Nutrient Profile, Energy Digestibility in Pigs, and In Vitro Degradation Characteristics of Wheat Flour Milling Co-Products
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1. In Vitro Digestibility
2.2. Experiment 2. In Vivo Digestibility
2.3. Experiment 3. In Vitro Fermentation
2.4. Chemical Analyses and Calculations
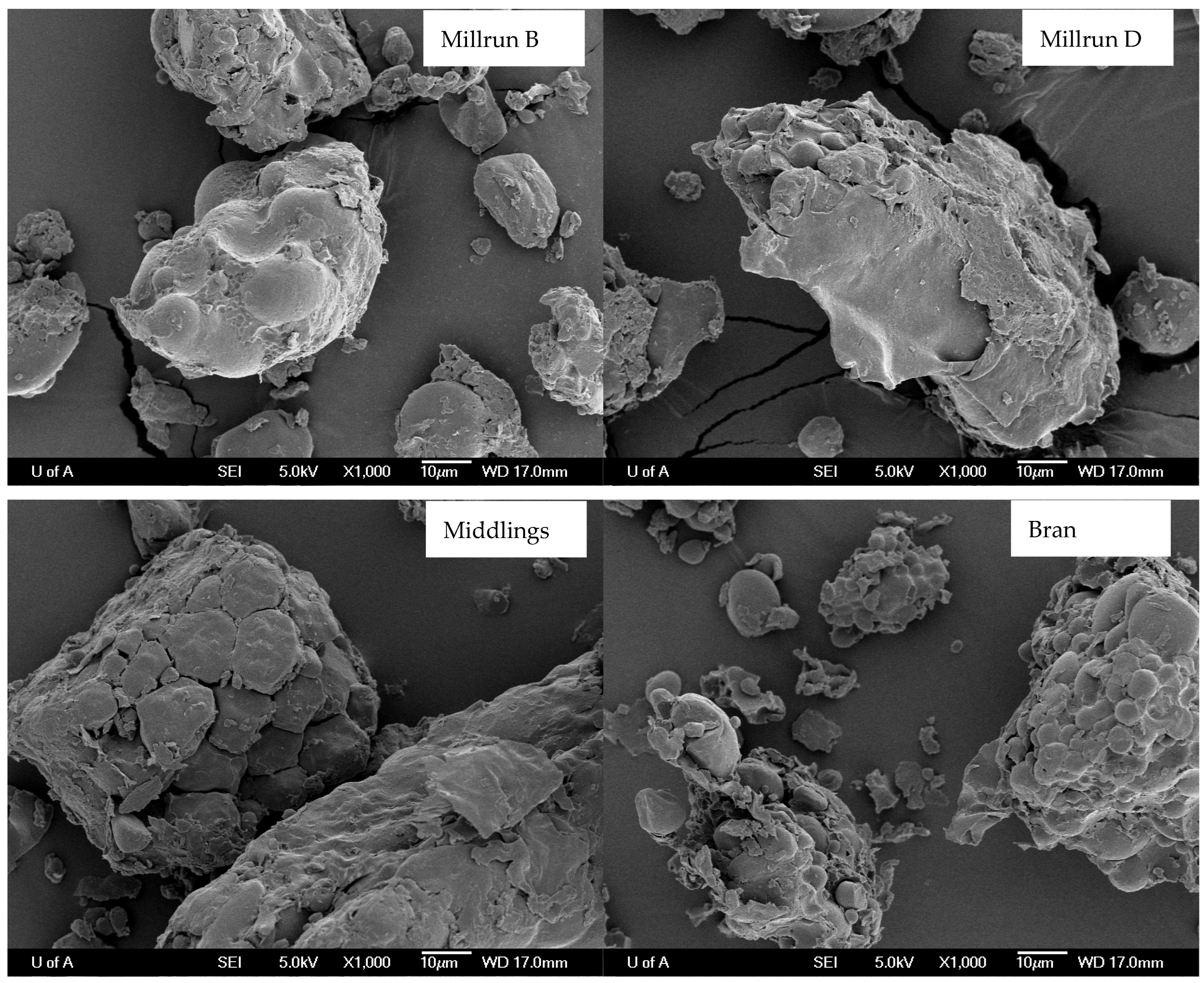
2.5. Scanning Electron Microscopy
2.6. Kinetics of Gas Production
2.7. Calculation of Digestibility
2.8. Statistical Analyses
3. Results
3.1. Chemical Characteristics
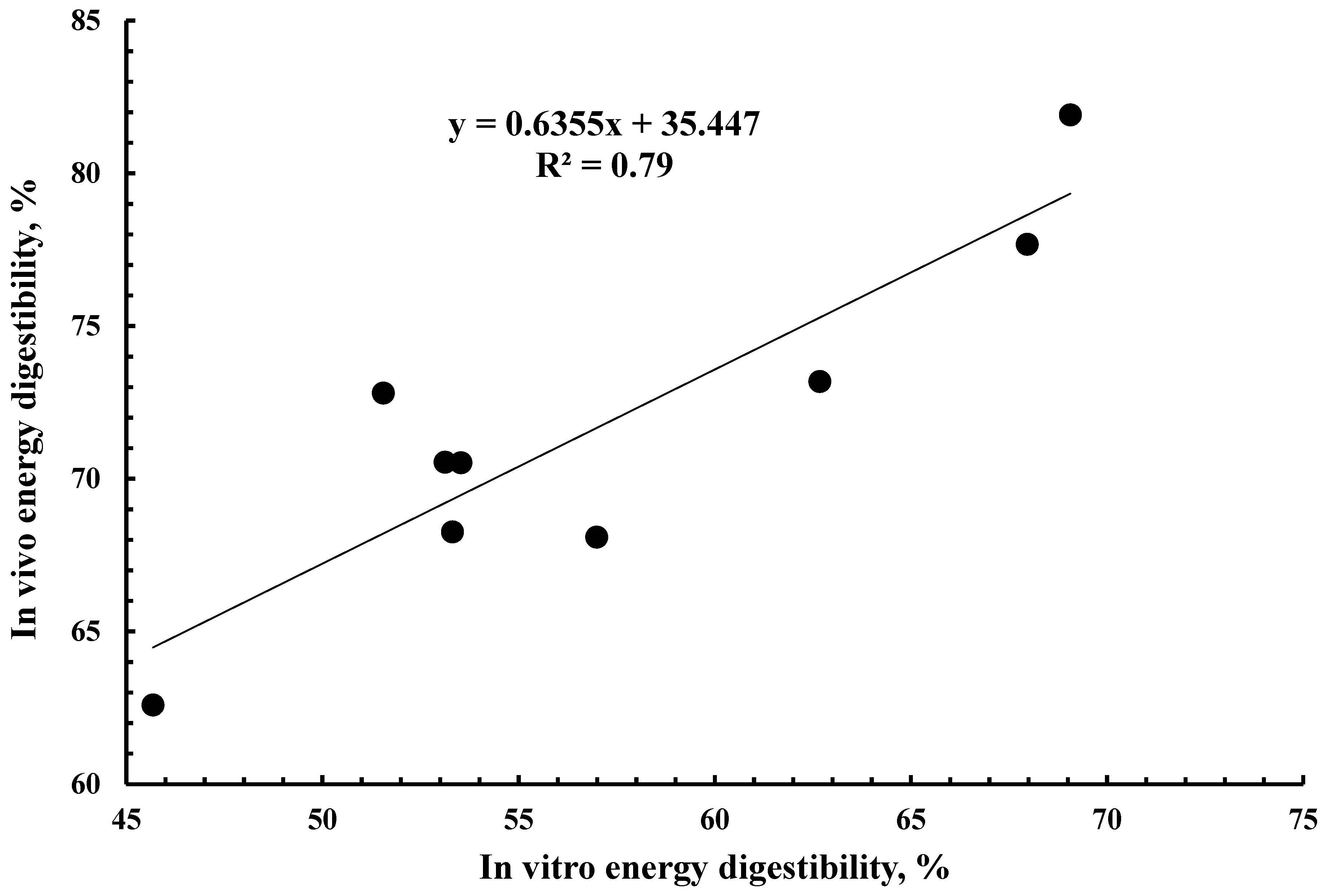
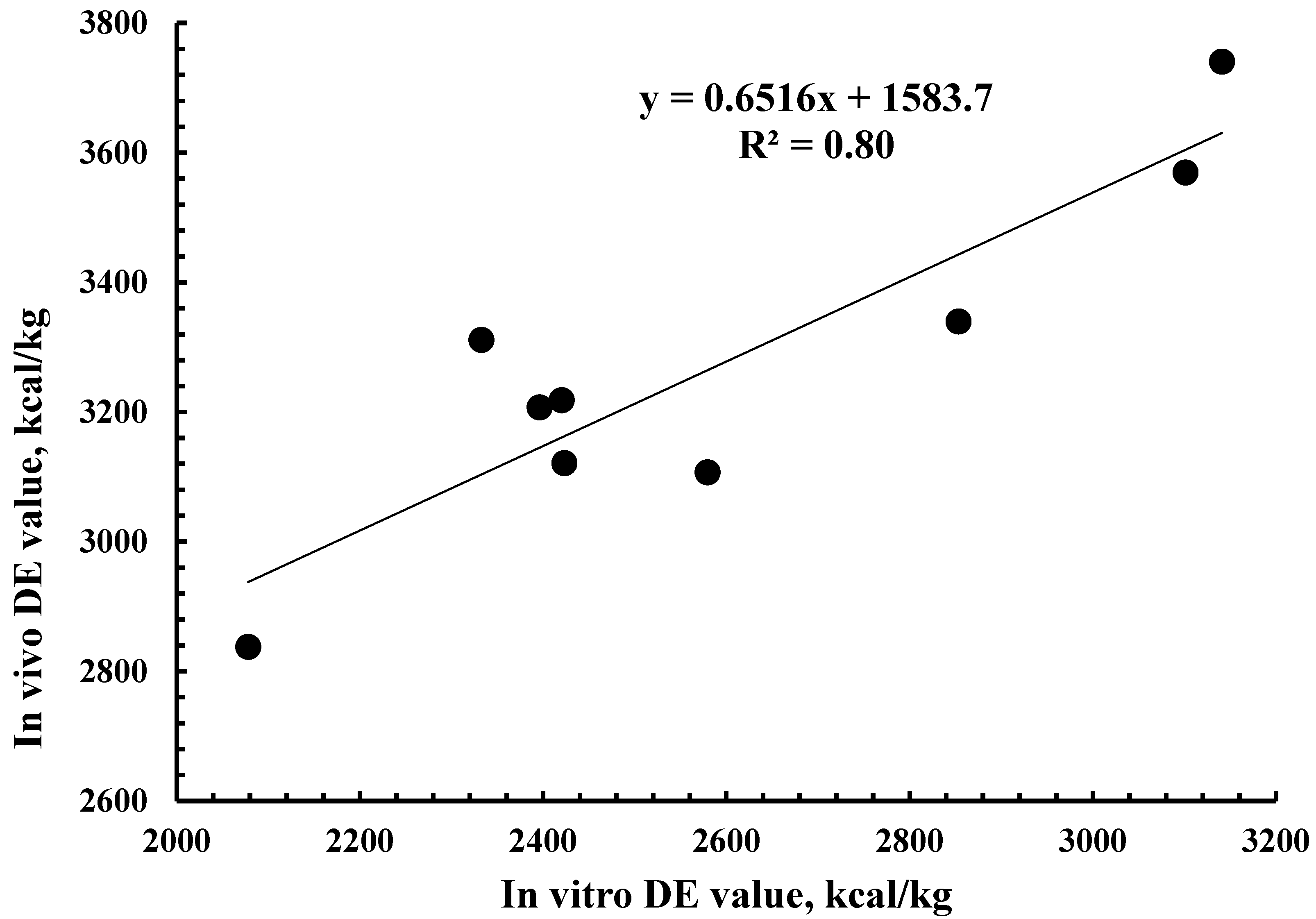
3.2. Energy Digestibility
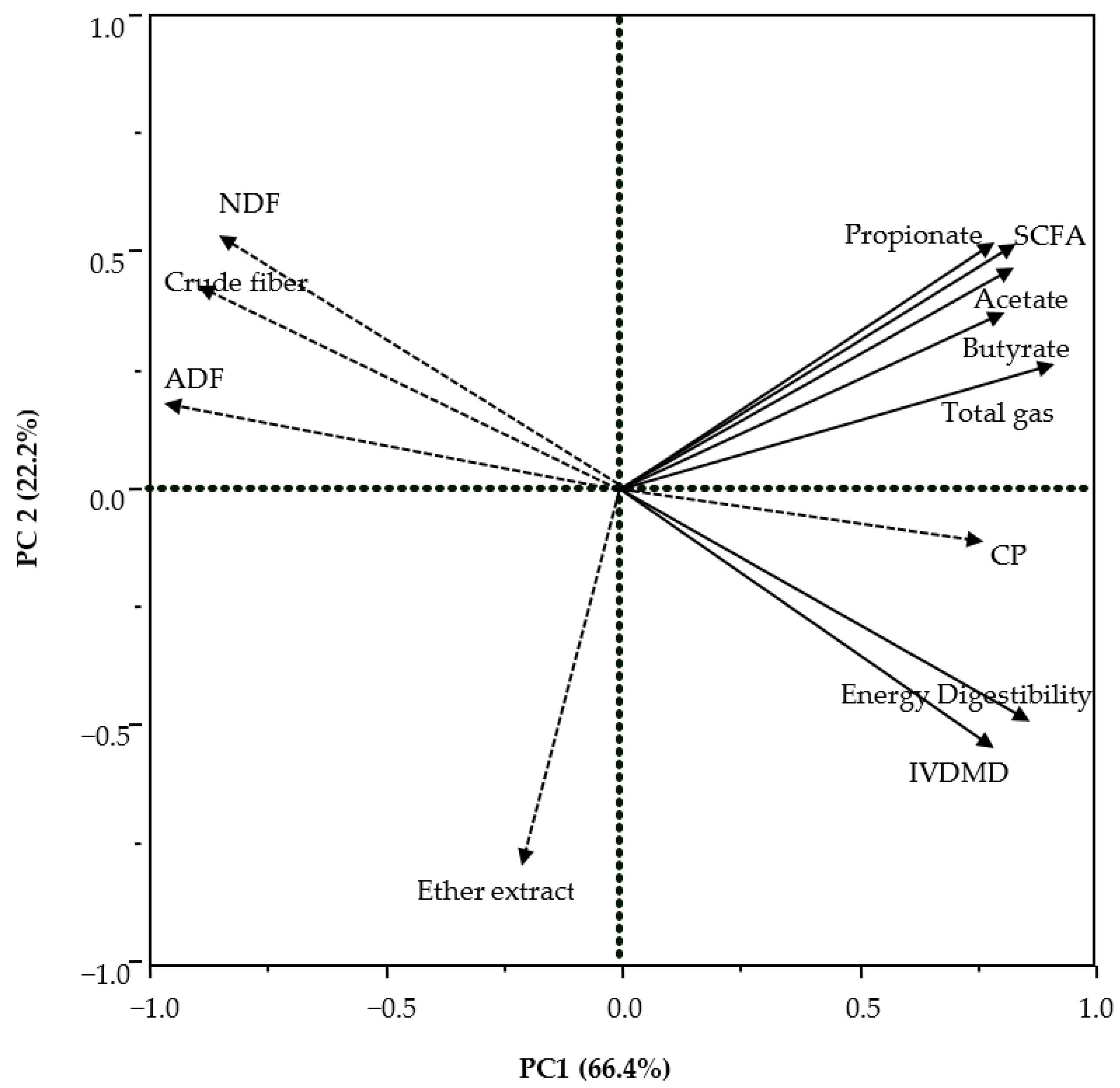
3.3. Relationship of Digestibility with Chemical and Fermentation Characteristics
3.4. In Vitro Fermentation Kinetics and Metabolites
3.5. Microscopic Matrix Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Van Zanten, H.H.E.; Van Ittersum, M.K.; De Boer, I.J.M. The role of farm animals in a circular food system. Glob. Food Sec. 2019, 21, 18–22. [Google Scholar] [CrossRef]
- Zijlstra, R.T.; Beltranena, E. Feeding co-products to pigs to reduce feed cost and reach sustainable food production. Anim. Front. 2022, 12, 18–22. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Kerr, B.J.; Shurson, G.C. Strategies to improve fiber utilization in swine. J. Anim. Sci. Biotechnol. 2013, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Woyengo, T.A.; Beltranena, E.; Zijlstra, R.T. Controlling feed cost by including alternative ingredients into pig diets: A review. J. Anim. Sci. 2014, 92, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Rosenfelder, R.; Eklund, M.; Mosenthin, R. Nutritive value of wheat and wheat by-products in pig nutrition: A review. Anim. Feed Sci. Technol. 2013, 185, 107–125. [Google Scholar] [CrossRef]
