Simple Summary
Schizothorax o’connori, Oxygymnocypris stewartii, and other schizothoracinae fish species in the Duoxiong Zangbo River—a tributary of the Yarlung Zangbo River—are confronting severe threats, including population decline. Our study provides the first comprehensive assessment of key schizothoracinae species in these waters, revealing trends of early maturation, miniaturization, and accelerated growth. Current fishing pressure exceeds sustainable limits, indicating mild overexploitation. These findings serve as an urgent ecological warning, prompting us to propose targeted management strategies to support the conservation and restoration of these fish populations.
Abstract
The Yarlung Zangbo River (With a total length of 2057 km, the river forms part of the Ganges–Brahmaputra River system), located in the core region of the Tibetan Plateau, hosts a unique yet fragile aquatic ecosystem. Fish populations inhabiting this ecosystem have been significantly impacted by external factors, leading to declining resources. This decline is particularly evident in local tributaries, including the DuoXiong Zangbo River—a main tributary where scientific research remains scarce due to its geographic remoteness. Consequently, the status of schizothoracinae in this river remains poorly understood, necessitating research on their population structure, growth characteristics, and resource dynamics, as well as the extent of external disturbances. During the 2023–2024 season, fishery surveys were conducted during two sampling periods: summer (June–July) and autumn (September–October). This study focuses on the Duoxiong Zangbo River, a tributary of the Yarlung Zangbo River, targeting three schizothoracinae fish species: Schizothorax o’connori, Oxygymnocypris stewartii, and Ptychobarbus dipogon. The results show that their body lengths ranged from 23.02 to 440.00 mm, 23.02 to 460.00 mm, and 45.18 to 418.00 mm, with body weights ranging from 0.30 to 1394.30 g, 0.20 to 1013.00 g, and 1.20 to 814.30 g. Age distributions spanned 0–14, 0–16, and 0–13 years, respectively, indicating a trend toward younger and smaller individuals. Von Bertalanffy growth modeling revealed asymptotic body lengths (L∞) of 591.233 mm, 507.557 mm, and 515.292 mm, with growth coefficients (k) of 0.098, 0.122, and 0.118, respectively. These parameters suggest that the populations are exhibiting accelerated growth strategies in response to fishing pressure. Using FiSAT II, exploitation rates (E) were calculated as 0.547, 0.758, and 0.711 for the three species, with predicted maximum sustainable exploitation rates of 0.579, 0.882, and 0.884, respectively. These findings indicate that the three schizothoracinae species have approached the threshold of overexploitation and are facing threats of overexploitation. In summary, this study demonstrates that schizothoracinae in the DuoXiong Zangbo River are experiencing adverse effects from external pressures, with populations at risk of decline. These results underscore the urgent need for targeted conservation and management strategies.
1. Introduction
The Tibetan Plateau serves as a vital ecological barrier in global geography [1,2] and represents one of the world’s highest and most complex plateaus [3,4]. Often referred to as the “Third Pole of the Earth” [1], its distinctive alpine climate system plays a pivotal role in global water vapor cycles and biodiversity maintenance [5]. However, Tibetan Plateau ecosystems are notably fragile and highly sensitive to environmental changes [6,7]. Under the dual pressures of climate change and human activities [4,8,9], significant ecological responses have emerged, including hydrological cycle disruption [10] and persistent declines in river runoff [11,12,13].
As a crucial water system on the southern Tibetan Plateau, the Yarlung Zangbo River originates from the Jema Yangzong Glacier [14]. With a total length of 2057 km, the river forms part of the Ganges–Brahmaputra River system. Its main channel and tributaries collectively form an intricate hydrological network [15], recognized as one of Asia’s most ecologically significant watersheds [16,17]. The region’s distinctive high-altitude geomorphology and climatic conditions [18,19,20] have fostered specialized aquatic organisms adapted to extreme environments [21], exhibiting unique evolutionary trajectories [22]. Notably, schizothoracinae have undergone exceptional evolutionary diversification here, developing distinct niche differentiation. Their survival strategies markedly differ from lowland fishes, including evolutionary adaptations such as slow growth [23,24], extended life cycles [25], delayed sexual maturity [26,27,28,29], and reduced fecundity [27,30]. These life-history traits result in limited resilience to environmental disturbances [31]. Furthermore, the plateau’s fragile aquatic ecosystem [32] faces compounded environmental stressors [33,34] that are highly sensitive to both climatic variability and anthropogenic impacts [4,8,35], posing severe threats to these species’ survival [31]. Consequently, research on the Yarlung Zangbo River Basin holds substantial ecological value and strategic importance.
Tributaries constitute a vital component of watershed systems, serving as primary recharge sources and playing a crucial role in maintaining ecological balance [3]. Among the Yarlung Zangbo River’s tributaries, the Lhasa and Nianchu Rivers feature open channels, elevated water temperatures [36], and gentle bank slopes [37]. However, these rivers flow through major urban centers (Lhasa and Nyingchi) [38] and face increasing pressures from water system fragmentation caused by intensive fishing, graded hydropower development, and invasive species. These anthropogenic activities have substantially reduced ecological niches and breeding habitats for indigenous fish species. Studies indicate that persistent external pressures have led to overexploitation of Schizopygopsis younghusbandi populations in the Nianchu, Lhasa, and Niyang rivers, resulting in population miniaturization and juvenilization [39]. In contrast, the Duoxiong Zangbo River tributary provides abundant habitats and biomass for indigenous fish due to its high altitude, low temperatures, sparse vegetation cover, remote location [29], and minimal human disturbance [36]. Historical records show stable populations of endemic schizothoracinae such as Oxygymnocypris stewartia (Lloyd, 1908), Ptychobarbus dipogon (Regan, 1905), and Schizothorax o’connori (Lloyd, 1908) [29,40]. However, recent regional economic development has spurred water conservancy projects [19], intensified fishing, and increased biological invasions [41], exceeding the aquatic ecosystem’s resilience threshold and causing a dramatic decline in schizothoracinae resources. These external pressures will inevitably impact the ecosystem of the Duoxiong Zangbo River waters. However, the extent of their effects on local fish stocks remains unclear. Thus, it is imperative to investigate the population structure, growth characteristics, resource dynamics, and degree of external disturbance in this region.
Fish community structure serves as a crucial biological indicator of aquatic ecosystem health [42,43]. Environmental pressures are increasingly altering community structures, jeopardizing fish survival and reproduction [44,45,46,47] and contributing to the degradation of river fishery function [45,48]. Consequently, establishing adaptive management mechanisms based on ecological carrying capacity has become imperative [29]. The effectiveness of such mechanisms relies on comprehensive understanding of key species’ population dynamics and their response to environmental stressors. Age structure, growth parameters, and mortality rates constitute fundamental elements for analyzing population dynamics and predicting resource trends [49,50,51], providing essential data for management strategies. The unique schizothoracinae fish resources in Yarlung Zangbo River tributaries, particularly S. o’connori, O. stewartii, and P. dipogon, hold particular ecological and resource management significance [52]. This study hypothesizes significant alterations in these species’ population structures and growth characteristics, with observed increases in total mortality primarily attributable to intensive anthropogenic activities (especially fishing pressure) that substantially outweigh natural mortality. To test these hypotheses and establish baseline ecological data, we conducted a two-year fishery survey in the Duoxiong Zangbo River, analyzing the age structure, growth patterns, and resource status of the target species (S. o’connori, O. stewartii, and P. dipogon). Furthermore, we developed a population dynamics model to quantitatively evaluate the relative impacts of natural versus anthropogenic disturbances (particularly fishing) on total mortality and its components (fishing mortality (F) and natural mortality (M)). These findings aim to elucidate schizothoracinae fish population responses to environmental stress while informing conservation strategies and adaptive fisheries management in the Duoxiong Zangbo and Yarlung Zangbo River basins.
