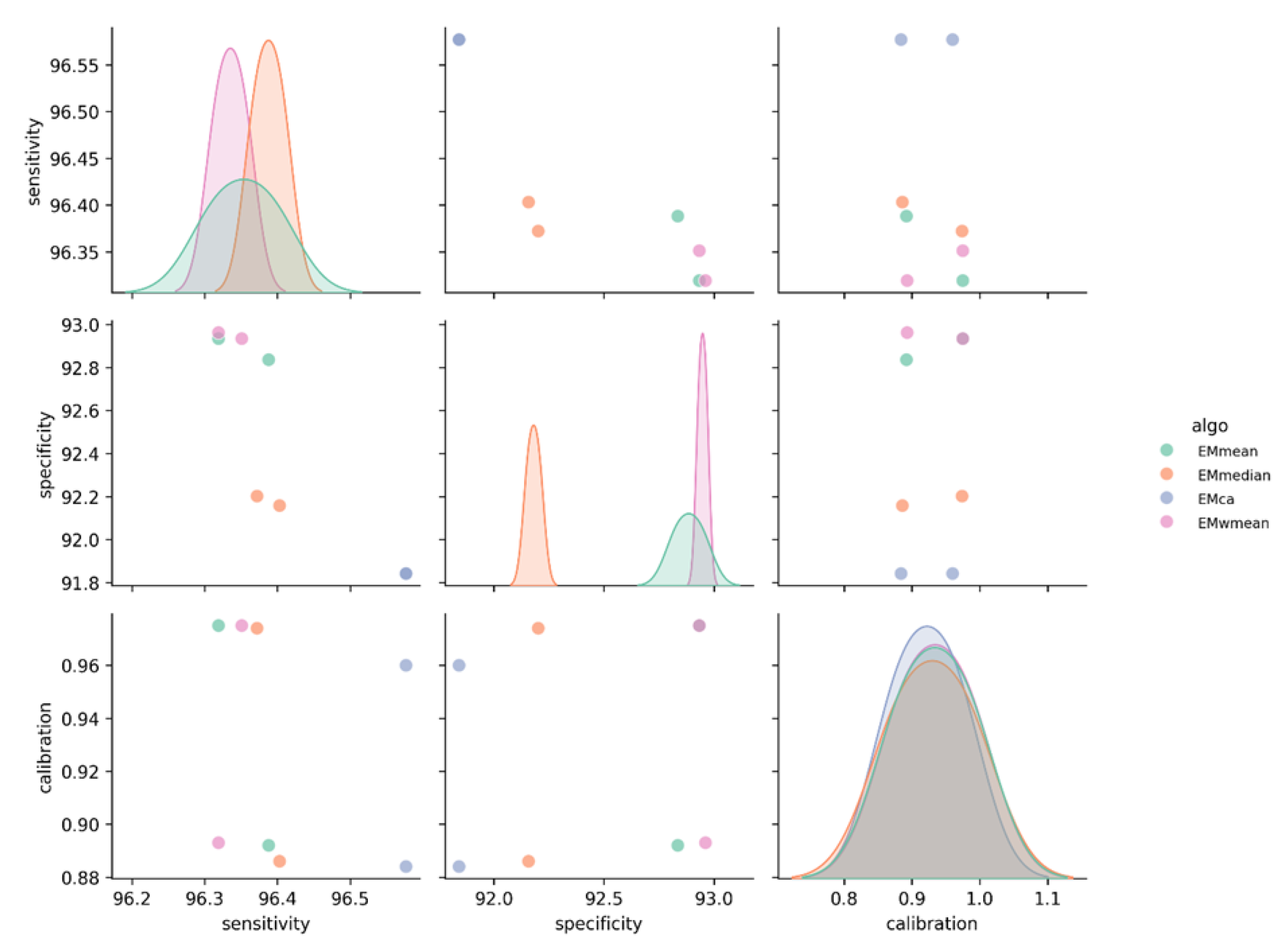
3.1. Model Performance
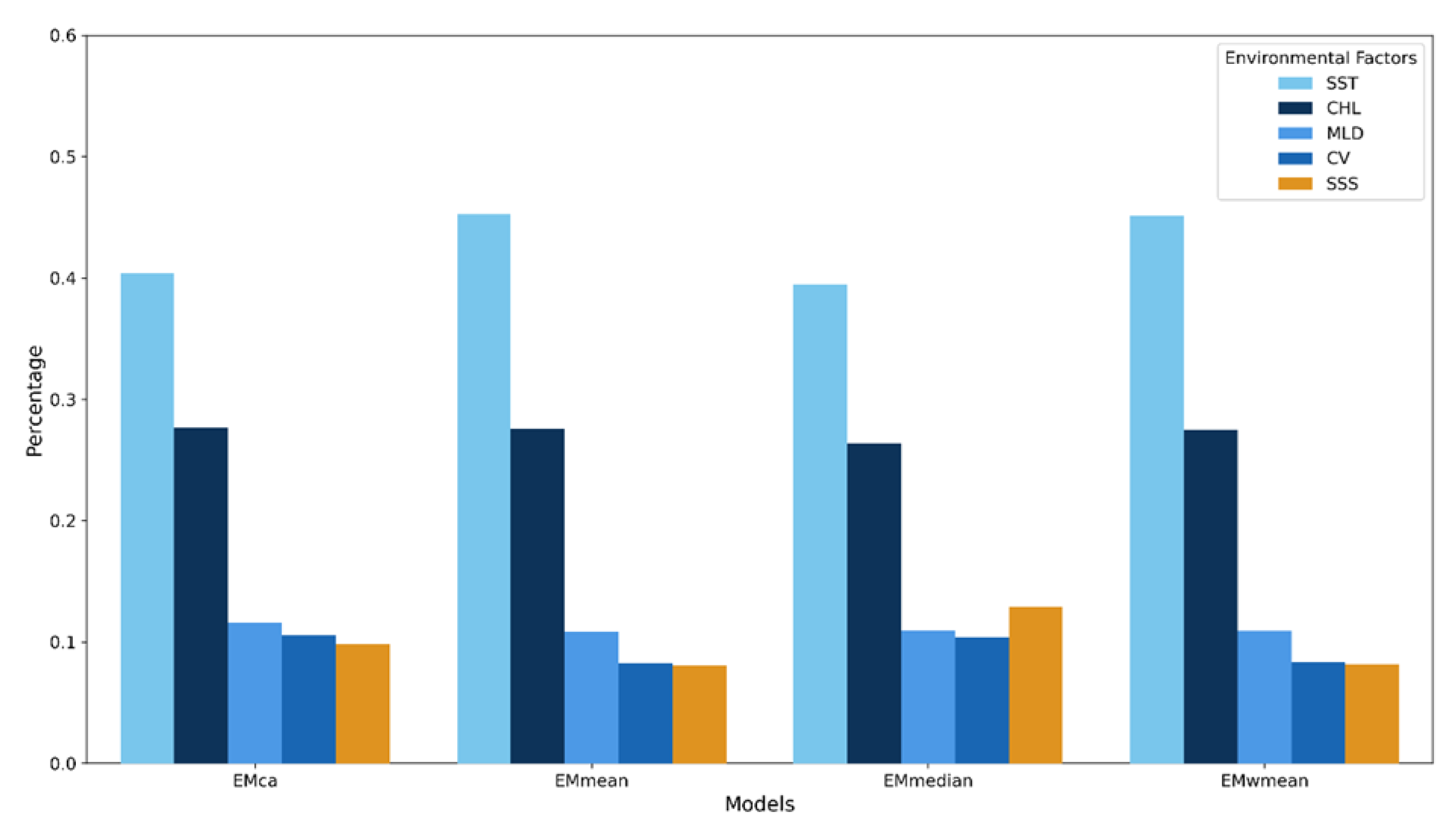
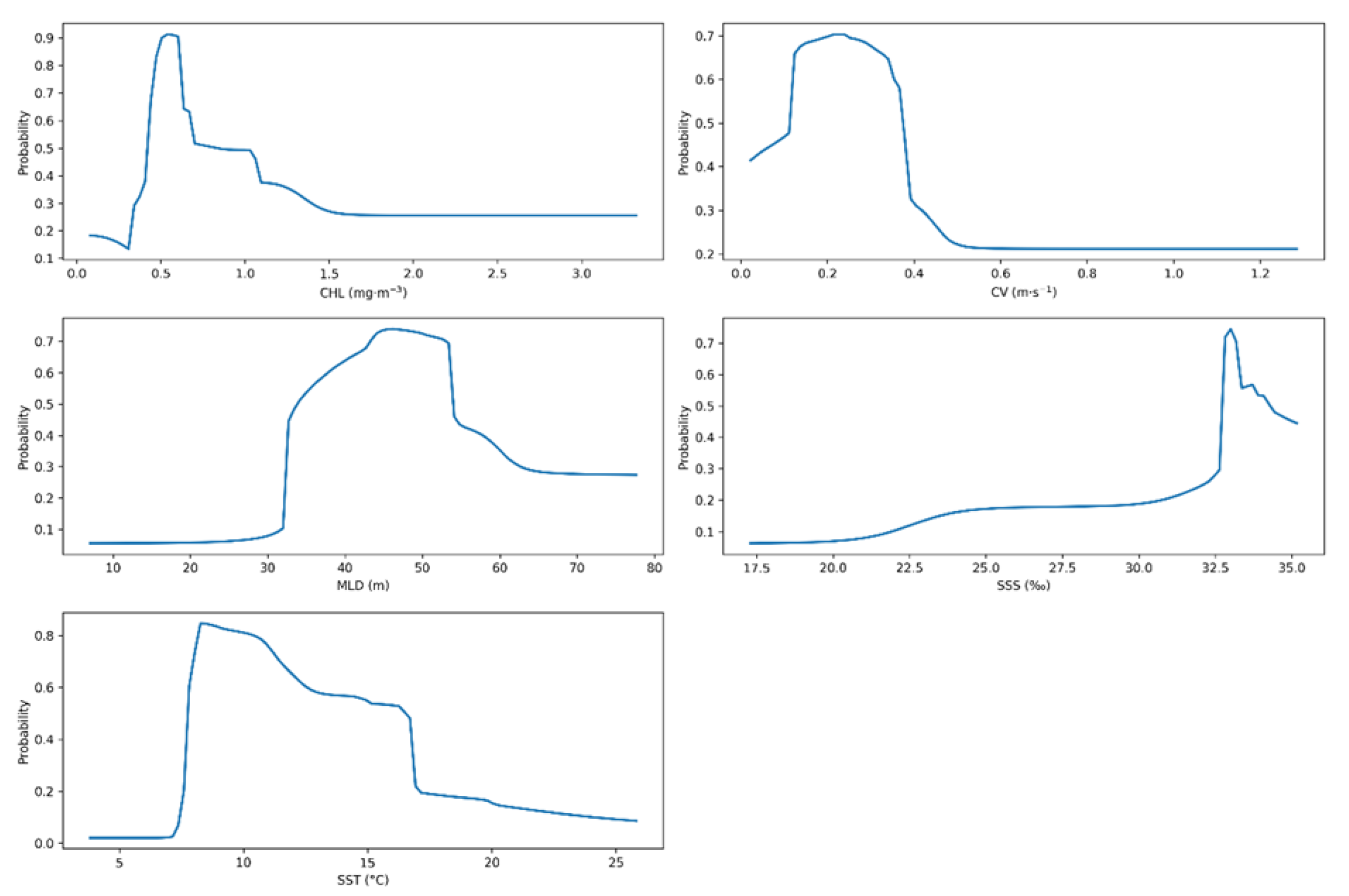
3.2. Environmental Factor Contributions
3.3. Habitat Distribution Prediction
4. Discussion
4.1. Model Robustness and Interpretation of Ecological Drivers
4.2. Future Spatiotemporal Habitat Shifts and Their Ecological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavole, L.M.; Demko, A.M.; Diner, R.E.; Giddings, A.; Koester, I.; Pagniello, C.M.; Paulsen, M.-L.; Ramirez-Valdez, A.; Schwenck, S.M.; Yen, N.K. Biological impacts of the 2013–2015 warm-water anomaly in the Northeast Pacific: Winners, losers, and the future. Oceanography 2016, 29, 273–285. [Google Scholar] [CrossRef]
- Gomes, D.G.; Ruzicka, J.J.; Crozier, L.G.; Huff, D.D.; Brodeur, R.D.; Stewart, J.D. Marine heatwaves disrupt ecosystem structure and function via altered food webs and energy flux. Nat. Commun. 2024, 15, 1988. [Google Scholar] [CrossRef]
- Barkhordarian, A.; Nielsen, D.M.; Baehr, J. Recent marine heatwaves in the North Pacific warming pool can be attributed to rising atmospheric levels of greenhouse gases. Commun. Earth Environ. 2022, 3, 131. [Google Scholar] [CrossRef]
- Buonanduci, M.S.; Buhle, E.R.; Case, M.J.; Howe, E.R.; Robertson, J.C.; Vanbuskirk, N.; Ettinger, A.K. Forest restoration can bolster salmon population persistence under climate change. Biol. Conserv. 2025, 305, 111099. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Worm, B.; Fogarty, M.J.; Sarmiento, J.L.; Levin, S.A. Marine taxa track local climate velocities. Science 2013, 341, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Han, S.; Fan, W. Saury resource and fishing grounds in the Northwest Pacific. Mar. Fish. 2004, 1, 61–65. [Google Scholar] [CrossRef]
- Chang, Y.J.; Lan, K.W.; Walsh, W.A.; Hsu, J.; Hsieh, C.H. Modelling the impacts of environmental variation on habitat suitability for Pacific saury in the Northwestern Pacific Ocean. Fish. Oceanogr. 2019, 28, 291–304. [Google Scholar] [CrossRef]
- Hua, C.; Zhu, Q.; Xu, W.; Song, L. Review of the life history, resources and fishing grounds of the Pacific saury in the North Pacific Ocean. J. Fish. Sci. China 2019, 26, 811–821. [Google Scholar]
- Xiang, D.; Sun, Y.; Zhu, H.; Wang, J.; Huang, S.; Zhang, S.; Zhang, F.; Zhang, H. Comparative Analysis of Prediction Models for Trawling Grounds of the Argentine Shortfin Squid Illex argentinus in the Southwest Atlantic High Seas Based on Vessel Position and Fishing Log Data. Biology 2025, 14, 35. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Zhu, H.; Xiang, D.; Wang, J.; Zhang, F.; Huang, S.; Li, Y. Simulation and Identification of the Habitat of Antarctic Krill Based on Vessel Position Data and Integrated Species Distribution Model: A Case Study of Pumping-Suction Beam Trawl Fishing Vessels. Animals 2025, 15, 1557. [Google Scholar] [CrossRef]
