Spatial Risk Distribution of Lumpy Skin Disease in Thailand Based on Maximum-Entropy Modeling
Simple Summary
Abstract
1. Introduction
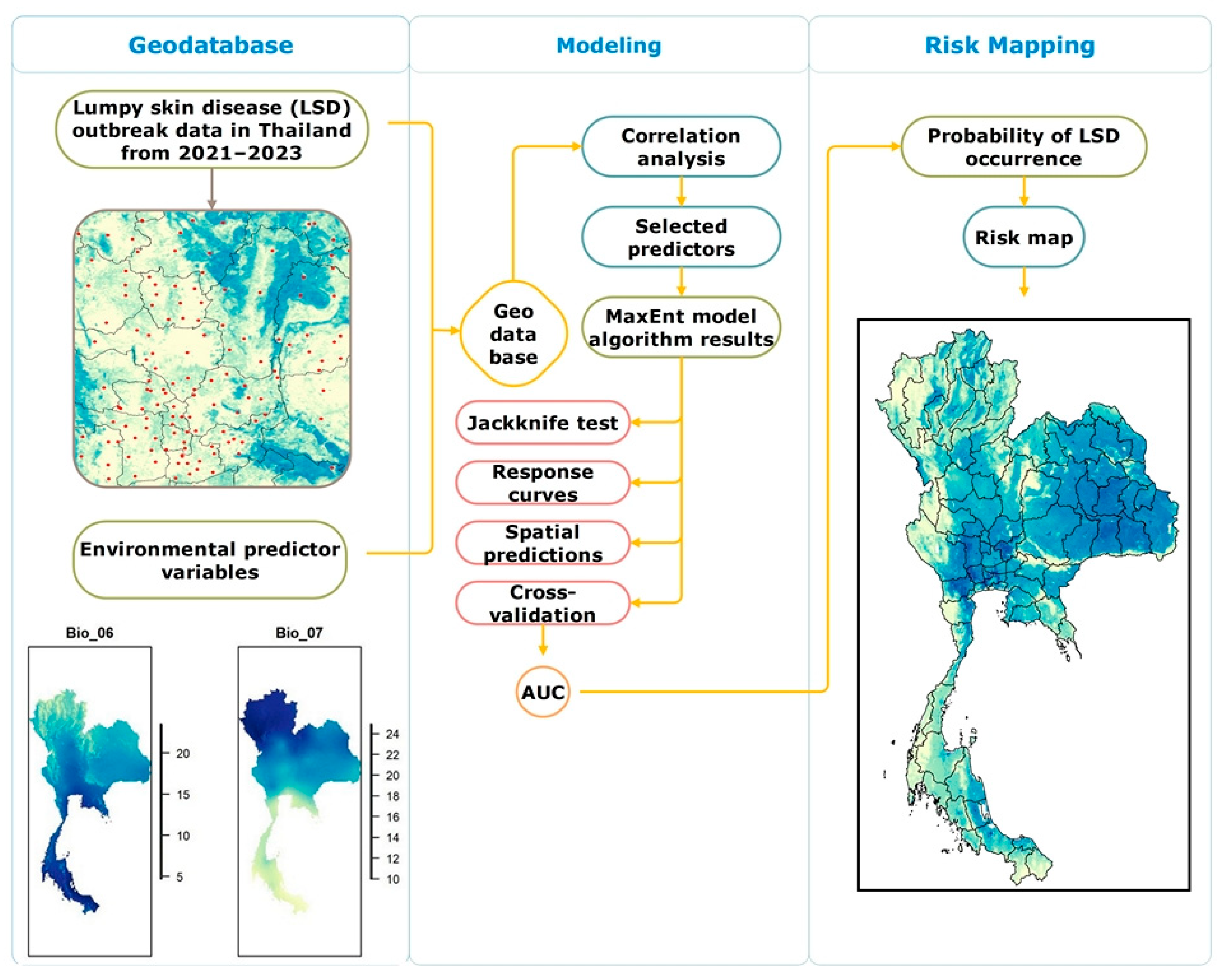
2. Materials and Methods
2.1. Study Area
2.2. Lumpy Skin Disease Data
2.3. Predictor Variable Data Collection and Processing
2.4. Variable Selection