2. Materials and Methods
2.1. Sample Collection and Processing
Six sampling sites were established along Duoxiong Zangbo River, a tributary of the upper Yarlung Zangbo River (Figure 1). Sampling points were established following standardized criteria that account for habitat variation, anthropogenic disturbance gradients, and tributary confluences. To ensure spatial independence, adjacent sampling points were positioned at minimum intervals of 20 km. Four field surveys were conducted between 2023 and 2024 during two seasons: summer (June–July) and autumn (September–October). Sampling activities were authorized by the fisheries authorities of the Tibet Autonomous Region. Standardized fishing gear combinations were employed at each site, including eight gillnets (two each with mesh sizes of 2–5 cm, 20 m long, and 1 m high) and two ground cages (6 m long, 40 cm wide, and 40 cm high). We conducted passive fishing for 12 consecutive hours from 18:00 to 06:00 the following day.
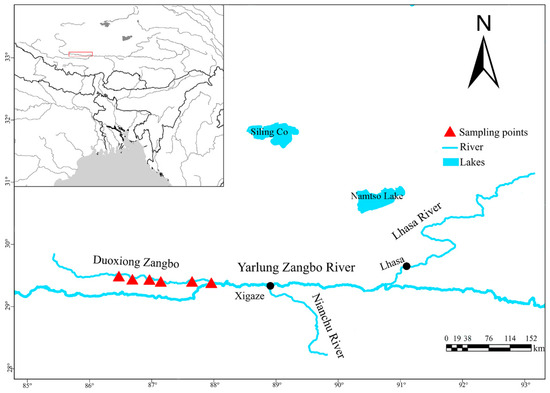
Figure 1.
Sampling site.
Each captured individual was measured for total length, body length (±1 mm), and body weight (±0.1 g). Following dissection for sex identification, otoliths were extracted and preserved in anhydrous ethanol to remove connective tissue, then air-dried at ambient temperature (25 ± 2 °C) before storage in 1.5 mL centrifuge tubes. In the laboratory, otoliths were mounted on slides using colorless nail polish; air-dried; and sequentially polished using 800-grit#, 1000-grit#, and 3000-grit# abrasive papers under real-time stereomicroscope observation. The polishing process continued until the central growth whorl patterns became clearly visible (repeated on both sides if necessary). Annual growth bands (dark and transparent bands) were identified under incident light, with the dark band serving as the annual marker for counting and microimaging.
2.2. Data Statistics and Analysis Methods
2.2.1. Body Length–Body Weight Relationship
The body length–weight relationship was modeled using a power function [53], and analysis of covariance (ANCOVA) was applied to test for significant differences in this relationship between males and females. The equations were derived as follows:
where W represents body weight (g), L denotes body length (mm), and a and b are constant.
W = a Lb
To assess the statistical significance of the difference between parameter “b” and value 3, the t-test formula (t = ××) was employed.
2.2.2. Growth Equation, Growth Rate Equation, and Growth Acceleration Equation
The von Bertalanffy growth equation was employed to model body length growth [54], while the body mass growth equation was derived from the length–mass relationship. The first and second derivatives of these growth equations were computed to obtain the corresponding growth rate and acceleration equations. Subsequently, the inflection point age, critical age, and growth performance index were calculated as follows:
where t represents the age; t0, ti, and tc represent the theoretical starting age, inflection point age, and critical age, respectively; Lt and Wt represent the body length (mm) and weight (g) at age t; L∞ represents the asymptotic body length (mm); W∞ represents the asymptotic body weight (g); and k represents the growth coefficient.
Body length growth equation: Lt = L∞ (1 − e−k(t−t0));
Body weight growth equation: Wt = W∞ (1 − e−k(t−t0))b;
Body length growth rate equation: dL/dt = L∞ k e−k(t−t0);
Body weight growth rate equation: dW/dt = b W∞ k e−k(t−t0) (1 − e−k(t−t0))b−1;
Body length growth acceleration equation: d2L/dt2 = −L∞k2e−k(t−t0);
Body weight Growth Acceleration Equation: d2W/dt2 = bW∞k2e − k (t − t0) (1 − e−k(t−t0))b−2 (be−k(t−t0) − 1);
Inflection point age: ti = ln b/k + t0;
Critical age: tc = [Kt0 − lnM + ln (bK + M)]/K;
Growth performance index: φ = lgk + 2 lgL∞;
Body weight growth equation: Wt = W∞ (1 − e−k(t−t0))b;
Body length growth rate equation: dL/dt = L∞ k e−k(t−t0);
Body weight growth rate equation: dW/dt = b W∞ k e−k(t−t0) (1 − e−k(t−t0))b−1;
Body length growth acceleration equation: d2L/dt2 = −L∞k2e−k(t−t0);
Body weight Growth Acceleration Equation: d2W/dt2 = bW∞k2e − k (t − t0) (1 − e−k(t−t0))b−2 (be−k(t−t0) − 1);
Inflection point age: ti = ln b/k + t0;
Critical age: tc = [Kt0 − lnM + ln (bK + M)]/K;
Growth performance index: φ = lgk + 2 lgL∞;
2.2.3. Mortality Characteristics and Exploitation Rate
The total mortality coefficient (Z) was calculated using the formula proposed by Beverton–Holt. The natural mortality coefficient (M) was estimated using Pauly’s formula [55,56,57]. The fishing mortality coefficient (F) was derived as the total mortality coefficient (Z) minus the natural mortality coefficient (M). The population exploitation rate (E) was calculated as the ratio of the fishing mortality coefficient (F) to the total mortality coefficient (Z), as follows:
where K represents the growth rate, L∞ represents the asymptotic body length (mm), Lmean represents the average body length of the sample (mm), Lc represents the starting body length (mm), and T represents the annual average water temperature (°C) of the habitat for the studied fish species.
Z = K (L∞ − Lmean)/(Lmean − Lc)
lnM = −0.0066 − 0.279 lnL∞ + 0.6543 lnK + 0.4634 lnT
F = Z − M
E = F/Z
2.2.4. Relative Yield per Recruit and Biomass per Recruit
Resource utilization by schizothoracinae was assessed using the Beverton–Holt dynamic pool model, specifically through relative yield per recruit (Y′/R) and relative biomass per recruit (B′/R) curves. The relevant model equations are expressed as follows [56]:
where E represents the exploitation rate, L∞ (mm) represents the asymptotic body length, Lc (mm) represents the length of the starting body, M represents the natural mortality factor, Z represents the total mortality coefficient, and k represents the growth factor.
Y′/R = E × (1 − Lc/L∞) M/k [1 − 3(1 − Lc/L∞)/(1 + k/Z) + 3 (1 − Lc/L∞)2/(1 + 2 k/Z) − (1 − Lc/L∞)3/(1 + 3 k/Z)]
B′/R = (Y′/R)/(Z − M)
3. Results
3.1. Group Structure
During this survey, we collected 51 S. o’connori, 45 O. stewartii, and 97 P. dipogon specimens from DuoXiong Zangbo River (Table S1). The schizothoracinae specimens exhibited body lengths ranging from 23.02 to 460.00 mm and body weights ranging from 0.20 to 1394.30 g. The male-to-female ratios were 0.57:1 for S. o’connori, 1:1 for O. stewartii, and 0.79:1 for P. dipogon. Age distributions spanned 0–13 years for S. o’connori, 0–16 years for O. stewartii, and 0–14 years for P. dipogon (Table 1, Figure 2).

Table 1.
The body lengths and body weights of three schizothoracinae species.
