Molecular Characterization and Protective Efficacy of a Novel Protein (EnSSB) Containing a Single-Stranded DNA-Binding Domain from Eimeria necatrix
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite, Animal and Antibody
2.2. Preparation of Parasites in Different Developmental Stages of E. necatrix
2.3. Transcript Levels of EnSSB in Different Developmental Stages of E. necatrix
2.4. Cloning and Sequence Analysis of EnSSB
2.5. Expression and Purification of the Recombinant EnSSB Protein
2.6. Preparation of Mouse Polyclonal Antibodies Against rEnSSB
2.7. Immunoblotting Analysis for rEnSSB and Native EnSSB in Different Developmental Stages of E. necatrix
2.8. Subcellular Localization of EnSSB Protein in E. necatrix Macrogametocytes
2.8.1. Indirect Immunofluorescence Assay
2.8.2. Immunogold Electron Microscopic Co-Localization
2.9. Evaluation of Immune Protection
2.9.1. Immunization Trail
2.9.2. Evaluation of the Protective Efficacy of rEnSSB Against Challenge Infection with E. necatrix
2.9.3. Detection of Serum Antibody Levels Against rEnSSB Using ELISA
2.9.4. Assessment of rEnSSB-Induced Alterations in CD4+ and CD8+ T Cells Using Flow Cytometry
2.10. Statistical Analysis
3. Results
3.1. Transcript Levels of EnSSB Gene
3.2. Cloning and Sequence Analysis of EnSSB Gene
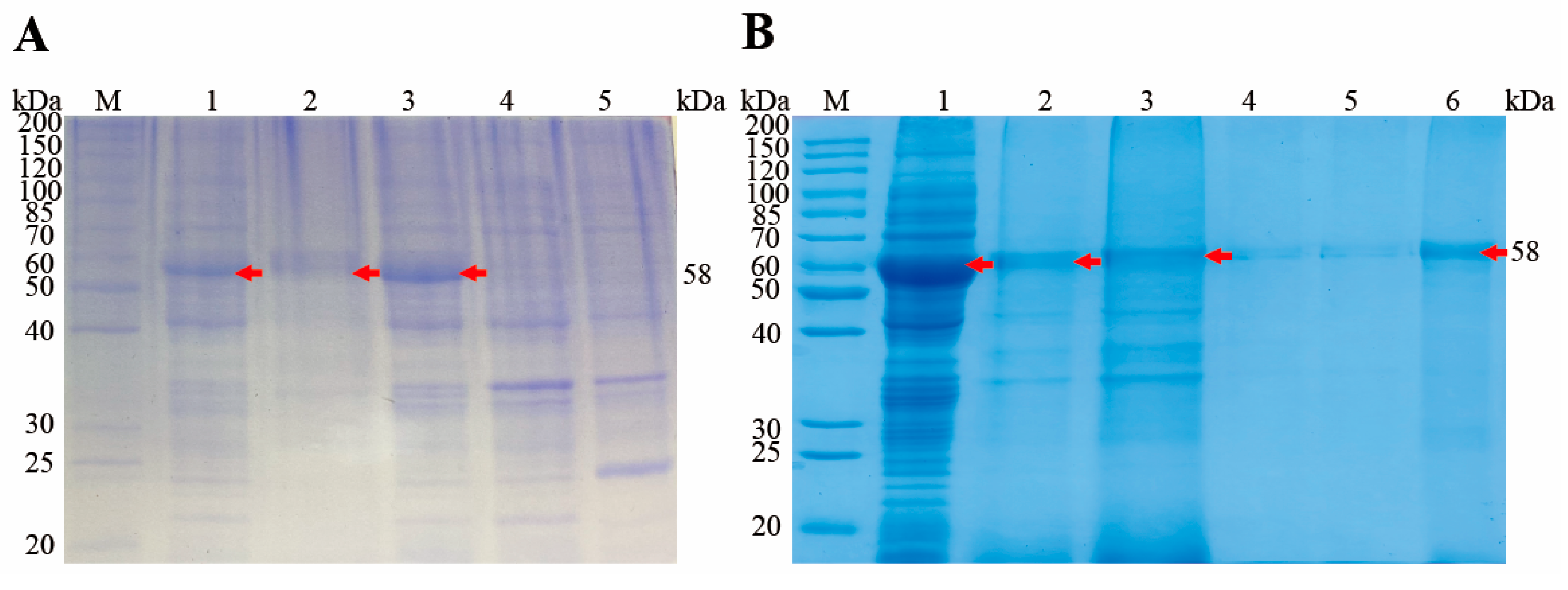
3.3. Expression and Purification of rEnSSB
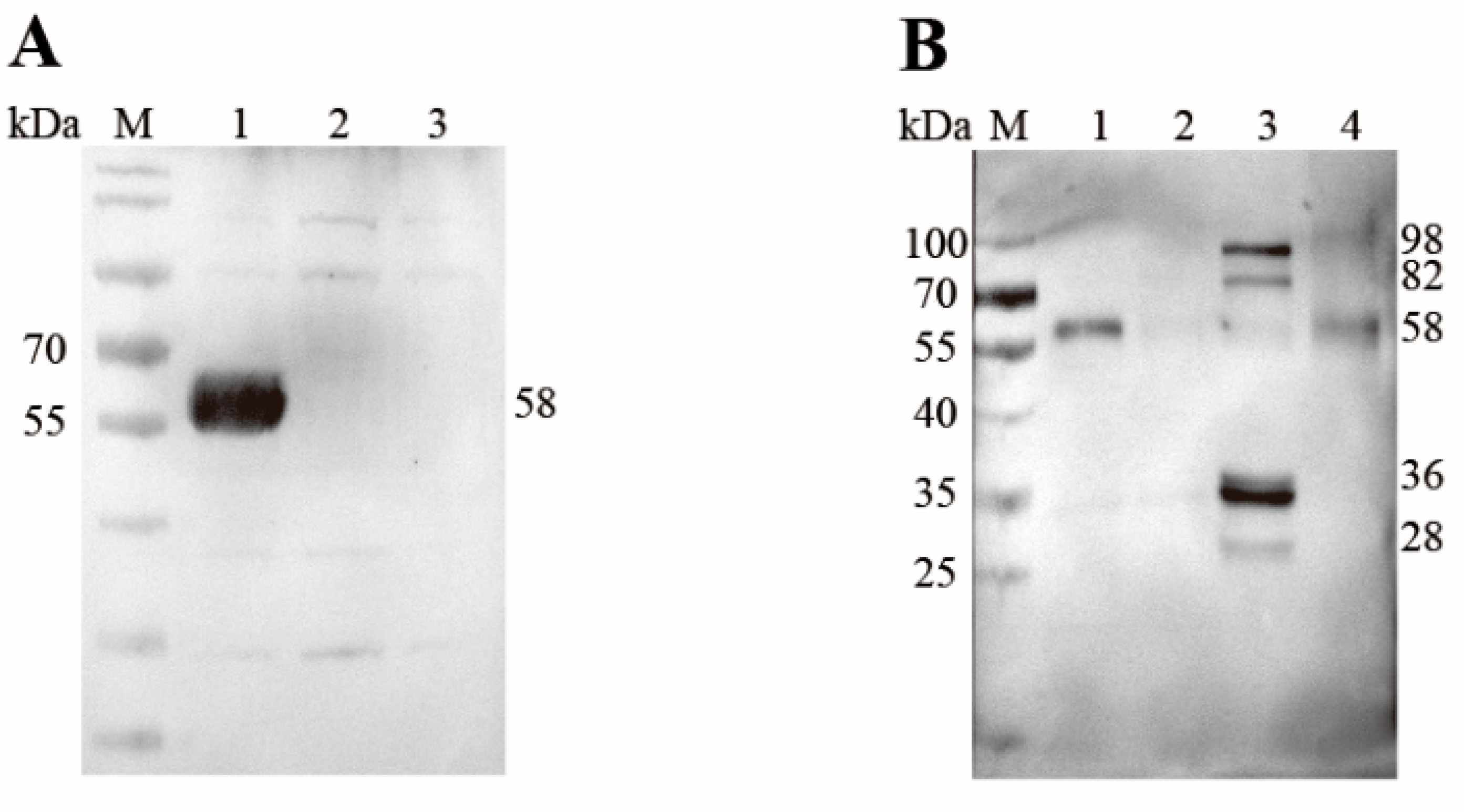
3.4. Western Blot Analysis for the Recombinant and Native EnSSB
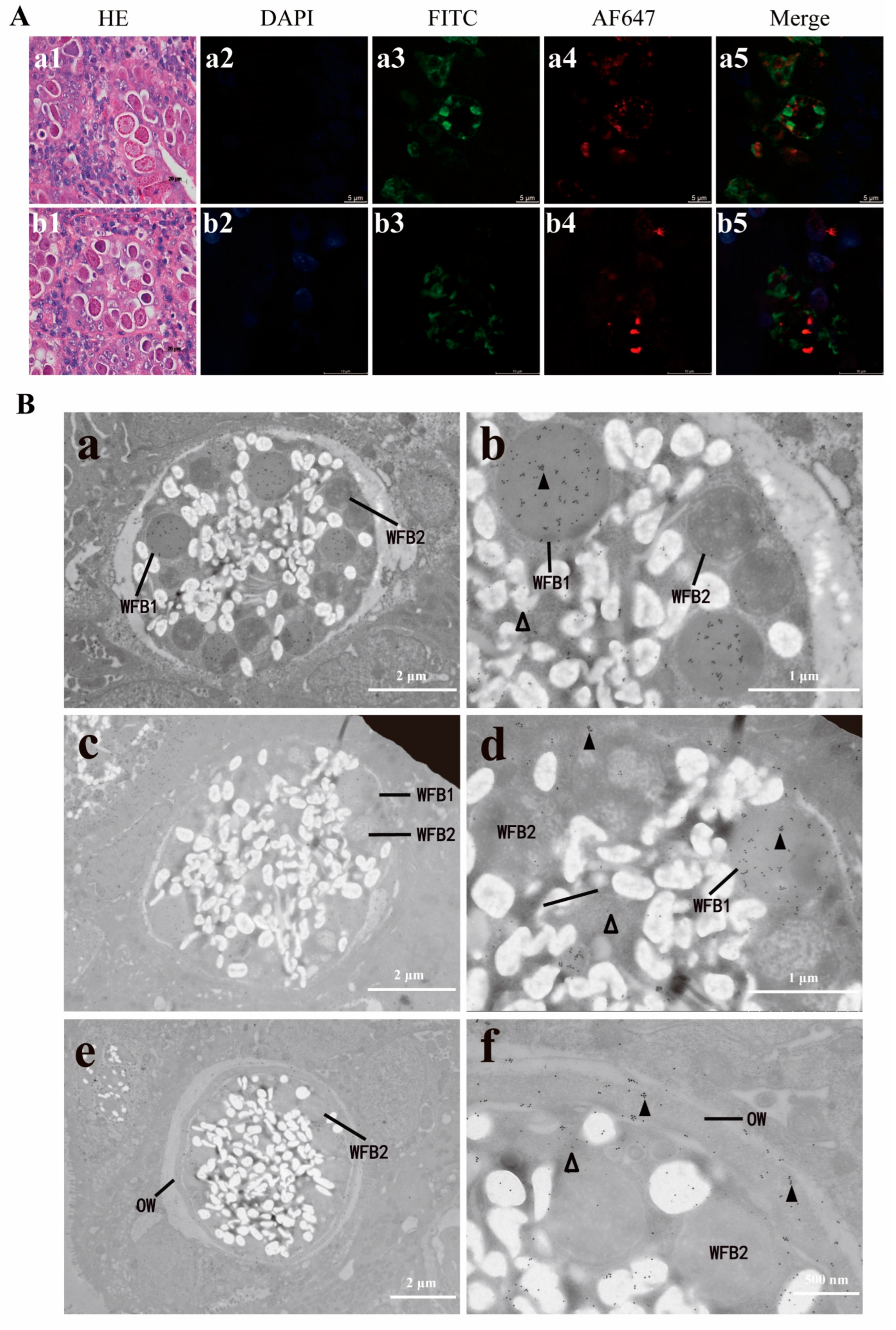
3.5. Subcellular Localization of Native EnSSB
3.6. Protective Efficacy of Vaccination on E. necatrix Challenge
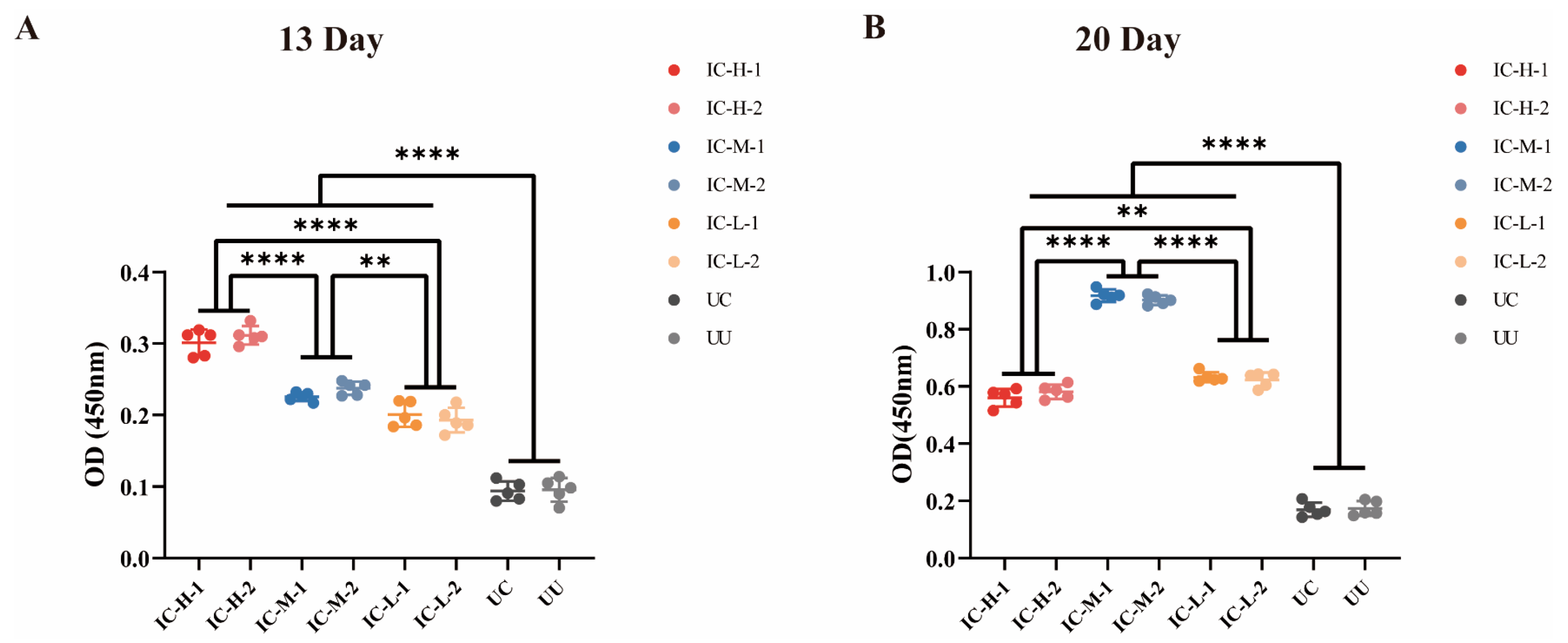
3.7. Serum Antibody Levels Against rEnSSB
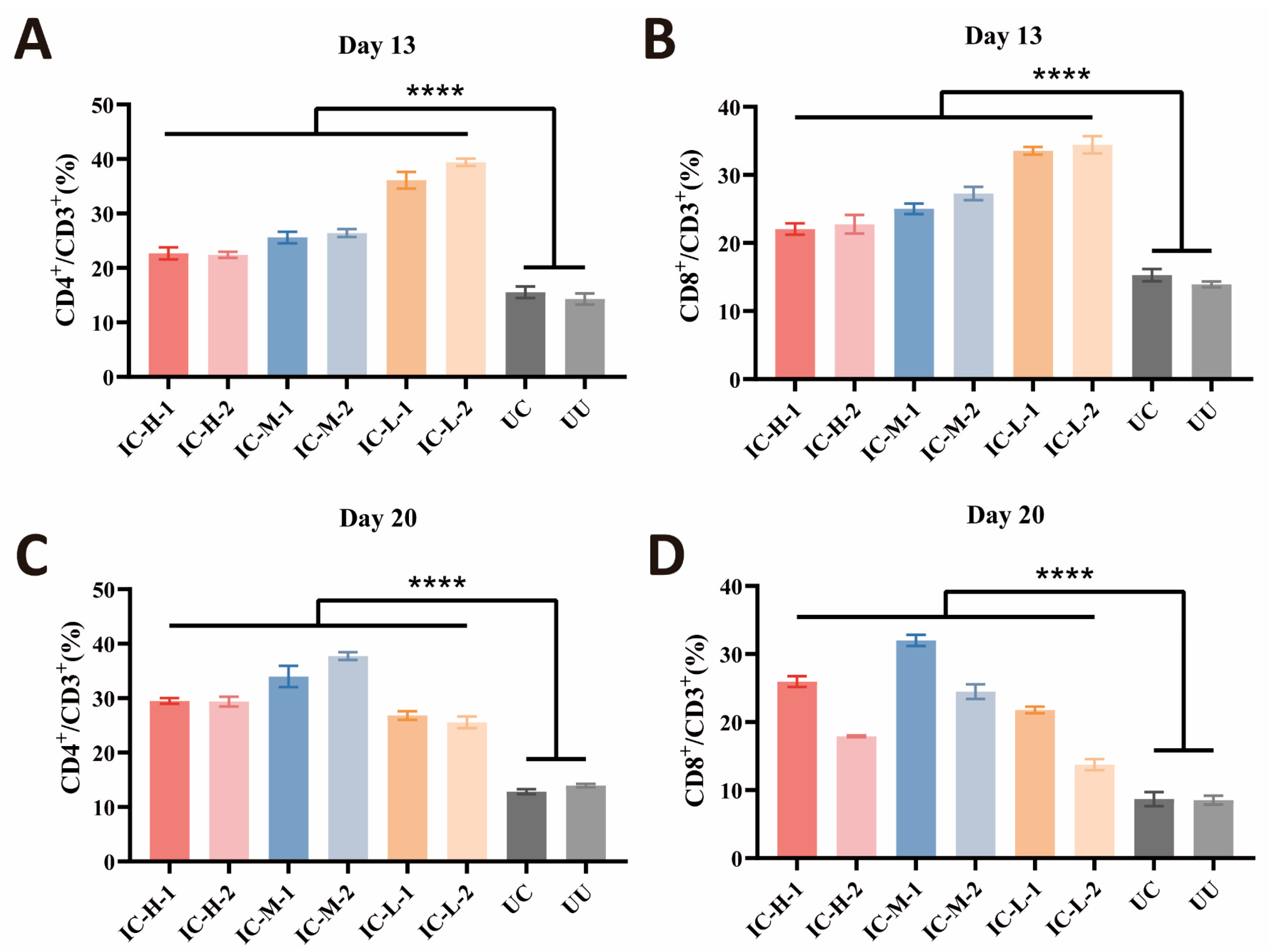
3.8. Effect of rEnSSB Immunization on CD4+/CD3+ and CD8+/CD3+ T Lymphocytes Subpopulation in Chickens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Group | IC-H-1 | IC-H-2 | IC-M-1 | IC-M-2 | IC-L-1 | IC-L-2 | UC | UU |
|---|---|---|---|---|---|---|---|---|