- Noblet, J.; Perez, J.M. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J. Anim. Sci. 1993, 71, 3389–3398. [Google Scholar] [CrossRef]
- Jha, R.; Leterme, P. Feed ingredients differing in fermentable fibre and indigestible protein content affect fermentation metabolites and faecal nitrogen excretion in growing pigs. Animal 2012, 6, 603–611. [Google Scholar] [CrossRef]
- Lindberg, J.E. Fiber effects in nutrition and gut health in pigs. J. Anim. Sci. Biotechnol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Nortey, T.N.; Patience, J.F.; Sands, J.S.; Trottier, N.L.; Zijlstra, R.T. Effects of xylanase supplementation on digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat by-products from dry milling in grower pigs. J. Anim. Sci. 2008, 86, 450–3464. [Google Scholar] [CrossRef]
- Boisen, S.; Fernandez, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Regmi, P.R.; Sauer, W.C.; Zijlstra, R.T. Prediction of in vivo apparent total tract energy digestibility of barley in grower pigs using an in vitro digestibility technique. J. Anim. Sci. 2008, 86, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.R.; Ferguson, N.S.; Zijlstra, R.T. In vitro digestibility techniques to predict apparent total tract energy digestibility of wheat in grower pigs. J. Anim. Sci. 2009, 87, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Zijlstra, R.T. Prediction of bioavailable nutrients and energy. In Feed Evaluation Science; Moughan, P.J., Hendriks, W.H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 337–386. [Google Scholar]