- Xue, M.; Tong, J.; Ma, W.; Zhu, Z.; Wang, W.; Lyu, S.; Chen, X. Reimagining habitat suitability modeling for Pacific saury (Cololabis saira) in the Northwest Pacific Ocean through acoustic data analysis from fishing vessels. Ecol. Inform. 2025, 85, 102971. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Han, Z. Predicting the effects of climate change on the suitable habitat of Japanese Spanish mackerel (Scomberomorus niphonius) based on the species distribution model. Front. Mar. Sci. 2022, 9, 927790. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Cai, Y.; Zhang, K.; Xu, Y.; Chen, Z.; Xu, S. Simulating Habitat Suitability Changes of Threadfin Porgy (Evynnis cardinalis) in the Northern South China Sea Using Ensemble Models Under Medium-to-Long-Term Future Climate Scenarios. Biology 2025, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Shepperson, J.L.; Hintzen, N.T.; Szostek, C.L.; Bell, E.; Murray, L.G.; Kaiser, M.J. A comparison of VMS and AIS data: The effect of data coverage and vessel position recording frequency on estimates of fishing footprints. ICES J. Mar. Sci. 2018, 75, 988–998. [Google Scholar] [CrossRef]
- Katara, I.; Silva, A. Mismatch between VMS data temporal resolution and fishing activity time scales. Fish. Res. 2017, 188, 1–5. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Huang, W.-B.; Lo, N.C.; Chiu, T.-S.; Chen, C.-S. Geographical distribution and abundance of Pacific saury, Cololabis saira (Brevoort)(Scomberesocidae), fishing stocks in the Northwestern Pacific in relation to sea temperatures. Zool. Stud. 2007, 46, 705. [Google Scholar]