| 13 Day | 0.3012 ± 0.01824 | 0.3116 ± 0.01299 | 0.2254 ± 0.006066 | 0.2374 ± 0.009370 | 0.2010 ± 0.01749 | 0.1930 ± 0.01718 | 0.09380 ± 0.01352 | 0.09540 ± 0.01673 |
| 20 Day | 0.5604 ± 0.03109 | 0.5816 ± 0.02503 | 0.9174 ± 0.02182 | 0.9018 ± 0.01663 | 0.6324 ± 0.01704 | 0.6232 ± 0.02567 | 0.1690 ± 0.02481 | 0.1736 ± 0.02586 |
References
- Shirley, M.W.; Smith, A.L.; Tomley, F.M. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv. Parasitol. 2005, 60, 285–330. [Google Scholar]
- Quiroz-Castañeda, R.E.; Dantán-González, E. Control of avian coccidiosis: Future and present natural alternatives. Biomed. Res. Int. 2015, 2015, 430610. [Google Scholar] [CrossRef]
- Haug, A.; Gjevre, A.G.; Skjerve, E.; Kaldhusdal, M. A survey of the economic impact of subclinical Eimeria infections in broiler chickens in Norway. Avian Pathol. 2008, 37, 333–341. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Hao, L.; Liu, X.; Zhou, X.; Li, J.; Suo, X. Transient transfection of Eimeria tenella using yellow or red fluorescent protein as a marker. Mol. Biochem. Parasitol. 2007, 153, 213–215. [Google Scholar] [CrossRef]
- McDougald, L.R.; Fuller, A.L.; McMurray, B.L. An outbreak of Eimeria necatrix coccidiosis in breeder pullets: Analysis of immediate and possible long-term effects on performance. Avian Dis. 1990, 34, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, R.; Boviez, J.D.; McDougald, L.R. Eimeria brunetti and Eimeria necatrix in chickens of Argentina and confirmation of seven species of Eimeria. Avian Dis. 2000, 44, 711–714. [Google Scholar] [CrossRef]
- Mathis, G.F.; Lumpkins, B.; Cervantes, H.M.; Fitz-Coy, S.H.; Jenkins, M.C.; Jones, M.K.; Price, K.R.; Dalloul, R.A. Coccidiosis in poultry: Disease mechanisms, control strategies, and future directions. Poult. Sci. 2025, 104, 104663. [Google Scholar] [CrossRef]
- Soutter, F.; Priestnall, S.L.; Catchpole, B.; Rycroft, A.N. An experimental dermal oedema model for apx toxins of Actinobacillus pleuropneumoniae. J. Comp. Pathol. 2022, 195, 12–18. [Google Scholar] [CrossRef]