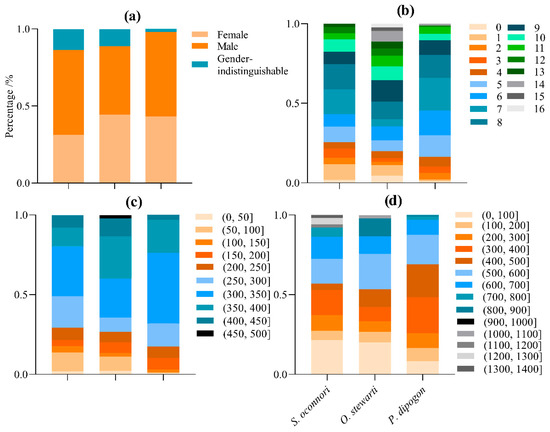
Figure 2.
The population structure of three schizothoracinae species. (a) Sex proportion; (b) age proportion; (c) proportion of the body length group; (d) proportion of the body weight group.
3.2. Body Length–Body Weight Relationship
Analysis of covariance revealed no significant sexual dimorphism in the length–weight relationship for any of the three schizothoracinae species: O. stewarti (F(1,37) = 0.901, p = 0.349), P. dipogon (F(1,92) = 1.409, p = 0.259), and S. o’connori (F(7,37) = 2.178, p = 0.059). Consequently, a unified length–mass relationship was established for each species (Figure 3).
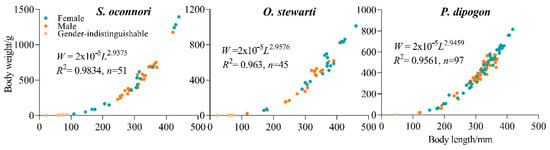
Figure 3.
Relationship between body length and body weight of three schizothoracinae species.
Independent-sample t-tests were conducted to compare the growth coefficient (b) of the length–weight regression equations among the three fish populations with the theoretical value of 3. The results showed no statistically significant differences for O. stewarti (t = 0.59, p > 0.05, df = 44), P. dipogon (t = 0.88, p > 0.05, df = 96), or S. o’connori (t = 1.07, p > 0.05, df = 50), as all t-values were below their respective critical values (t0.05 = 2.021, 1.987, and 2.009). This suggests that the growth patterns of these three fish species are consistent with isometric growth (b ≈ 3) (Table 2).

Table 2.
Confidence intervals for relevant growth parameters of three schizothoracinae species.
3.3. Growth Equation, Growth Velocity Equation, and Growth Acceleration Equation
This study employed empirical measurements to successfully model the growth patterns of three schizothoracinae fish species using the von Bertalanffy growth function (VBGF) (Figure 4 and Figure 5, Table 2). Model derivation yielded equations characterizing both growth velocity (first derivative) and acceleration (second derivative). Analytical results demonstrated that while the three species shared similar overall growth trends, they exhibited significant differences in dynamic details. Analysis of length growth revealed continuously decreasing velocity and acceleration throughout ontogeny, with persistent negative acceleration indicating a monotonically decelerating process devoid of inflection points. In contrast, mass growth displayed distinct dynamics, featuring a characteristic inflection point (Figure 5). Pre-inflection growth showed annually increasing velocity (positive acceleration), marking a phase of progressive weight gain. Maximum growth velocity occurred at the inflection age, followed by declining growth rates (negative acceleration) with diminishing mass accumulation efficiency. Key growth parameters were quantitatively characterized (Table 3). S. o’connori exhibited significantly greater values for both mass growth inflection age (maximum growth velocity) and critical age (theoretical growth cessation) compared to the other two species. However, growth performance indices (φ’) showed no significant interspecific variation (p > 0.05), indicating comparable efficiencies in energy conversion to somatic growth across all three taxa.
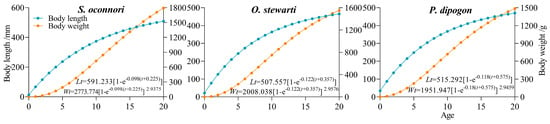
Figure 4.
Growth equation of three schizothoracinae species.
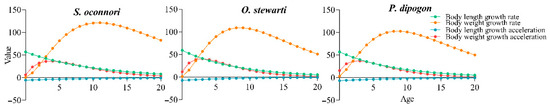
Figure 5.
Growth rate and growth acceleration of three schizothoracinae species.

Table 3.
The inflection point age, critical age, and growth performance index of three schizothoracinae species.
3.4. Mortality Characteristics and Exploitation Rate
Exploitation rates (E) of the three schizothoracinae species ranged from 0.547 to 0.758. O. stewartii showed the highest exploitation rate (0.758), followed by P. dipogon (0.711), while S. o’connori showed the lowest (0.547). All rates were below E-max (Table 4).

Table 4.
Mortality coefficient and exploitation rate of three schizothoracinae species.
3.5. Relative Yield per Recruit and Biomass per Recruit
The relationship between the relative yield per recruit (Y′/R) and relative biomass per recruit (B′/R) for the three schizothoracinae species as a function of the exploitation rate (E) is presented in Figure 6. Y′/R peaked when the exploitation rate reached its maximum value (S. o’connori: 0.579; O. stewartii: 0.882; P. dipogon: 0.884), declining sightly as the rate decreased to E-50. At maximum exploitation (E-max), relative biomass was below 25% for all species but reached 50% at E-50. Contour analysis of Y′/R versus E and Lc revealed that, under the current exploitation rate, Y′/R increased with Lc, attaining its maximum at Lc/L∞ = 0.6 (Figure 7). The corresponding optimal catch lengths at this point were 355 mm for S. o’connori, 305 mm for O. stewartii, and 309 mm for P. dipogon.
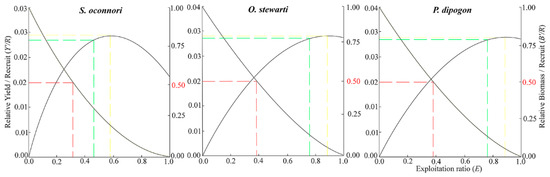
Figure 6.
Two-dimensional analysis of Y′/R and B′/R of three schizothoracinae species.
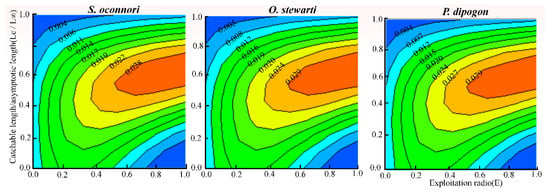
Figure 7.
Relative yield per recruit (Y′/R) of three schizothoracinae species in relation to exploitation rate and catchable length.
4. Discussion
4.1. Population Structure and Growth Characteristics
The growth process of fish is jointly regulated by genetic and environmental factors [58,59]. Notably, conspecific fish often exhibit significant growth variations across different distribution areas or environmental conditions. Our analysis of growth patterns in fish from the Duoxiong Zangbo River waterbody revealed that the allometric coefficient (b) values for S. o’connori (b = 2.9375 [2.886, 2.989]), O. stewarti (b = 2.9576 [2.902, 3.014]), and P. dipogon (b = 2.9459 [2.875, 3.017]) all include the theoretical isometric value of 3 within their 95% confidence intervals. This strongly supports an isometric growth model, indicating that these species maintain geometric similarity in body shape during development, with minimal interspecific differences. The slightly lower point estimates suggest a potential weak negative allometric growth trend, likely influenced by environmental conditions in the Duoxiong Zangbo River [60,61]. Compared to the middle reaches of the Niyang and Lhasa Rivers [37], this high-altitude waterbody exhibits lower temperatures and reduced food availability [34,36,38], which may lead to preferential energy allocation toward longitudinal growth rather than mass accumulation [61]. However, since all confidence intervals encompass a value of 3, this minor deviation requires further investigation to determine its biological significance.