- CCAC. Guidelines on: The care and Use of Farm Animals in Research, Teaching and Testing; Canadian Council on Animal Care: Ottawa, ON, Canada, 2009. [Google Scholar]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- NRC. Nutrient Requirements of Swine, 10th ed.; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Jha, R.; Bindelle, J.; Van Kessel, A.; Leterme, P. In vitro fibre fermentation of feed ingredients with varying fermentable carbohydrate and protein levels and protein synthesis by colonic bacteria isolated from pigs. Anim. Feed Sci. Technol. 2011, 165, 191–200. [Google Scholar] [CrossRef]
- Jha, R.; Bindelle, J.; Rossnagel, B.; Van Kessel, A.; Leterme, P. In vitro evaluation of the fermentation characteristics of the carbohydrate fractions of hulless barley and other cereals in the gastrointestinal tract of pigs. Anim. Feed Sci. Technol. 2011, 163, 185–193. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 1999, 79, 321–330. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2006. [Google Scholar]
- Fenton, T.W.; Fenton, M. An improved procedure for the determination of chromic oxide in feed and feces. Can. J. Anim. Sci. 1979, 59, 631–634. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- France, J.; Dhanoa, M.S.; Theodorou, M.K.; Lister, S.J.; Davies, D.R.; Isac, D. A model to interpret gas accumulation profiles associated with in vitro degradation of ruminant feeds. J. Theor. Biol. 1993, 163, 99–111. [Google Scholar] [CrossRef]