- Tian, Y.; Akamine, T.; Suda, M. Variations in the abundance of Pacific saury (Cololabis saira) from the northwestern Pacific in relation to oceanic-climate changes. Fish. Res. 2003, 60, 439–454. [Google Scholar] [CrossRef]
- Tseng, C.-T.; Su, N.-J.; Sun, C.-L.; Punt, A.E.; Yeh, S.-Z.; Liu, D.-C.; Su, W.-C. Spatial and temporal variability of the Pacific saury (Cololabis saira) distribution in the northwestern Pacific Ocean. ICES J. Mar. Sci. 2013, 70, 991–999. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Jiang, K.; Xiang, D.; Shi, Y.; Huang, S.; Li, Y.; Han, H. Simulating the changes of the habitats suitability of chub mackerel (Scomber japonicus) in the high seas of the North Pacific Ocean using ensemble models under medium to long-term future climate scenarios. Mar. Pollut. Bull. 2024, 207, 116873. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Sun, Y.; Xiang, D.; Wang, J.; Jiang, K.; Zhu, H.; Huang, S.; Zhang, F.; Li, Y.; Zhang, H. Comparative analysis of modeling methods and prediction accuracy for Japanese sardine habitat under three climate scenarios with differing greenhouse emission pathways. Mar. Pollut. Bull. 2025, 215, 117867. [Google Scholar] [CrossRef] [PubMed]