- Chapman, H.D.; Jeffers, T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 214–217. [Google Scholar] [CrossRef]
- Blake, D.P.; Pastor-Fernández, I.; Nolan, M.J.; Tomley, F.M. Recombinant anticoccidial vaccines—A cup half full? Infect. Genet. Evol. 2017, 55, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Jeffers, T.K.; Williams, R.B. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010, 89, 1788–1801. [Google Scholar] [CrossRef]
- Muthamilselvan, T.; Kuo, T.F.; Wu, Y.C.; Yang, W.C. Herbal remedies for coccidiosis control: A review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Alternat. Med. 2016, 2016, 2657981. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Zhu, Y.; Wang, F.; Cai, W.; Feng, Q.; Su, S.; Hou, Z.; Xu, J.; Hu, J.; et al. Comparative proteomic analysis of wall-forming bodies and oocyst wall reveals the molecular basis underlying oocyst wall formation in Eimeria necatrix. Parasit. Vectors 2023, 16, 460. [Google Scholar] [CrossRef]
- Gao, Y.; Suding, Z.; Wang, L.; Liu, D.; Su, S.; Xu, J.; Hu, J.; Tao, J. Full-length transcriptome sequence analysis of Eimeria necatrix unsporulated oocysts and sporozoites identifies genes involved in cellular invasion. Vet. Parasitol. 2021, 296, 109480. [Google Scholar] [CrossRef]
- Gao, Y.; Suding, Z.; Wang, L.; Liu, D.; Su, S.; Xu, J.; Hu, J.; Tao, J. Full-length transcriptome analysis and identification of transcript structures in Eimeria necatrix from different developmental stages by single-molecule real-time sequencing. Parasit. Vectors 2021, 14, 502. [Google Scholar] [CrossRef]
- Su, S.; Hou, Z.; Wang, L.; Liu, D.; Hu, J.; Xu, J.; Tao, J. Further confirmation of second- and third-generation Eimeria necatrix merozoite DEGs using suppression subtractive hybridization. Parasitol. Res. 2019, 118, 1159–1169. [Google Scholar] [CrossRef]