- Jørgensen, H.; Sauer, W.C.; Thacker, P.A. Amino acid availabilities in soybean meal, sunflower meal, fish meal and meat and bone meal fed to growing pigs. J. Anim. Sci. 1984, 58, 926–934. [Google Scholar] [CrossRef]
- Adeola, O. Digestion and balance techniques in pigs. In Swine Nutrition; Lewis, A.J., Southern, L.L., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2001; pp. 903–916. [Google Scholar]
- SAS. SAS/STAT User’s Guide. Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Saxton, A.M. A Macro for Converting Mean Separation Output to Letter Groupings in Proc Mixed. In Proceedings of 23rd SAS User Group International; SAS Institute: Cary, NC, USA, 1998; pp. 1243–1246. [Google Scholar]
- Tervila-Wilo, A.; Parkkonen, T.; Morgan, A.; Hopeakoski-Nurminen, M.; Poutanen, K.; Heikkinen, P.; Autio, K. In vitro digestion of wheat microstructure with xylanase and cellulase from Trichoderma reesei. J. Cereal Sci. 1996, 24, 215–225. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef] [PubMed]
- Slominski, B.A.; Boros, D.; Campbell, L.D.; Guenter, W.; Jones, O. Wheat by-products in poultry nutrition. Part I. Chemical and nutritive composition of wheat screenings, bakery by-products and wheat mill run. Can. J. Anim. Sci. 2004, 84, 421–428. [Google Scholar] [CrossRef]
- Noblet, J.; Le Goff, G. Effect of dietary fibre on the energy value of feeds for pigs. Anim. Feed Sci. Technol. 2001, 90, 35–52. [Google Scholar] [CrossRef]
- Jaworski, N.W.; Lærke, H.N.; Bach Knudsen, K.E.; Stein, H.H. Carbohydrate composition and in vitro digestibility of dry matter and non-starch polysaccharides in corn, sorghum, and wheat and co-products from these grains. J. Anim. Sci. 2015, 93, 1103–1113. [Google Scholar] [CrossRef]