Fish growth parameters serve as fundamental data for the study of biological characteristics, population structure, and resource assessment and are critical components in constructing fishery resource models. These parameters directly influence population biomass and distribution patterns [62,63,64,65]. Among them, the growth coefficient (k) is a key indicator of fish population growth potential [66]. Branstetter [67] classified fish growth rates into three types based on k-values: slow (k = 0.05–0.10), medium (k = 0.10–0.20), and fast (k = 0.20–0.50). In the Duoxiong Zangbo River, O. stewartii (k = 0.122) and P. dipogon (k = 0.118) exhibit medium growth rates, while S. o’connori (k = 0.098) falls into the slow-growth category, suggesting limited population growth potential. Comparative analysis reveals that most S. o’connori subfamily members have k values around 0.1, indicating a generally slow growth rate. Consequently, the populations of this subfamily may struggle to recover following resource depletion.
Comparative temporal analysis revealed that the growth coefficient (k) of S. o’connori in the Duoxiong Zangbo River watershed was significantly higher than that of the 2008–2009 Yarlung Zangbo River mainstem population (kmale = 0.095; kfemale = 0.081) [68]. Similarly, the k value of P. dipogon in this watershed exceeded that of the 2019 Yarlung Zangbo River mainstem population (kmale = 0.069; kfemale = 0.048) [69]. This acceleration stems from two primary factors: habitat environmental changes [70] and fishing pressure [71]. Specifically, rising water temperatures due to climate warming have enhanced fish metabolism and growth rates [9,35], while intensive fishing has prompted schizothoracinae populations to adapt through phenotypic plasticity [62], manifesting as compensatory growth to maintain population viability [72,73]. Regarding environmental adaptations, fish often adjust their population age structure. Our comparison of historical data for three schizothoracinae species in the Yarlung Zangbo River Basin showed maximum ages of 50 years for S. o’connori [68] and 47 years for P. dipogon [74], whereas the maximum age in our study was only 16 years. This substantial discrepancy suggests significant alterations in age structure and lifespan, likely attributable to environmental changes and increased fishing pressure. The elevated growth rates observed in these schizothoracinae species directly reflect intense fishing pressure [75], with these adaptive responses causing marked shifts in life-history parameters. Collectively, these changes indicate a trend toward younger and smaller population structures.
The growth coefficient (k) is sensitive to variations in population age structure, whereas the growth performance index (φ), which integrates information from both k and L∞, is commonly used to evaluate the reliability of growth parameter estimates. Notably, φ values tend to be similar among closely related species [76]. In this study, we employed φ values to assess the accuracy of growth parameters for S. o’connori, O. stewartii, and P. dipogon in the Duoxiong Zangbo River watershed and to examine their interspecific growth variation. The φ’ values for these three schizothoracinae species ranged narrowly from 4.50 to 4.53, suggesting robust and reliable growth parameter estimates. Comparative analysis with other Tibetan Plateau schizothoracinae populations revealed that our φ’ values fell within the median range (4.37–4.75) of previously reported values [77,78,79,80,81,82,83]. This indicates that the growth performance of these species in Duoxiong Zangbo River is intermediate among highland schizothoracinae. Their life-history strategies appear primarily constrained by phylogenetic factors, maintaining energy allocation patterns consistent with their sympatric relatives. The observed growth patterns likely reflect phenotypic plasticity in response to local environmental conditions rather than profound genetic adaptation. These findings provide crucial quantitative support for the development of watershed-specific aquatic resource restoration strategies based on ecological characteristics.
4.2. Resource Dynamics and Exploration Rate
Fish mortality is a critical driver of population dynamics, directly influencing the magnitude of population decline. In this study, we evaluated mortality parameters for S. o’connori, O. stewartii, and P. dipogon in the Duoxiong Zangbo River watershed using established reliability criteria: the growth equation validity threshold (e−k < 1) [84], the natural mortality confidence range (M/K = 1.5–2.5), and the mortality-type discrimination threshold (Z/K > 3) [84]. The e−k values (0.89–0.91) confirmed the validity of the growth model, while M/K ratios (2.07–2.14) aligned with theoretical expectations for natural mortality. These results support the reliability of our growth parameter (k, L∞) estimates and natural mortality (M) calculations. Notably, the Z/K ratios (4.72–8.53) for all three species substantially exceeded the discrimination threshold (Z/K > 3), indicating that total mortality (Z) is predominantly driven by anthropogenic fishing pressure. This suggests severe overexploitation of these schizothoracinae populations, necessitating immediate fisheries management interventions to mitigate further resource depletion. This study primarily aims to elucidate the driving mechanisms of overfishing through mortality structure analysis (Z/K >> 3). Although direct reports on natural mortality rates (M) of these endemic fish species are scarce in the literature, the estimated M values in this study were rigorously validated against biological criteria (M/K = 2.07–2.14 ∈ 1.5–2.5), thereby providing robust support for our conclusions. The determination of population-critical thresholds (e.g., minimum viable population, MVP) requires long-term integration of population viability analysis (PVA) data, which constitutes a distinct research focus. The substantial exceedance of the Z/K threshold (>3) observed here (4.72–8.53) provides conclusive evidence of overfishing, underscoring the need for immediate management interventions. Future studies should build upon these findings to quantify population recovery targets more precisely.
The definition of overexploitation thresholds for fish resources remains debated. While Gulland [85] proposed an exploitation rate threshold of 0.5 to identify overexploited populations, Mehanna [86] suggested that rates below E-max indicate sustainable exploitation. In this study, the exploitation rates of S. o’connori (0.547), O. stewartii (0.758), and P. dipogon (0.711) exceeded Gulland’s threshold but remained below Mehanna’s proposed Emax for riverine systems, suggesting moderate exploitation. This intermediate status may reflect the remote location of the Duoxiong Zangbo River watershed, which lacks major urban centers and experiences limited anthropogenic pressure. Additionally, traditional Tibetan religious practices may contribute to informal fish conservation, distinguishing this region from more heavily exploited tributaries in the middle and lower Yarlung Zangbo River basin [39].
Analysis using the Beverton-Holt dynamic synthesis model identified suboptimal exploitation patterns in the stock resources of three schizothoracinae species (S. o’connori, O. stewartii, and P. dipogon). Based on integrated exploitation rate analysis, current fishing practices exhibit excessive intensity and small size at capture. Thus, management optimization requires an increase in the minimum capture size and reduced fishing pressure. Notably, adjusting size limits may offer greater conservation benefits than simply decreasing fishing intensity [87,88]. We propose three science-based methods for determining optimal size at capture: the inflection point length for maximum commercial yield, the critical length at critical age for generational biomass conservation, and the length corresponding to peak yield per recruit in the Beverton–Holt model. By averaging these metrics, we derived optimal opening lengths of 353 mm (S. o’connori), 313 mm (O. stewartii), and 317 mm (P. dipogon). Implementing these size restrictions would enhance stock recruitment while maintaining sustainable harvests, allowing individuals to realize their growth potential, preventing growth overfishing, and ensuring long-term resource sustainability [87].
4.3. Conservation Measures
Based on analyses of growth characteristics in S. o’connori, O. stewartii, and P. dipogon, coupled with assessments of aquatic ecosystem vulnerability on the Qinghai-Tibet Plateau, this study proposes four core conservation strategies: (1) Protected area establishment: Designate no-fishing zones in critical habitats (spawning and feeding grounds) [89,90,91,92,93], complemented by artificial breeding and stock enhancement programs [94,95]. This integrated approach aims to rebuild natural populations and reverse resource depletion trends. (2) Stocking regulation: Develop science-based release protocols with rigorous approval and monitoring systems [93,96], prioritizing prevention of non-native species introductions to maintain ecosystem integrity. (3) Fisheries management: Implement catch quotas and seasonal closures [87,92,97,98,99,100,101,102] to protect reproductive cycles and growth phases while reducing habitat degradation and illegal harvests. (4) Ecological monitoring: Create a comprehensive aquatic monitoring network [95] for systematic tracking of hydrological conditions and fish community dynamics, enabling adaptive management.