- Su, S.; Hou, Z.; Liu, D.; Jia, C.; Wang, L.; Xu, J.; Tao, J. Comparative transcriptome analysis of Eimeria necatrix third-generation merozoites and gametocytes reveals genes involved in sexual differentiation and gametocyte development. Vet. Parasitol. 2018, 252, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wobbe, C.R.; Weissbach, L.; Borowiec, J.A.; Dean, F.B.; Murakami, Y.; Bullock, P.; Hurwitz, J. Replication of simian virus 40 origin-containing DNA in vitro with purified proteins. Proc. Natl. Acad. Sci. USA 1987, 84, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Wold, M.S.; Kelly, T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc. Natl. Acad. Sci. USA 1988, 85, 2523–2527. [Google Scholar] [CrossRef]
- Alberts, B.M.; Frey, L. T4 bacteriophage gene 32: A structural protein in the replication and recombination of DNA. Nature 1970, 227, 1313–1318. [Google Scholar] [CrossRef]
- Wold, M.S. Replication protein A: A heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 1997, 66, 61–92. [Google Scholar] [CrossRef]
- Lohman, T.M.; Ferrari, M.E. Escherichia coli single-stranded DNA-binding protein: Multiple DNA-binding modes and cooperativities. Annu. Rev. Biochem. 1994, 63, 527–570. [Google Scholar] [CrossRef]
- Cha, T.A.; Alberts, B.M. The bacteriophage T4 DNA replication fork. Only DNA helicase is required for leading strand DNA synthesis by the DNA polymerase holoenzyme. J. Biol. Chem. 1989, 264, 12220–12225. [Google Scholar] [CrossRef]
- Kim, Y.T.; Richardson, C.C. Bacteriophage T7 gene 2.5 protein: An essential protein for DNA replication. Proc. Natl. Acad. Sci. USA 1993, 90, 10173–10177. [Google Scholar] [CrossRef]
- Pestryakov, P.E.; Lavrik, O.I. Mechanisms of single-stranded DNA-binding protein functioning in cellular DNA metabolism. Biochemistry 2008, 73, 1388–1404. [Google Scholar] [CrossRef] [PubMed]
- Kur, J.; Olszewski, M.; Długołecka, A.; Filipkowski, P. Single-stranded DNA-binding proteins (SSBs)—Sources and applications in molecular biology. Acta Biochim. Pol. 2005, 52, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Roberts, B.; Shirley, M.W.; Williams, R.B. Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathol. 2005, 34, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.W.; Melendy, T.E.; Ray, D.S. Conservation of structure and function of DNA replication protein A in the trypanosomatid Crithidia fasciculata. Proc. Natl. Acad. Sci. USA 1992, 89, 10227–10231. [Google Scholar] [CrossRef]