- Jaworski, N.W.; Liu, D.W.; Li, D.F.; Stein, H.H. Wheat bran reduces concentrations of digestible, metabolizable, and net energy in diets fed to pigs, but energy values in wheat bran determined by the difference procedure are not different from values estimated from a linear regression procedure. J. Anim. Sci. 2016, 94, 3012–3021. [Google Scholar] [CrossRef]
- Lee, G.I.; Hedemann, M.S.; Jorgensen, H.; Bach Knudsen, K.E. Influence of dietary fibre on nutrient digestibility and energy utilisation in growing pigs fed diets varying in soluble and insoluble fibres from co-products. Animal 2022, 16, 100511. [Google Scholar] [CrossRef]
- Jha, R.; Woyengo, T.A.; Li, J.; Bedford, M.R.; Vasanthan, T.; Zijlstra, R.T. Enzymes enhance degradation of the fiber-starch-protein matrix of distillers dried grains with solubles as revealed by a porcine in vitro fermentation model and microscopy. J. Anim. Sci. 2015, 93, 1039–1051. [Google Scholar] [CrossRef]
- Jha, R.; Zijlstra, R.T. Physico-chemical properties of purifies fiber and their in vitro fermentation characteristics and are linked to in vivo characteristics in pigs. Can. J. Anim. Sci. 2018, 98, 394–398. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. The nutritional significance of “dietary fibre” analysis. Anim. Feed Sci. Technol. 2001, 90, 3–20. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]

| Ingredient, %, As-Fed Basis | Test Diet | Control Diet |
|---|---|---|
| Corn | 560 | 960 |
| WFM co-products 1 | 400 | – |
| Limestone | 12 | 12 |
| CaHPO4 | 8 | 8 |
| Salt | 5 | 5 |