5. Conclusions
This study presents a comprehensive evaluation of resource status, growth characteristics, and exploitation intensity for three schizothoracine fish species (S. oconnori, O. stewarti, and P. dipogon) in the Duoxiong Zangbo River basin. Key findings indicate the following: (1) Growth parameter analysis (asymptotic length L∞ = 507.577–591.233 mm; growth coefficient k = 0.098–0.122), combined with exploitation rate assessment (current values = 0.547–0.758), reveals all three populations are moderately exploited. While current exploitation rates exceed the general sustainability threshold (0.5), none has reached the maximum sustainable rate (E-max) predicted by the Beverton–Holt model. (2) Beverton–Holt dynamic modeling identifies critical management deficiencies, particularly excessive fishing pressure and suboptimal minimum size limits, which threaten long-term population stability if unaddressed. (3) Considering species-specific growth parameters (inflection age and critical age) and sustainable yield optimization, we recommend raising minimum size limits to 355 mm (S. oconnori), 305 mm (O. stewarti), and 309 mm (P. dipogon). These adjustments would protect pre-reproductive individuals, ensure recruitment, and balance ecological sustainability with economic benefits.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani15162340/s1: Table S1: Catch data from sampling sites.
Author Contributions
Conceptualization, methodology, investigation and writing—original draft, H.H.; investigation, L.W.; validation and investigation, C.Z.; investigation, H.L.; conceptualization, project administration, writing—review and editing, funding acquisition and supervision, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Project of Agricultural Finance Special Project of the Ministry of Agriculture and Rural Affairs “Fisheries Resources and Environment Survey in Key Waters of Southwest”; Xizang Science and Technology Department’s Central Government-Guided Local Projects (XZ202301YD0017C); Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD07).
Institutional Review Board Statement
This study was conducted in accordance with animal care laws and guidelines (Directive 2010(63) EU); all procedures were approved by the Laboratory Animal Ethics Committee of Heilongjiang River Fisheries Research Institute (No. 20230212-001, 12 February 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, F.P.; Zhang, Y.Q.; Xu, Z.X.; Teng, J.; Liu, C.M.; Liu, W.F.; Mpelasoka, F. The impact of climate change on runoff in the southeastern Tibetan Plateau. J. Hydrol. 2013, 505, 188–201. [Google Scholar] [CrossRef]
- Zhang, J.W.; Yan, Y.N.; Zhao, Z.Q.; Liu, X.M.; Li, X.D.; Zhang, D.; Ding, H.; Meng, J.L.; Liu, C.Q. Spatiotemporal variation of Li isotopes in the Yarlung Tsangpo River basin (upper reaches of the Brahmaputra River): Source and process. Earth. Planet. Sci. Lett. 2022, 600, 117875. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, P.; Li, X.D.; Yang, S.X.; Chao, X.; Liu, H.Q.; Ba, S. Distribution patterns and community assembly processes of eukaryotic microorganisms along an altitudinal gradient in the middle reaches of the Yarlung Zangbo River. Water Res. 2023, 239, 120047. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Hao, J.S.; Zhang, G.T.; Fang, H.Y.; Wang, Y.; Lu, H.J. Runoff variations affected by climate change and human activities in Yarlung Zangbo River, southeastern Tibetan Plateau. Catena 2023, 230, 107184. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Ye, C.; Liu, W. Bacterial community and climate change implication affected the diversity and abundance of antibiotic resistance genes in wetlands on the Qinghai-Tibetan Plateau. J. Hazard. Mater. 2019, 361, 283–293. [Google Scholar] [CrossRef]
- Tian, P.P.; Lu, H.W.; Feng, W.; Guan, Y.L.; Xue, Y.X. Large decrease in streamflow and sediment load of Qinghai-Tibetan Plateau driven by future climate change: A case study in Lhasa River Basin. Catena 2020, 187, 104340. [Google Scholar] [CrossRef]
- Yao, Z.J.; Liu, J.; Huang, H.Q.; Song, X.F.; Dong, X.H.; Liu, X. Characteristics of isotope in precipitation, river water and lake water in the Manasarovar basin of Qinghai-Tibet Plateau. Environ. Geol. 2009, 57, 551–556. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhong, X.H.; Liu, S.Z.; Liu, J.G.; Wang, Z.Y.; Li, M.H. Regional assessment of environmental vulnerability in the Tibetan Plateau. Development and application of a new method. J. Arid Environ. 2008, 72, 1929–1939. [Google Scholar] [CrossRef]
- You, Q.L.; Kang, S.C.; Wu, Y.H.; Yan, Y.P. Climate change over the Yarlung Zangbo River Basin during 1961–2005. J. Geosci. 2007, 17, 409–420. [Google Scholar] [CrossRef]
- Chien, H.; Yeh, P.J.F.; Knouft, J.H. Modeling the potential impacts of climate change on streamflow in agricultural watersheds of the Midwestern United States. J. Hydrol. 2013, 491, 73–88. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Hao, F.; Wu, Y.; Li, C.; Xu, Y. Evaluating the contributions of climate change and human activities to runoff in typical semi-arid area, China. J. Hydrol. 2020, 590, 125555. [Google Scholar] [CrossRef]
- Feng, X.; Cheng, W.; Fu, B.; Lü, Y. The role of climatic and anthropogenic stresses on long-term runoff reduction from the Loess Plateau, China. Sci. Total Environ. 2016, 571, 688–698. [Google Scholar] [CrossRef]
- Bao, Z.; Zhang, J.; Wang, G.; Fu, G.; He, R.; Yan, X.; Jin, J.; Liu, Y.; Zhang, A. Attribution for decreasing streamflow of the Haihe River basin, northern China: Climate variability or human activities. J. Hydrol. 2012, 460–461, 117–129. [Google Scholar] [CrossRef]
- Cui, L.; Kou, X.; Sun, J.; Liu, R.; Gao, F.; Tan, J.; Soomro, S.; Wang, Y.; Kattle, G.R.; Shi, X. Fishway assessment and monitoring for endemic migratory fish using multiple techniques in high-altitude river systems: A case study from the Yarlung Zangbo River, Southeastern Tibetan Plateau. Glob. Ecol. Conserv. 2024, 56, e03325. [Google Scholar] [CrossRef]
- Li, F.P.; Xu, Z.X.; Feng, Y.C.; Liu, M.; Liu, W.F. Changes of land cover in the Yarlung Zangbo River basin from 1985 to 2005. Environ. Earth Sci. 2013, 68, 181–188. [Google Scholar] [CrossRef]