- Zhu, G.; Marchewka, M.J.; Keithly, J.S. Cryptosporidium parvum possesses a short-type replication protein A large subunit that differs from its host. FEMS Microbiol. Lett. 1999, 176, 367–372. [Google Scholar] [CrossRef]
- Millership, J.J.; Zhu, G. Heterogeneous expression and functional analysis of two distinct replication protein A large subunits from Cryptosporidium parvum. Int. J. Parasitol. 2002, 32, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.S.; Mini, T.; Jenoe, P.; Beck, H.P. Plasmodium falciparum possesses a cell cycle-regulated short type replication protein A large subunit encoded by an unusual transcript. J. Biol. Chem. 2002, 277, 17493–17501. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cao, L.; Zhu, Y.; Deng, C.; Su, S.; Xu, J.; Jin, W.; Li, J.; Wu, L.; Tao, J. Cloning and characterization of an Eimeria necatrix gene encoding a gametocyte protein and associated with oocyst wall formation. Parasit. Vectors 2014, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, D.; Gao, Y.; Hou, Z.; Zhu, Y.; Wang, F.; Li, W.; Zhang, A.; Xu, J.; Hu, J.; et al. Examination of gametocyte protein 22 localization and oocyst wall formation in Eimeria necatrix using laser confocal microscopy and scanning electron microscopy. Parasit. Vectors 2023, 16, 124. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, D.; Wang, L.; Feng, Q.; Wang, F.; Xue, N.; Hou, Z.; Xu, J.; Hu, J.; Tao, J. Observations of the fine structural changes associated with merogony and gametogony in Eimeria necatrix and localization of two gametocyte proteins. Microorganisms 2025, 13, 1135. [Google Scholar] [CrossRef]