- Wisz, M.S.; Hijmans, R.; Li, J.; Peterson, A.T.; Graham, C.; Guisan, A.; Group, N.P.S.D.W. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Davies, S.C.; Thompson, P.L.; Gomez, C.; Nephin, J.; Knudby, A.; Park, A.E.; Friesen, S.K.; Pollock, L.J.; Rubidge, E.M.; Anderson, S.C. Addressing uncertainty when projecting marine species’ distributions under climate change. Ecography 2023, 2023, e06731. [Google Scholar] [CrossRef]
- Hua, C.; Li, F.; Zhu, Q.; Zhu, G.; Meng, L. Habitat suitability of Pacific saury (Cololabis saira) based on a yield-density model and weighted analysis. Fish. Res. 2020, 221, 105408. [Google Scholar] [CrossRef]
- Karp, M.A.; Brodie, S.; Smith, J.A.; Richerson, K.; Selden, R.L.; Liu, O.R.; Muhling, B.A.; Samhouri, J.F.; Barnett, L.A.; Hazen, E.L. Projecting species distributions using fishery-dependent data. Fish Fish. 2023, 24, 71–92. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Farrell, A.P. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef]
- Chassot, E.; Bonhommeau, S.; Dulvy, N.K.; Mélin, F.; Watson, R.; Gascuel, D.; Le Pape, O. Global marine primary production constrains fisheries catches. Ecol. Lett. 2010, 13, 495–505. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Knust, R. Climate Change Affects Marine Fishes Through the Oxygen Limitation of Thermal Tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Yang, C.; Fang, Z. Differences in habitat distribution of Sardinops melanostictus and Scomber japonicus in the northwest Pacific based on a maximum entropy model. J. Shanghai Ocean Univ. 2023, 32, 806–817. [Google Scholar] [CrossRef]