| Mineral premix 2 | 5 | 5 |
| Vitamin premix 3 | 5 | 5 |
| Chromic oxide | 5 | 5 |
| Shorts | Millrun | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyzed Content | A | B | A | B | C | D | E | Middlings | Bran | Corn |
| Ingredient | ||||||||||
| Dry matter | 901 | 899 | 904 | 891 | 892 | 904 | 902 | 886 | 910 | 854 |
| Ash | 73 | 55 | 75 | 65 | 54 | 62 | 52 | 53 | 67 | 21 |
| Ether extract | 29 | 34 | 56 | 48 | 45 | 41 | 44 | 41 | 30 | 33 |
| Crude protein | 278 | 249 | 200 | 187 | 171 | 190 | 165 | 221 | 159 | 94 |
| Acid-detergent fiber | 115 | 80 | 114 | 129 | 130 | 150 | 128 | 108 | 155 | 36 |
| Neutral-detergent fiber | 317 | 229 | 326 | 340 | 352 | 363 | 321 | 266 | 492 | 87 |
| Crude fiber | 79 | 52 | 77 | 83 | 88 | 99 | 82 | 71 | 120 | 18 |
| GE (Mcal/kg DM) | 4.56 | 4.57 | 4.55 | 4.55 | 4.57 | 4.56 | 4.56 | 4.60 | 4.53 | 4.35 |
| Diet | ||||||||||
| Dry matter | 855 | 867 | 876 | 874 | 875 | 874 | 875 | 868 | 876 | 858 |
| Ash | 20 | 75 | 74 | 72 | 66 | 68 | 65 | 67 | 70 | 55 |
| Ether extract | 34 | 31 | 41 | 38 | 38 | 36 | 39 | 41 | 36 | 36 |
| Crude protein | 93 | 160 | 131 | 124 | 119 | 129 | 118 | 147 | 115 | 91 |
| Acid-detergent fiber | 34 | 66 | 71 | 75 | 81 | 82 | 73 | 66 | 79 | 42 |
| Neutral-detergent fiber | 87 | 165 | 187 | 202 | 197 | 196 | 195 | 164 | 240 | 98 |
| Crude fiber | 20 | 43 | 46 | 50 | 49 | 52 | 48 | 42 | 56 | 23 |
| GE (Mcal/kg DM) | 4.30 | 4.33 | 4.36 | 4.30 | 4.31 | 4.35 | 4.31 | 4.38 | 4.29 | 4.17 |
| Shorts | Millrun | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | A | B | A | B | C | D | E | Middlings | Bran | Corn | SEM | p-Value |
| In vitro | ||||||||||||