- Su, S.; Hou, Z.; Liu, D.; Jia, C.; Wang, L.; Xu, J.; Tao, J. Comparative transcriptome analysis of second- and third-generation merozoites of Eimeria necatrix. Parasit. Vectors 2017, 10, 388. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, D.; Wang, F.; Cao, L.; Wang, L.; Su, S.; Hou, Z.; Xu, J.; Hu, J.; Tao, J. Identification and characterization of a cDNA encoding a gametocyte-specific protein of the avian coccidial parasite Eimeria necatrix. Mol. Biochem. Parasitol. 2020, 240, 111318. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- McManus, E.C.; Campbell, W.C.; Cuckler, A.C. Development of resistance to quinoline coccidiostats under field and laboratory conditions. J. Parasitol. 1968, 54, 1190–1193. [Google Scholar] [CrossRef]
- Shereda, R.D.; Kozlov, A.G.; Lohman, T.M.; Cox, M.M.; Keck, J.L. SSB as an organizer/mobilizer of genome maintenance complexes. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 289–318. [Google Scholar] [CrossRef]
- Murzin, A.G. OB(oligonucleotide/oligosaccharide binding)-fold: Common structural and functional solution for non-homologous sequences. EMBO J. 1993, 12, 861–867. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Kaguni, L.S. Functional roles of the N- and C-terminal regions of the human mitochondrial single-stranded DNA-binding protein. PLoS ONE 2010, 5, e15379. [Google Scholar] [CrossRef]
- Millership, J.J.; Cai, X.; Zhu, G. Functional characterization of replication protein A2 (RPA2) from Cryptosporidium parvum. Microbiol. (Read. ) 2004, 150 Pt 5, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Rider, S.D., Jr.; Cai, X.; Sullivan, W.J., Jr.; Smith, A.T.; Radke, J.; White, M.; Zhu, G. The protozoan parasite Cryptosporidium parvum possesses two functionally and evolutionarily divergent replication protein A large subunits. J. Biol. Chem. 2005, 280, 31460–31469. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, E.; Foury, F.; Stillman, B.; Brill, S.J. A single-stranded DNA binding protein required for mitochondrial DNA replication in S. cerevisiae is homologous to E. coli SSB. EMBO J. 1992, 11, 3421–3430. [Google Scholar] [CrossRef]
- Prusty, D.; Dar, A.; Priya, R.; Sharma, A.; Dana, S.; Choudhury, N.R.; Rao, N.S.; Dhar, S.K. Single-stranded DNA binding protein from human malarial parasite Plasmodium falciparum is encoded in the nucleus and targeted to the apicoplast. Nucleic Acids Res. 2010, 38, 7037–7053. [Google Scholar] [CrossRef] [PubMed]