- Edwards, M.; Richardson, A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 2004, 430, 881–884. [Google Scholar] [CrossRef]
- Pecquerie, L.; Petitgas, P.; Kooijman, S.A. Modeling fish growth and reproduction in the context of the Dynamic Energy Budget theory to predict environmental impact on anchovy spawning duration. J. Sea Res. 2009, 62, 93–105. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Jusup, M.; Klanjscek, T.; Pecquerie, L. Integrating dynamic energy budget (DEB) theory with traditional bioenergetic models. J. Exp. Biol. 2012, 215, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.D.; Smith, A.B. Modeling the rarest of the rare: A comparison between multi-species distribution models, ensembles of small models, and single-species models at extremely low sample sizes. Ecography 2023, 2023, e06500. [Google Scholar] [CrossRef]
- Panzeri, D.; Russo, T.; Arneri, E.; Carlucci, R.; Cossarini, G.; Isajlović, I.; Krstulović Šifner, S.; Manfredi, C.; Masnadi, F.; Reale, M. Identifying priority areas for spatial management of mixed fisheries using ensemble of multi-species distribution models. Fish Fish. 2024, 25, 187–204. [Google Scholar] [CrossRef]
- McMillan, M.N.; Leahy, S.M.; Hillcoat, K.B.; Wickens, M.; Roberts, E.M.; Daniell, J.J. Untangling multi-species fisheries data with species distribution models. Rev. Fish Biol. Fish. 2024, 34, 1133–1148. [Google Scholar] [CrossRef]