- Sun, W.C.; Wang, Y.Y.; Fu, Y.S.H.; Xue, B.L.; Wang, G.Q.; Yu, J.S.; Zuo, D.P.; Xu, Z.X. Spatial heterogeneity of changes in vegetation growth and their driving forces based on satellite observations of the Yarlung Zangbo River Basin in the Tibetan Plateau. J. Hydrol. 2019, 574, 324–332. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.H.; Zhao, L.; Liu, Y.; Gao, F.Y.; Tang, J.M.; Wang, H.Y.; Chen, Z.X.; Wang, S.Y.; Li, G.H.; et al. Aeolian activity in the Yarlung Zangbo River Basin, southern Tibetan Plateau, began at 584 ka: Implications for the glaciation of the Tibetan Plateau. Quat. Sci. Rev. 2024, 337, 108799. [Google Scholar] [CrossRef]
- Liu, C.C.; Witonsky, D.; Gosling, A.; Hyeon, L.J.; Ringbauer, H.; Hagan, R.; Patel, N.; Stahl, R.; Novembre, J.; Aldenderfer, M.; et al. Ancient genomes from the Himalayas illuminate the genetic history of Tibetans and their Tibeto-Burman speaking neighbors. Nat. Commun. 2022, 13, 1203. [Google Scholar] [CrossRef]
- Duan, Y.J.; Xie, C.X.; Zhou, X.; Ma, B.S.; Huo, B. Age and growth characteristics of Schizopygopsis younghusbandi Regan, 1905 in the Yarlung Tsangpo River in Tibet, China. J. Appl. Ichthyol. 2014, 30, 948–954. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, X.J.; Zhang, D.F.; Giorgi, F. Climate change over the Yarlung Zangbo-Brahmaputra River Basin in the 21st century as simulated by a high-resolution regional climate model. Quat. Int. 2011, 244, 159–168. [Google Scholar] [CrossRef]
- Ji, F.; Ma, B.; Wang, B. The Fisheries Resources and Environment Research Report of the Middle Reaches of the Yarlung Zangbo River; China Agriculture Press: Beijing, China, 2019. [Google Scholar]
- Jiang, X.; Xie, Z.; Chen, Y. Longitudinal patterns of macroinvertebrate communities in relation to environmental factors in a Tibetan-Plateau River system. Quat. Int. 2013, 304, 107–114. [Google Scholar] [CrossRef]
- Yi, X.; Lai, Q.; Shi, J.; Gao, P.; Zhou, K.; Qi, H.; Wang, H.; Me, Z. Nitrogenous waste excretion and gene expression of nitrogen transporter in Gymnocypris przewalskii in high alkaline environment. J. Fish. Sci. China 2017, 24, 681–689. [Google Scholar]
- Huo, B.; Xie, C.X.; Ma, B.S.; Yang, X.F.; Huang, H.P. Age and growth of Oxygymnocypris stewartii in the Yarlung Tsangpo River, Tibet, China. Zool. Stud. 2012, 51, 185–194. [Google Scholar]
- Zhou, X.; Xie, C.; Huo, B.; Duan, Y.; Yang, X.; Ma, B. Age and growth of Schizothorax waltoni (Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River, China. J. Appl. Anim. Res. 2017, 45, 346–354. [Google Scholar] [CrossRef]
- Ma, B.S.; Xie, C.X.; Huo, B.; Yang, X.F.; Chen, S.S. Reproductive biology of Schizothorax o’connori (Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River, Tibet. Zool. Stud. 2012, 51, 1066–1076. [Google Scholar]
- Zhou, X.J.; Xie, C.X.; Huo, B.; Duan, Y.J.; Yang, X.; Ma, B.S. Reproductive biology of Schizothorax waltoni (Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River in Tibet, China. Environ. Biol. Fish. 2015, 98, 597–609. [Google Scholar] [CrossRef]
- Feng, X.; Jia, Y.T.; Zhu, R.; Chen, K.; Chen, Y.F. Characterization and analysis of the transcriptome in Gymnocypris selincuoensis on the Qinghai-Tibetan Plateau using single-molecule long-read sequencing and RNA-seq. DNA Res. 2019, 26, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, M.Z.; Wang, J.; Gong, Z.; Liu, M.; Liu, H.Z.; Lin, P.C. Species composition and longitudinal patterns of fish assemblages in the middle and lower Yarlung Zangbo River, Tibetan Plateau, China. Ecol. Indic. 2021, 125, 107542. [Google Scholar] [CrossRef]
- Li, L.; Ma, B.; Jin, X.; Wang, P.; Chen, Z.; Wang, N.; Wu, S.; Zhang, C.; Gong, J. Quantitative assessment of the priority conservation of Schizothoracinae fishes in the middle Yarlung Zangbo River. J. Fish. Sci. China 2019, 26, 914–924. [Google Scholar]
- He, D.K.; Sui, X.Y.; Sun, H.Y.; Tao, J.; Ding, C.Z.; Chen, Y.F.; Chen, Y.Y. Diversity, pattern and ecological drivers of freshwater fish in China and adjacent areas. Rev. Fish Biol. Fish. 2020, 30, 387–404. [Google Scholar] [CrossRef]
- Chen, Y.F.; He, D.K.; Cao, W.X.; Duan, Z.H. Growth of Selincuo schizothoracini (Gymnocypris selincuoensis) in Selincuo Lake. Tibet Platean. Acta Zool. Sin. 2002, 48, 667–676. [Google Scholar]
- Shen, W.S.; Li, H.D.; Sun, M.; Jiang, J. Dynamics of aeolian sandy land in the Yarlung Zangbo River basin of Tibet, China from 1975 to 2008. Global. Planet. Change 2012, 86, 37–44. [Google Scholar] [CrossRef]
- Zhang, P.; Xiong, J.; Qiao, N.Q.; An, R.Z.; Da, Z.; Miao, W.; Ba, S. Spatiotemporal distribution of protists in the Yarlung Zangbo River, Tibetan Plateau. Water Biol. Secur. 2022, 1, 100064. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, Z.; Huang, H.; Wu, S.; Liu, G. Land use and climate changes and their impacts on runoff in the Yarlung Zangbo River basin, China. Land Degrad. Dev. 2012, 25, 203–215. [Google Scholar] [CrossRef]
- Yu, Z.L.; Yan, N.; Wu, G.J.; Xu, T.L.; Li, F. Chemical weathering in the upstream and midstream reaches of the Yarlung Tsangpo basin, southern Tibetan Plateau. Chem. Geol. 2021, 559, 119906. [Google Scholar] [CrossRef]
- Bai, Q.Q.; Wang, L.; Cidang, Y.Z. Spatial and Temporal Variability of Rainfall Erosivity in the Niyang River Basin. Atmosphere 2024, 15, 1032. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Liu, K.F.; Dan, Z.; Gao, Q.Z.; Mima, C.; Lu, C.H. Runoff spatiotemporal variability driven by climate change and human activity for the Nianchu River Basin in Southwestern Tibet. J. Hydrol. Reg. Stud. 2025, 58, 102301. [Google Scholar] [CrossRef]
- Han, H.H.; Wang, L.; Zhang, C.; Li, H.C.; Ma, B. Population Structure, Growth Characteristics, Resource Dynamics, and Management Strategies of Schizopygopsis younghusbandi in Four Tributaries of the Yarlung Zangbo River, Tibet. Biology 2025, 14, 707. [Google Scholar] [CrossRef]
- Shao, J.; Ma, B.S.; Duan, Y.J.; Xie, C.X.; Lin, S.Q.; Zhou, X.J.; Huo, B. Population resources and fishery management policies of Schizopygopsis younghusbandi in the Yarlung Zangbo River, China. J. Appl. Ecol. 2019, 30, 2437–2446. [Google Scholar]
- Zheng, G.; Zhai, D.D.; Chen, J.; Liu, B.; Zhu, T.S. Landscape determinants of genetic structure for Schizopygopsis younghusbandi in the Yarlung Tsangpo River drainage, Tibetan Plateau. Ecol. Indic. 2023, 151, 110309. [Google Scholar] [CrossRef]
- Guo, C.; Wang, R.; Qu, X.; Xin, W.; Chen, Y.; Li, Z. Assessing fish assemblages in a shallow Yangtze River Lake using mulei-mesh gillnets and dense-mesh weirs. Acta Hydrobiol. Sin. 2018, 42, 1116–1123. [Google Scholar]
- Liu, Y.; Tang, S.; Li, D.; Gu, X.; Zhu, B.; Mao, G.; Zhang, T. Characteristics of the fish community structure in Jiangsu reach of the Huaihe River. J. Fish. Sci. China 2020, 27, 224–235. [Google Scholar]