- Jong, A.Y.; Aebersold, R.; Campbell, J.L. Multiple species of single-stranded nucleic acid-binding proteins in Saccharomyces cerevisiae. J. Biol. Chem. 1985, 260, 16367–16374. [Google Scholar] [CrossRef]
- Wu, Y.; Zhan, H.; Zhang, S.; Wang, C.; Zhang, M.; Wang, M.; Tian, B.; Yang, Q.; Jia, R.; Chen, S.; et al. The third conserved cysteine residue in the zinc-binding domain of duck plague virus ICP8 is responsible for its single-stranded DNA-binding ability, viral attenuation and protection against lethal challenge. FASEB J. 2025, 39, e70610. [Google Scholar] [CrossRef]


| Primer Name | Sequence 5′-3′ |
|---|---|
| EnSSB-F | ATGGCGGAGTCCTTCAC |
| EnSSB-R | TTATATGCCTCTTCTCCCTCG |
| pET-EnSSB-F | ggaattccatatgATGGCGGAGTCCTTCAC |
| pET-EnSSB-R | cgcggatccTTATATGCCTCTTCTCCCTCG |
| qEnSSB-F | CATACAGCAGGTCAAGCCAGAGATC |
| qEnSSB-R | CTGAGTTCCGCAGCACGATGAG |
| pMD-18T-RV-M | AGCGGATAACAATTTCACACAGGA |
| pMD-18T-M13-47 | CGCCAGGGTTTTCCCAGTCACGAC |
| pET-28a-T7 | TAATACGACTCACTATAGGG |
| pET-28a-T7ter | TGCTAGTTATTGCTCAGCGG |
| qEn18S-F | GAAACTGCGAATGGCTCATT |
| qEn18S-R | CTTGCGCGTACTAGGCATTC |
| Groups | Names | No. of Chickens | Immunization-Dose (μg) and Route | Challenge-Oocyst Dose (×104) and Route | |
|---|---|---|---|---|---|
| 6 Days Old * | 13 Days Old ** | 20 Days Old | |||
| 1 | IC-H-1 | 20 | 200/S.C *** | 200/S.C | 2/Oral gavage |
| 2 | IC-H-2 | 20 | 200/S.C | 200/S.C | 2/Oral gavage |
| 3 | IC-M-1 | 20 | 100/S.C | 100/S.C | 2/Oral gavage |
| 4 | IC-M-2 | 20 | 100/S.C | 100/S.C | 2/Oral gavage |
| 5 | IC-L-1 | 20 | 50/S.C | 50/S.C | 2/Oral gavage |
| 6 | IC-L-2 | 20 | 50/S.C | 50/S.C | 2/Oral gavage |
| 7 | UC | 20 | PBS/S.C | PBS/S.C | 2/Oral gavage |
| 8 | UU | 20 | PBS/S.C | PBS/S.C | PBS/Oral gavage |
| Groups | Names | SR (%) |
BWG (g) | RBWG (%) | OP Per Bird (×106) | ROP (%) | LS | LI | OI | ACI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IC-H-1 | 100 | 47.99 ± 8.39 abc | 82.94 | 0.92 | 71.25 | 2.30 ± 0.54 bcd | 23.00 | 10.00 | 149.94 |