- Rourke, M.L.; Fowler, A.M.; Hughes, J.M.; Broadhurst, M.K.; DiBattista, J.D.; Fielder, S.; Wilkes Walburn, J.; Furlan, E.M. Environmental DNA (eDNA) as a tool for assessing fish biomass: A review of approaches and future considerations for resource surveys. Environ. DNA 2022, 4, 9–33. [Google Scholar] [CrossRef]
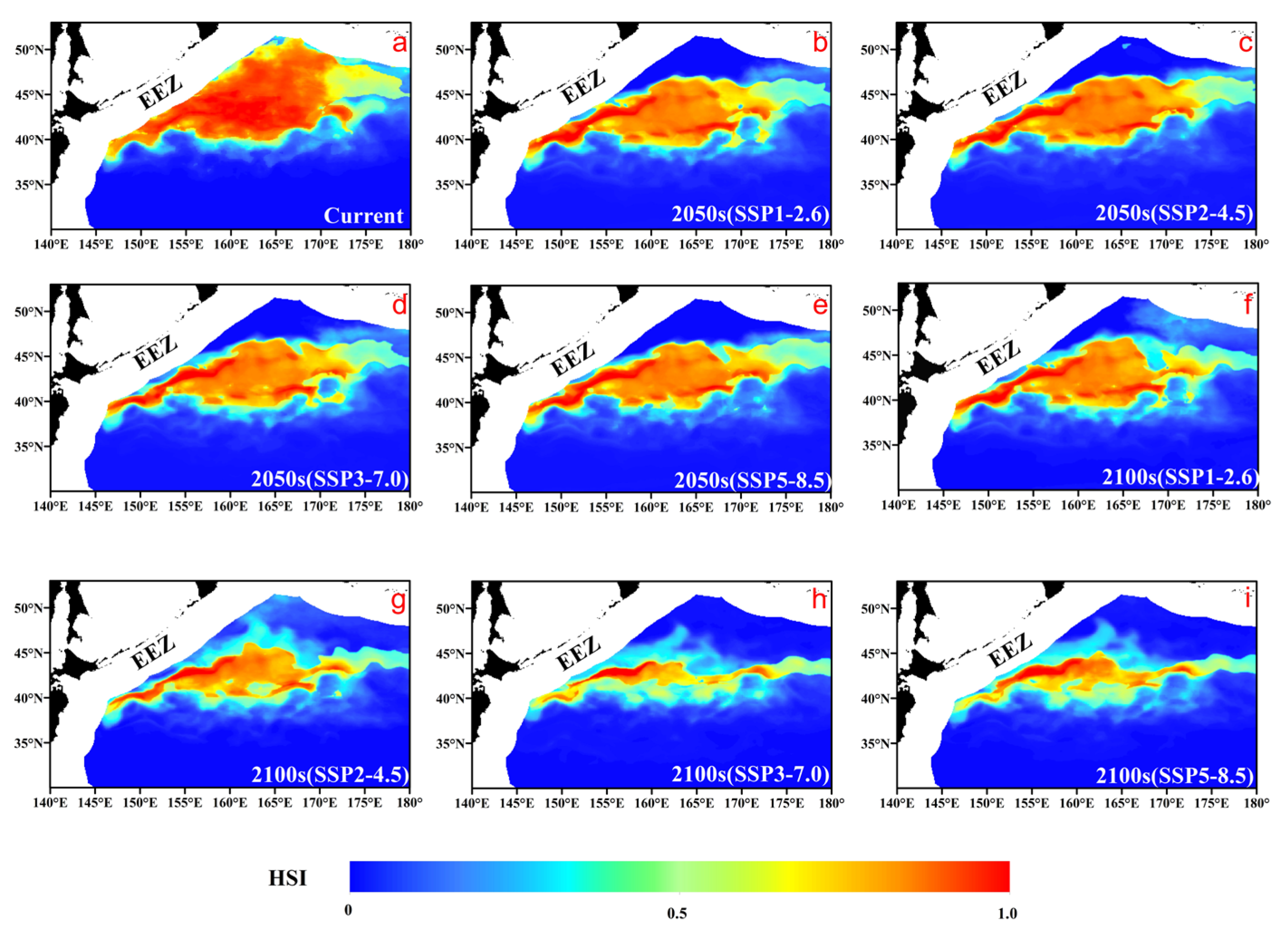
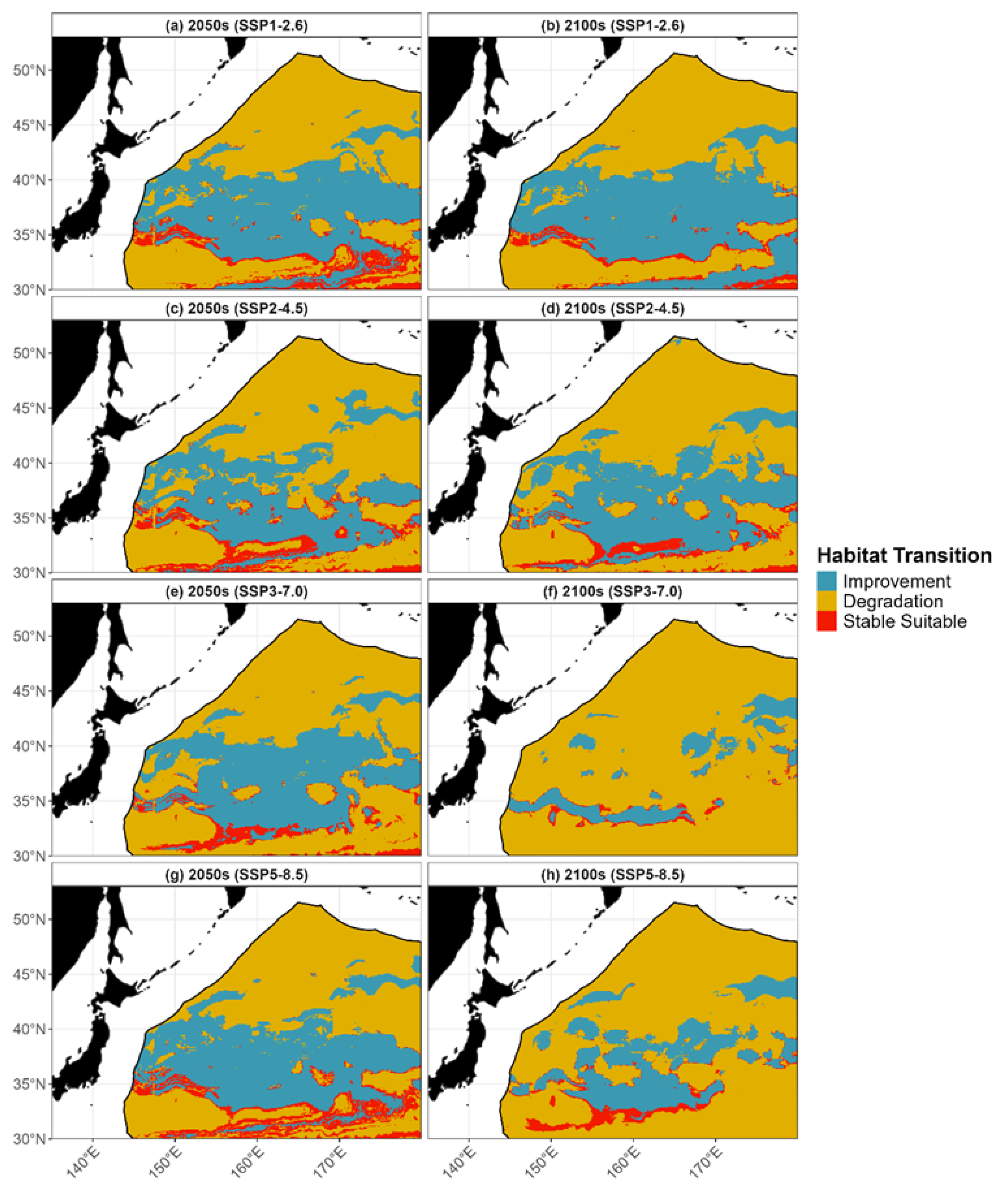
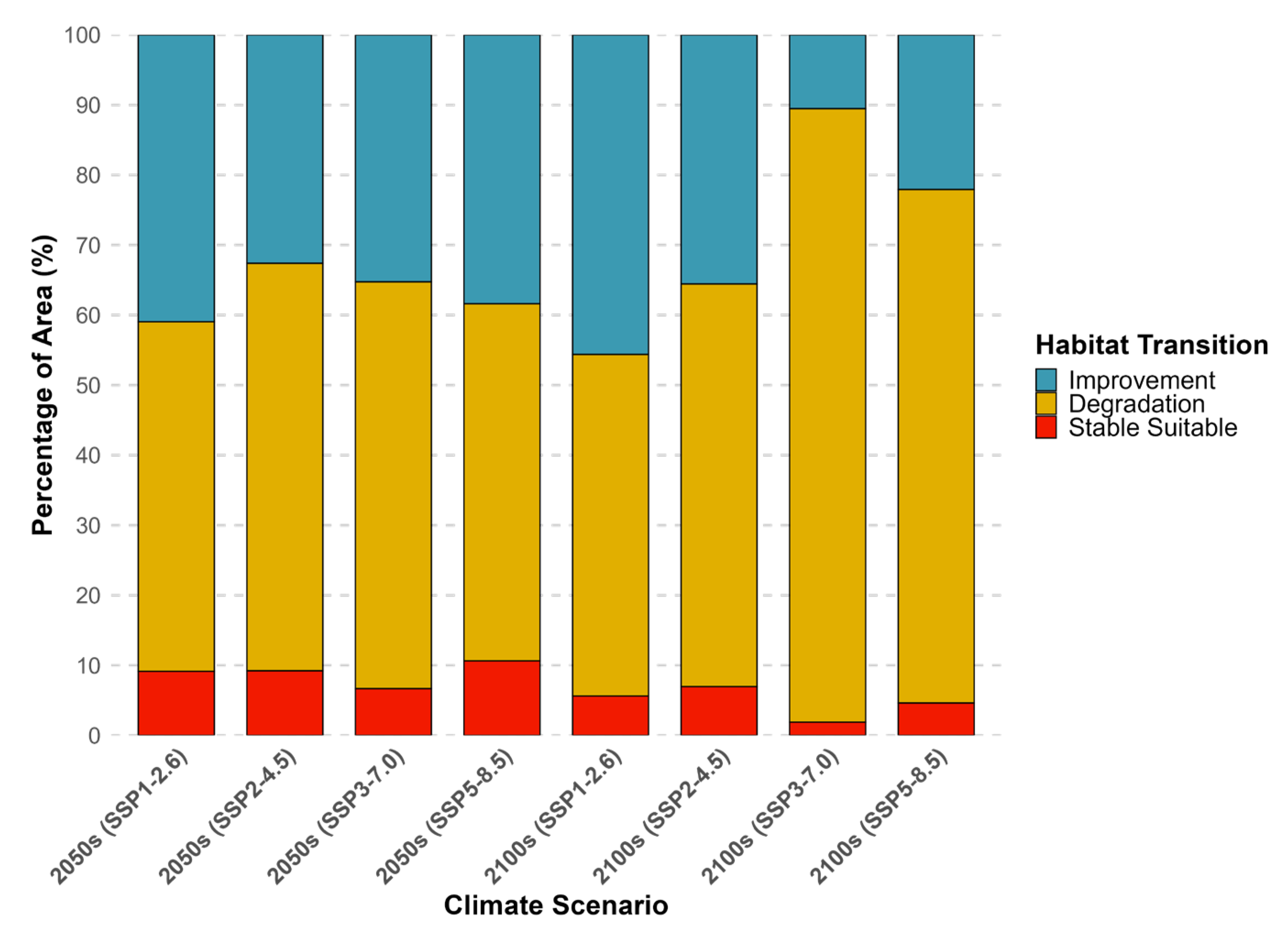
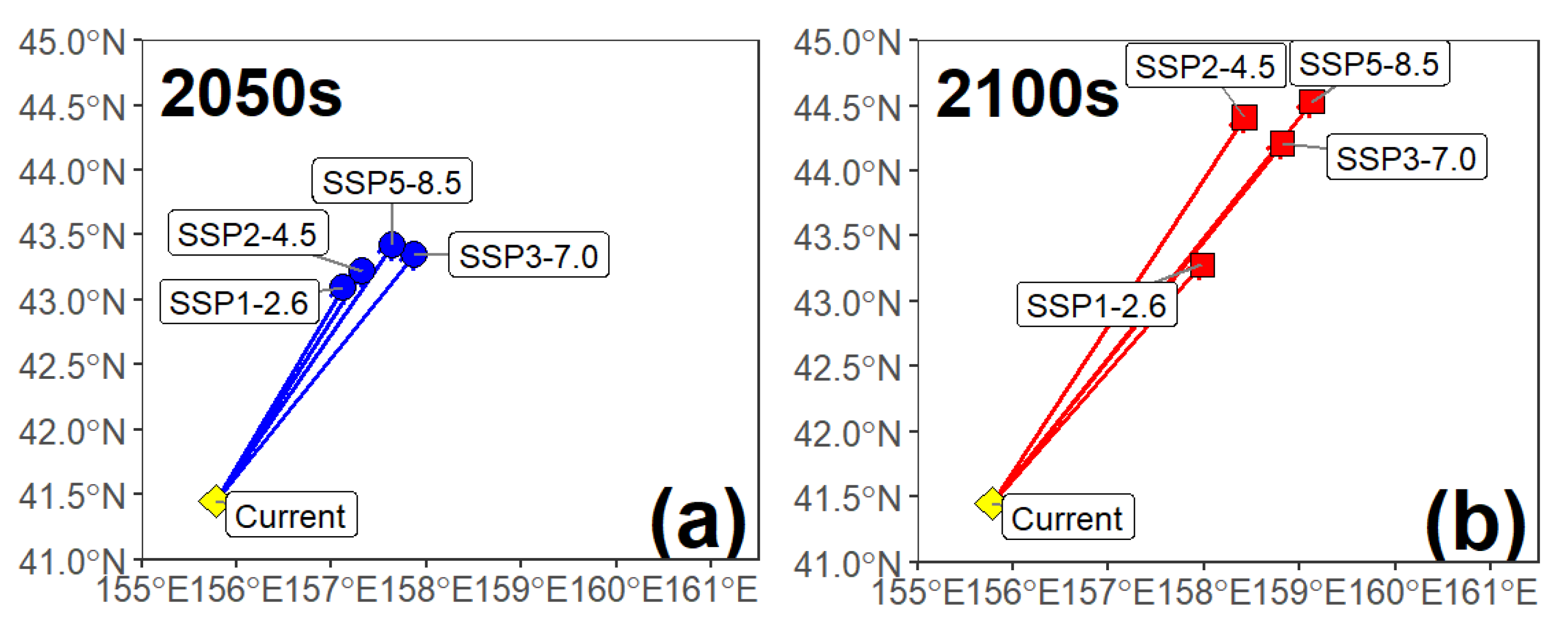
| Period and Climate Scenarios | Habitat Suitability Index (HSI) Range | |||
|---|---|---|---|---|
| Non-Suitable Areas (0–0.4) (km2) | Low Suitable Area (0.4–0.6) (km2) | Moderately Suitable Area (0.6–0.8) (km2) | Highly Suitable Area (0.8–1.0) (km2) | |
| Current | 10,894,427 | 471,397 | 360,939 | 1,200,282 |
| 2050s SSP1-2.6 | 11,386,961 | 345,528 | 465,098 | 719,241 |
| 2050s SSP2-4.5 | 11,432,190 | 343,446 | 375,633 | 765,558 |
| 2050s SSP3-7.0 | 11,528,263 | 343,727 | 467,651 | 577,186 |
| 2050s SSP5-8.5 | 11,500,569 | 376,849 | 366,425 | 672,985 |
| 2100s SSP1-2.6 | 11,566,635 | 337,221 | 388,768 | 624,203 |
| 2100s SSP2-4.5 | 11,847,665 | 412,899 | 371,439 | 284,824 |
| 2100s SSP3-7.0 | 12,213,550 | 417,520 | 218,893 | 66,865 |