- Lynch, A.J.; Myers, B.J.E.; Chu, C.; Eby, L.A.; Falke, J.A.; Kovach, R.P.; Krabbenhoft, T.J.; Kwak, T.J.; Lyons, J.; Paukert, C.P.; et al. Climate Change Effects on North American Inland Fish Populations and Assemblages. Fisheries 2016, 41, 346–361. [Google Scholar] [CrossRef]
- Nyboer, E.A.; Liang, C.; Chapman, L.J. Assessing the vulnerability of Africa’s freshwater fishes to climate change: A continent-wide trait-based analysis. Biol. Conserv. 2019, 236, 505–520. [Google Scholar] [CrossRef]
- Jaeger, K.L.; Olden, J.D.; Pelland, N.A. Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc. Natl. Acad. Sci. USA 2014, 111, 13894–13899. [Google Scholar] [CrossRef]
- Isaak, D.J.; Wollrab, S.; Horan, D.; Chandler, G. Climate change effects on stream and river temperatures across the northwest U.S. from 1980–2009 and implications for salmonid fishes. Clim. Change 2011, 113, 499–524. [Google Scholar] [CrossRef]
- Domisch, S.; Araújo, M.B.; Bonada, N.; Pauls, S.U.; Jähnig, S.C.; Affiliation, P.H. Modelling distribution in European stream macroinvertebrates under future climates. Glob. Change Biol. 2013, 19, 752–762. [Google Scholar] [CrossRef]
- Murua, H.; Rodriguez-Marin, E.; Neilson, J.D.; Farley, J.H.; Juan-Jordá, M.J. Fast versus slow growing tuna species: Age, growth, and implications for population dynamics and fisheries management. Rev. Fish Biol. Fish. 2017, 27, 733–773. [Google Scholar] [CrossRef]
- Denechaud, C.; Smoliński, S.; Geffen, A.J.; Godiksen, J.A.; Campana, S.E. A century of fish growth in relation to climate change, population dynamics and exploitation. Glob. Change Biol. 2020, 26, 5661–5678. [Google Scholar] [CrossRef] [PubMed]
- Foubert, A.; Lecomte, F.; Legendre, P.; Cusson, M. Spatial organization of fish communities in the St. Lawrence River: A test for longitudinal gradients and spatial heterogeneities in a large river system. Hydrobiologia 2018, 809, 155–173. [Google Scholar] [CrossRef]
- Zhou, C.W.; Zhou, Y.; Xu, L.H.; Liu, F.; Lei, L.; Gao, H.; Li, J.T.; Fu, S.X.; Duan, Y.T.; Tan, Y.G.; et al. Chromosome-level genome assembly and population genomic analysis provide insights into the genetic diversity and adaption of Schizopygopsis younghusbandi on the Tibetan Plateau. Integr. Zool. 2024, 0, 1–19. [Google Scholar] [CrossRef]
- Ricker, W.E. Linear regressions in fishery research. J. Fish. Res. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. A quantitative theory of organicgrowth (inquiries on growth laws. II). Hum. Biology 1973, 10, 181–213. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality growth parameters and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Pauly, D. Fish population dynamics in tropical waters: A manual for use with programmable calculators. RICLARM Stud. Rev. 1984, 8, 1–325. [Google Scholar]
- Pauly, D. Length converted catch curves and the sea-sonal growth of fishes. ICLARM Fish. 1990, 8, 33. [Google Scholar]
- Buj, I.; Marčić, Z.; Flauder, E.; Šanda, R.; Vukić, J. Population Genetic Structure of Endemic Fish Species Facilitating Their Survival in Changing Environments—A Case Study on the Genus Telestes in Croatia. Diversity 2022, 14, 529. [Google Scholar] [CrossRef]
- Add: Andreychev, A.V.; Zhalilov, A.B.; Kuznetsov, V.A. The state of local steepe woodchuck (Marmota bobak) populations in the Republic of Mordovia. Zool. Zhurnal. 2015, 94, 723–730. [Google Scholar]
- Pecuchet, L.; Lindegren, M.; Hidalgo, M.; Delgado, M.; Esteban, A.; Fock, H.O.; Salo, L.G.D.; Punzón, A.; Sólmundsson, J.; Payne, M.R. From traits to life-history strategies: Deconstructing fish community composition across European seas. Glob. Ecol. Biogeogr. 2017, 26, 812–822. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.S.; Wang, X.A.; Wang, M.J.; He, R.H.; Wang, M. Distribution Pattern of Fish Richness in the Yarlung Zangbo River Basin. Diversity 2022, 14, 1142. [Google Scholar] [CrossRef]
- Rochet, M.J. A comparative approach to life-history strategies and tactics among four orders of teleost fish. ICES J. Mar. Sci. 2000, 27, 228–239. [Google Scholar] [CrossRef]
- Rochet, M.; Trenkel, V. Which community indicators can measure the impact of fishing? A review and proposals. Can. J. Fish. Aquat. Sci. 2003, 60, 86–99. [Google Scholar] [CrossRef]
- Mercier, L.; Panfili, J.; Paillin, C.; Ndiaye, A.; Mouillot, D.; Darnaude, A. Otolith reading and multi-model inference for improved estimation of age and growth in the gilthead seabream Sparus aurata (L.). Estuar. Coast. Shelf. Sci. 2011, 92, 534–545. [Google Scholar] [CrossRef]
- Rountrey, A.N.; Coulsin, P.G.; Meeuwig, J.J.; Meekan, M. Water temperature and fish growth: Otoliths predict growth patterns of a marine fish in a changing climate. Glob. Change Biol. 2014, 20, 2450–2458. [Google Scholar] [CrossRef]
- Musick, J.A. Ecology and conservation of long-lived marine animals. Am. Fish. Soc. Symp. 1999, 23, 1–10. [Google Scholar]
- Branstetter, S. Age and growth estimates for blacktip, Carcharhinus limbatus and spinner C. brevipinna, sharks from the northwestern Gulf of Mexico. Copeia 1987, 4, 964–974. [Google Scholar] [CrossRef]
- Ma, B.S.; Xie, C.X.; Huo, B. Life History Pattern and Exploitation Status of Apoulation of Schizothorax O’connori in the Yarlung Zangbo River. Resour. Environ. Yangtze Basin 2014, 23, 1558–1565. [Google Scholar]
- He, W.J.; Gao, H.; Zhou, C.W. A Review of the Age, Growth Characteristics, and Population Resources of Ptychobarbus dipogon in the Middle and Upper Reaches of the Yarlung Zangbo River. Water 2023, 15, 1713. [Google Scholar] [CrossRef]
- Brown, C.J.; Broadley, A.; Adame, M.F.; Branch, T.A.; Turschwell, M.P.; Connolly, M.R. The assessment of fishery status depends on fish habitats. Fish Fish. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Mercedes González-Wangüemert, M.; Lenfant, P.; Marcos, C.; García-Charton, J.A. Effects of fishing protection on the genetic structure of fish populations. Biol. Conserv. 2006, 129, 244–255. [Google Scholar] [CrossRef]
- Margarida, C.M.; Lawing, W. A study of sampling strategies for estimating growth parameters in fish populations. Fish. Res. 1995, 22, 59–75. [Google Scholar] [CrossRef]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, Y.F. Age structure, growth and mortality estimates of an endemic Ptychobarbus dipogon (Regan, 1905) (Cyprinidae: Schizothoracinae) in the Lhasa River, Tibet. Chin. Fish. 2008, 28, 97–105. [Google Scholar]