| DM digestibility, % | 65.2 b | 71.8 a | 60.7 d | 59.4 e | 58.7 ef | 58.3 f | 62.5 c | 71.1 a | 50.4 g | NA | 0.2 | <0.001 |
| GE digestibility, % | 62.7 b | 69.1 a | 53.1 d | 51.6 e | 53.3 d | 53.5 d | 57.0 c | 68.0 a | 45.7 f | NA | 0.3 | <0.001 |
| In vivo 2 | ||||||||||||
| Diet DE value, Mcal/kg DM | 3.41 bcd | 3.56 a | 3.40 bcd | 3.39 cd | 3.31 de | 3.39 cd | 3.30 de | 3.53 ab | 3.21 e | 3.46 abc | 0.03 | <0.001 |
| ATTD of GE of ingredient, % | 73.2 bc | 81.9 a | 70.5 c | 72.8 bc | 68.3 c | 70.5 c | 68.1 c | 77.7 ab | 62.6 d | 82.9 a | 1.1 | <0.001 |
| ATTD of GE of diet, % | 78.8 bc | 82.5 a | 77.7 c | 78.7 bc | 76.8 c | 77.7 c | 76.7 c | 80.7 ab | 74.4 d | 82.9 a | 0.5 | <0.001 |
| Variable | Mean | SD | CV | Lowest | Highest | Adj. R2 with In Vitro DE Value 1 | Adj. R2 with In Vivo DE Value 2 |
|---|---|---|---|---|---|---|---|
| Ash | 62 | 9 | 14.4 | 52 | 75 | 0.02 | 0.06 |
| Ether extract | 41 | 9 | 21.2 | 29 | 56 | 0.07 | 0.14 |
| Crude protein | 202 | 40 | 19.8 | 160 | 278 | 0.53 | 0.48 |
| Acid-detergent fiber | 123 | 23 | 18.5 | 80 | 155 | 0.66 | 0.72 |
| Neutral-detergent fiber | 334 | 73 | 21.7 | 229 | 492 | 0.74 | 0.81 |
| Crude fiber | 83 | 19 | 22.4 | 52 | 120 | 0.66 | 0.78 |
| Shorts | Millrun | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | A | B | B | D | Middlings | Bran | SEM 2 | p-Value |
| Fermentation kinetics | ||||||||
| Lag time 3 | 4.01 ab | 3.95 b | 3.44 c | 3.45 c | 3.39 c | 4.29 a | 0.12 | <0.001 |
| Half time 4 | 11.5 a | 10.2 b | 10.2 b | 10.4 b | 9.82 b | 10.1 b | 1.89 | <0.001 |
| FRD 5 | 0.075 c | 0.097 ab | 0.085 bc | 0.093 b | 0.094 b | 0.111 a | 0.028 | <0.001 |