| 2100s SSP5-8.5 | 11,927,266 | 538,183 | 265,671 | 185,707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Sun, Y.; Li, Y.; Xiang, D.; Gao, M.; Zhang, F.; Wang, J.; Huang, S.; Zhang, H.; Li, L. Habitat Shifts in the Pacific Saury (Cololabis saira) Population in the High Seas of the North Pacific Under Medium-to-Long-Term Climate Scenarios Based on Vessel Position Data and Ensemble Species Distribution Models. Animals 2025, 15, 2828. https://doi.org/10.3390/ani15192828
Zhu H, Sun Y, Li Y, Xiang D, Gao M, Zhang F, Wang J, Huang S, Zhang H, Li L. Habitat Shifts in the Pacific Saury (Cololabis saira) Population in the High Seas of the North Pacific Under Medium-to-Long-Term Climate Scenarios Based on Vessel Position Data and Ensemble Species Distribution Models. Animals. 2025; 15(19):2828. https://doi.org/10.3390/ani15192828
Chicago/Turabian StyleZhu, Hanji, Yuyan Sun, Yang Li, Delong Xiang, Ming Gao, Famou Zhang, Jianhua Wang, Sisi Huang, Heng Zhang, and Lingzhi Li. 2025. "Habitat Shifts in the Pacific Saury (Cololabis saira) Population in the High Seas of the North Pacific Under Medium-to-Long-Term Climate Scenarios Based on Vessel Position Data and Ensemble Species Distribution Models" Animals 15, no. 19: 2828. https://doi.org/10.3390/ani15192828
APA StyleZhu, H., Sun, Y., Li, Y., Xiang, D., Gao, M., Zhang, F., Wang, J., Huang, S., Zhang, H., & Li, L. (2025). Habitat Shifts in the Pacific Saury (Cololabis saira) Population in the High Seas of the North Pacific Under Medium-to-Long-Term Climate Scenarios Based on Vessel Position Data and Ensemble Species Distribution Models. Animals, 15(19), 2828. https://doi.org/10.3390/ani15192828