- Schindler, D.E.; Geib, S.I.; Willians, M.R. Patterns of Fish Growth along a Residential Development Gradient in North Temperate Lakes. Ecosystems 2000, 3, 229–237. [Google Scholar] [CrossRef]
- Ama-Abasi, D.; Holzloehner, S.; Enin, U. The dynamics of the exploited population of Ethmalosa fimbriata (Bowdich, 1825, Clupeidae) in the Cross River Estuary and adjacent Gulf of Guinea. Fish. Res. 2004, 68, 225–235. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhu, F.Y.; Liu, M.D.; Duan, X.B.; Liu, S.P.; Chen, D.Q.; Wang, D.Q. AgeStructure and Growth Characteristics of Schizopygopsis thermalis in the Upper Nujiang River. J. Hydrobiol. 2022, 43, 111–118. [Google Scholar]
- Yang, X.; Huo, B.; Duan, Y.J.; Ma, B.S.; Xie, C.X. Age structure and growth characteristics of Ptychobarbus dipogon in the Yarlung Tsangpo River, Tibet. J. Fish. Sci. China 2015, 22, 1085–1094. [Google Scholar]
- Yao, J.L.; Chen, Y.F.; Chen, F.; He, D.K. Age and growth of an endemic Tibetan fish, Schizothorax o’connori, in the Yarlung Tsangpo River. J. Freshw. Ecol. 2009, 24, 343–345. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.B.; Hu, H.M.; Gong, Z.; Cao, W.X.; Lin, P.C. Characteristics of age and growth of Schizothorax curcilabiatus in the lower reaches of the Yarlung Zangbo River. Acta Hydrobiol. Sin. 2022, 46, 1770–1779. [Google Scholar]
- Jia, Y.T.; Chen, Y.F. Age Structure and Growth Characteristics of the Endemic Fish Oxygymnocypris stewartii (Cypriniformes: Cyprinidae: Schizothoracinae) in the Yarlung Tsangpo River, Tibet. Zool. Stud. 2011, 50, 69–75. [Google Scholar]
- Tan, B.Z.; Yang, X.F.; Yang, R.B. Age structure and growth characteristics of Gymnocypris waddelli in the Zhegu Lake, Tibet. J. Fish. Sci. China 2020, 27, 879–885. [Google Scholar]
- Zhu, Q.G.; Tang, H.Y.; Lin, H.; Gong, Y.; Li, X.N.; Yang, Z. Age Structure, Growth Characteristics and Population Dynamic of Schizothorax chongi in Middle and Lower Jinsha River. J. Hydrobiol. 2021, 42, 56–63. [Google Scholar]
- Li, X.F.; Jiang, R.J.; Bing, Y.; Wang, Y.L.; Sun, H.Q.; Yin, R.; Li, K.; Hu, Z.J. Growth, Mortaily and optimum catahable size of Coilia mystus in Oujiang river estuary. Acta Hydrobiol. Sin. 2022, 46, 1393–1401. [Google Scholar]
- Gulland, J.A. Fish stock assessment: A manual of basic methods. J. Mar. Biol. Assoc. United Kingd. 1984, 64, 249. [Google Scholar] [CrossRef]
- Mehanna, S.F. Stock assessment and management of the Egyptian sole Solea aegyptiaca Chabanaud, 1927(Osteichthyes:Soleidae), in the Southeastern Mediterranean, Egypt. Turk. J. Zool. 2007, 31, 379–388. [Google Scholar]
- Halliday, R.G.; Pinhorn, A.T. A review of the scientific and technical bases for policies on the capture of small fish in North Atlantic groundfish fisheries. Fish. Res. 2002, 57, 211–222. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, M.; Shen, L.; Wu, J.M.; Li, J.Y.; Du, H.; Wang, C.Y.; Yang, H.L.; Zhou, Q.; Liu, Z.G.; et al. Rapid change in Yangtze fisheries and its implications for global freshwater ecosystem management. Fish Fish. 2020, 21, 601–620. [Google Scholar] [CrossRef]
- Collas, F.P.L.; Buijse, A.D.; van den Heuvel, L.; van Kessel, N.; Schoor, M.M.; Eerden, H.; Leuven, R.S.E.W. Longitudinal training dams mitigate effects of shipping on environmental conditions and fish density in the littoral zones of the river Rhine. Sci. Total Environ. 2018, 619–620, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fujiwara, M.; Pawluk, M.; Liu, H.; Cao, W.; Gao, X. Changes in taxonomic and functional diversity of fish communities after catastrophic habitat alteration caused by construction of Three Gorges Dam. Ecol. Evol. 2020, 10, 5829–5839. [Google Scholar] [CrossRef]
- Gao, X.; Fujiwara, M.; Winemiller, K.O.; Lin, P.; Li, M.; Liu, H. Regime shift in fish assemblage structure in the Yangtze River following construction of the Three Gorges Dam. Sci. Rep. 2019, 9, 4212. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Zhu, F.; Chen, D.; Liu, S.; Duan, X.; Liu, M. Fish Community Structure and Diversity in the Xizang Section of Nujiang River. J. Hydroecol. 2024, 45, 73–81. [Google Scholar]
- Wang, J.; Liao, C.; Lian, Y.; Lin, X.; Zhang, Y.; Bi, Y.; Liu, J.; Ye, S. Characteristics and historical of fish community structure in Xiangxi River, Three Gorges Reservoir, China. J. Lake Sci. 2023, 35, 2082–2091. [Google Scholar]
- Yang, J.; Yan, D.; Yang, Q.; Gong, S.; Shi, Z.; Qiu, Q.; Huang, S.; Zhou, S.; Hu, M. Fish species composition, distribution and community structure in the Fuhe River Basin, Jiangxi Province, China. Glob. Ecol. Conserv. 2021, 27, e01559. [Google Scholar] [CrossRef]
- Zhu, T.; Hu, F.; Gong, J.; Wang, X.; Chen, K.; Du, H.; Yang, D.; Wu, X. Community structure and species diversity of fishes in the Tibet reach of the Lancang River, China. J. Fish. Sci. China 2022, 29, 304–313. [Google Scholar]
- Scott, M.C.; Helfman, G.S. Native Invasions, Homogenization, and the Mismeasure of Integrity of Fish Assemblages. Fisheries 2001, 26, 6–15. [Google Scholar] [CrossRef]
- Alma, V.; Romagnoni, G.; Wolff, M. Exploration of fisheries management policies in the Gulf of Nicoya (Costa Rica) using ecosystem modelling. Ocean Coast. Manag. 2022, 230, 106349. [Google Scholar] [CrossRef]
- Comdie, H.M.; Grant, A.; Catchpole, T.L. Incentivising selective fishing under a policy to ban discards; lessons from European and global fisheries. Mar. Pol. 2014, 45, 287–292. [Google Scholar] [CrossRef]
- Macusi, E.D.; Liguez, A.K.O.; Digal, L.N. Factors influencing catch and support for the implementation of the closed fishing season in Davao Gulf, Philippines. Mar. Pol. 2021, 217, 105997. [Google Scholar] [CrossRef]
- Zorrozua, N.; Granado, I.; Fernandes-Salvador, J.A.A.; Louzao, M.; Basterretxea, M.; Arizaga, J. Evaluating the dependence of opportunistic Yellow-legged Gulls (Larus michahellis) on marine habitat and fishing discards. Int. J. Avian Sci. 2023, 166, 112–128. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Cottalorda, J.M.; Dominici, J.M.; Harmelin, J.G.; Diréach, L.L.; Ruitton, S. Effects of reserve protection level on the vulnerable fish species Sciaena umbra and implications for fishing management and policy. Glob. Ecol. Conserv. 2015, 3, 279–287. [Google Scholar] [CrossRef]
- Muawanah, U.; Gellwynn Yusuf, G.; Adrianto, L.; Kalther, J.; Pomeroy, R.; Abdullah, H.; Ruchimat, T. Review of national laws and regulation in Indonesia in relation to an ecosystem approach to fisheries management. Mar. Pol. 2018, 91, 150–160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).