| Total gas 6 | 136 b | 147 a | 125 c | 101 d | 148 a | 125 c | 10.4 | <0.001 |
| Total SCFA 7 | 2.6 ab | 3.0 a | 2.1 b | 2.0 b | 2.9 a | 2.7 ab | 0.2 | <0.001 |
| Individual SCFA 8 | ||||||||
| Acetate | 60.8 a | 57.5 b | 61.4 a | 60.8 a | 60.8 a | 59.9 a | 1.1 | <0.001 |
| Propionate | 24.6 c | 25.8 ab | 25.3 bc | 25.6 ab | 26.4 a | 25.3 bc | 1.6 | <0.001 |
| Butyrate | 10.2 c | 12.7 a | 10.3 bc | 10.4 bc | 9.8 c | 10.9 b | 0.7 | <0.001 |
| BCFA | 2.9 a | 2.7 ab | 2.1 bc | 2.0 c | 1.9 c | 2.7 ab | 0.1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, R.; Regmi, P.R.; Wang, L.F.; Pharazyn, A.; Zijlstra, R.T. Nutrient Profile, Energy Digestibility in Pigs, and In Vitro Degradation Characteristics of Wheat Flour Milling Co-Products. Animals 2025, 15, 2460. https://doi.org/10.3390/ani15162460
Jha R, Regmi PR, Wang LF, Pharazyn A, Zijlstra RT. Nutrient Profile, Energy Digestibility in Pigs, and In Vitro Degradation Characteristics of Wheat Flour Milling Co-Products. Animals. 2025; 15(16):2460. https://doi.org/10.3390/ani15162460
Chicago/Turabian StyleJha, Rajesh, Prajwal R. Regmi, Li F. Wang, Andrew Pharazyn, and Ruurd T. Zijlstra. 2025. "Nutrient Profile, Energy Digestibility in Pigs, and In Vitro Degradation Characteristics of Wheat Flour Milling Co-Products" Animals 15, no. 16: 2460. https://doi.org/10.3390/ani15162460
APA StyleJha, R., Regmi, P. R., Wang, L. F., Pharazyn, A., & Zijlstra, R. T. (2025). Nutrient Profile, Energy Digestibility in Pigs, and In Vitro Degradation Characteristics of Wheat Flour Milling Co-Products. Animals, 15(16), 2460. https://doi.org/10.3390/ani15162460