| 2 | IC-H-2 | 100 | 47.29 ± 8.33 abc | 81.73 | 0.94 | 70.63 | 2.15 ± 0.58 bcd | 21.50 | 10.00 | 150.23 |
| 3 | IC-M-1 | 100 | 54.48 ± 9.99 c | 94.16 | 1.53 | 52.19 | 1.90 ± 0.52 b | 19.00 | 10.00 | 165.16 |
| 4 | IC-M-2 | 100 | 54.19 ± 8.92 bc | 93.66 | 1.35 | 57.81 | 1.85 ± 0.67 b | 18.50 | 10.00 | 165.16 |
| 5 | IC-L-1 | 100 | 39.33 ± 14.09 a | 67.97 | 2.38 | 25.63 | 2.65 ± 0.41 cd | 26.50 | 20.00 | 121.47 |
| 6 | IC-L-2 | 100 | 42.38 ± 15.63 a | 73.25 | 2.17 | 32.19 | 2.55 ± 0.64 bcd | 25.50 | 20.00 | 127.75 |
| 7 | UC | 100 | 43.93 ± 11.50 a | 75.92 | 3.20 | 0.00 | 2.90 ± 0.39 d | 29.00 | 40.00 | 106.92 |
| 8 | UU | 100 | 57.86 ± 8.82 bc | 100.00 | NA | 100.00 | 0.00 ± 0.00 a | 0.00 | 0.00 | 200.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Liu, D.; Wang, L.; Feng, Q.; Xue, N.; Hou, Z.; Xu, J.; Tao, J. Molecular Characterization and Protective Efficacy of a Novel Protein (EnSSB) Containing a Single-Stranded DNA-Binding Domain from Eimeria necatrix. Animals 2025, 15, 2482. https://doi.org/10.3390/ani15172482
Zhu Y, Liu D, Wang L, Feng Q, Xue N, Hou Z, Xu J, Tao J. Molecular Characterization and Protective Efficacy of a Novel Protein (EnSSB) Containing a Single-Stranded DNA-Binding Domain from Eimeria necatrix. Animals. 2025; 15(17):2482. https://doi.org/10.3390/ani15172482
Chicago/Turabian StyleZhu, Yu, Dandan Liu, Lele Wang, Qianqian Feng, Nianyu Xue, Zhaofeng Hou, Jinjun Xu, and Jianping Tao. 2025. "Molecular Characterization and Protective Efficacy of a Novel Protein (EnSSB) Containing a Single-Stranded DNA-Binding Domain from Eimeria necatrix" Animals 15, no. 17: 2482. https://doi.org/10.3390/ani15172482
APA StyleZhu, Y., Liu, D., Wang, L., Feng, Q., Xue, N., Hou, Z., Xu, J., & Tao, J. (2025). Molecular Characterization and Protective Efficacy of a Novel Protein (EnSSB) Containing a Single-Stranded DNA-Binding Domain from Eimeria necatrix. Animals, 15(17), 2482. https://doi.org/10.3390/ani15172482