2.5. Modeling Using MaxEnt
2.6. Parameter Setting
2.7. Model Performance Evaluation
3. Results
3.1. Model Performance and Variables Used in the Final Model
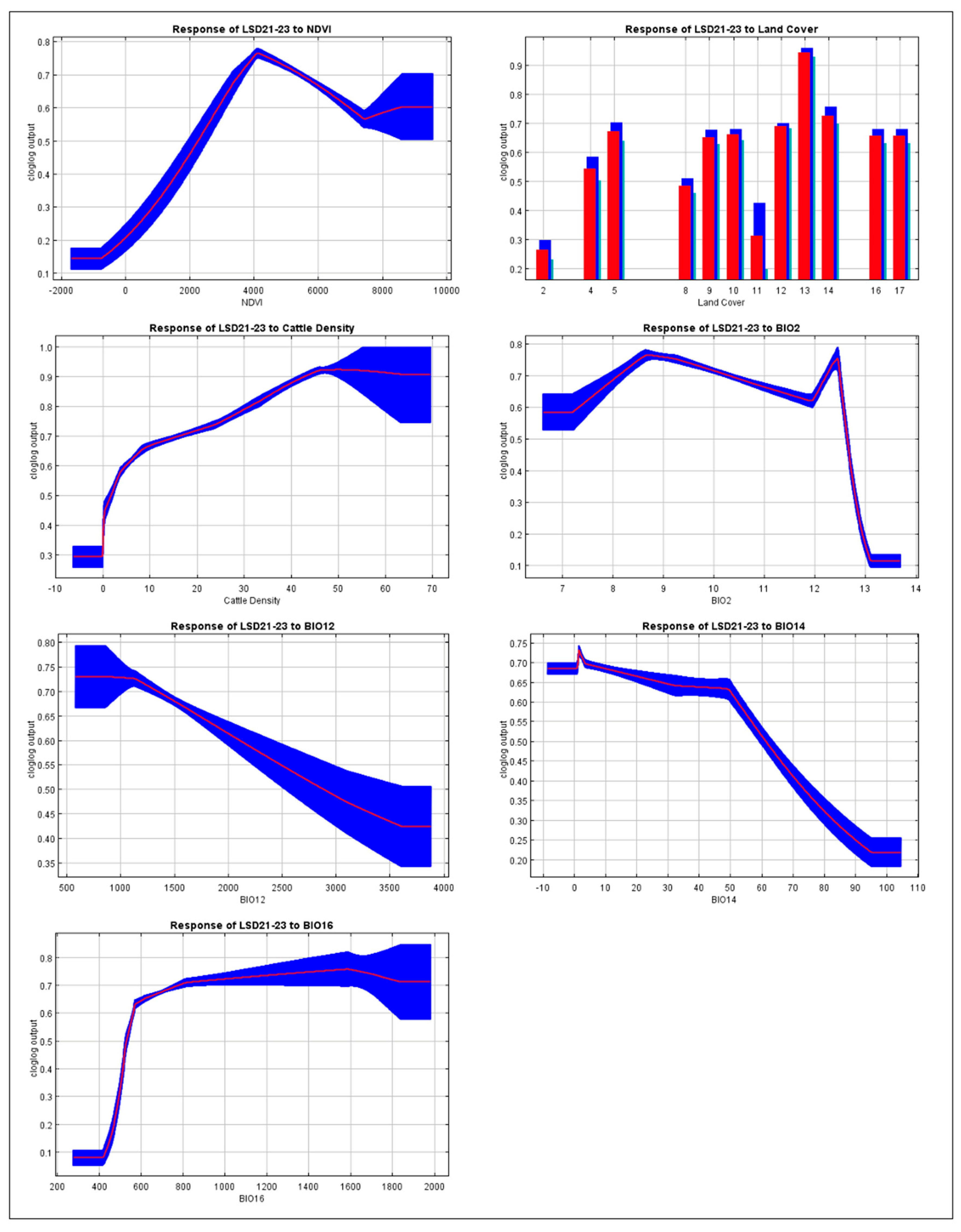
3.2. Response Curves for Important Variables in the LSD Model
3.3. The Contribution of Each Environmental Variable to the Model
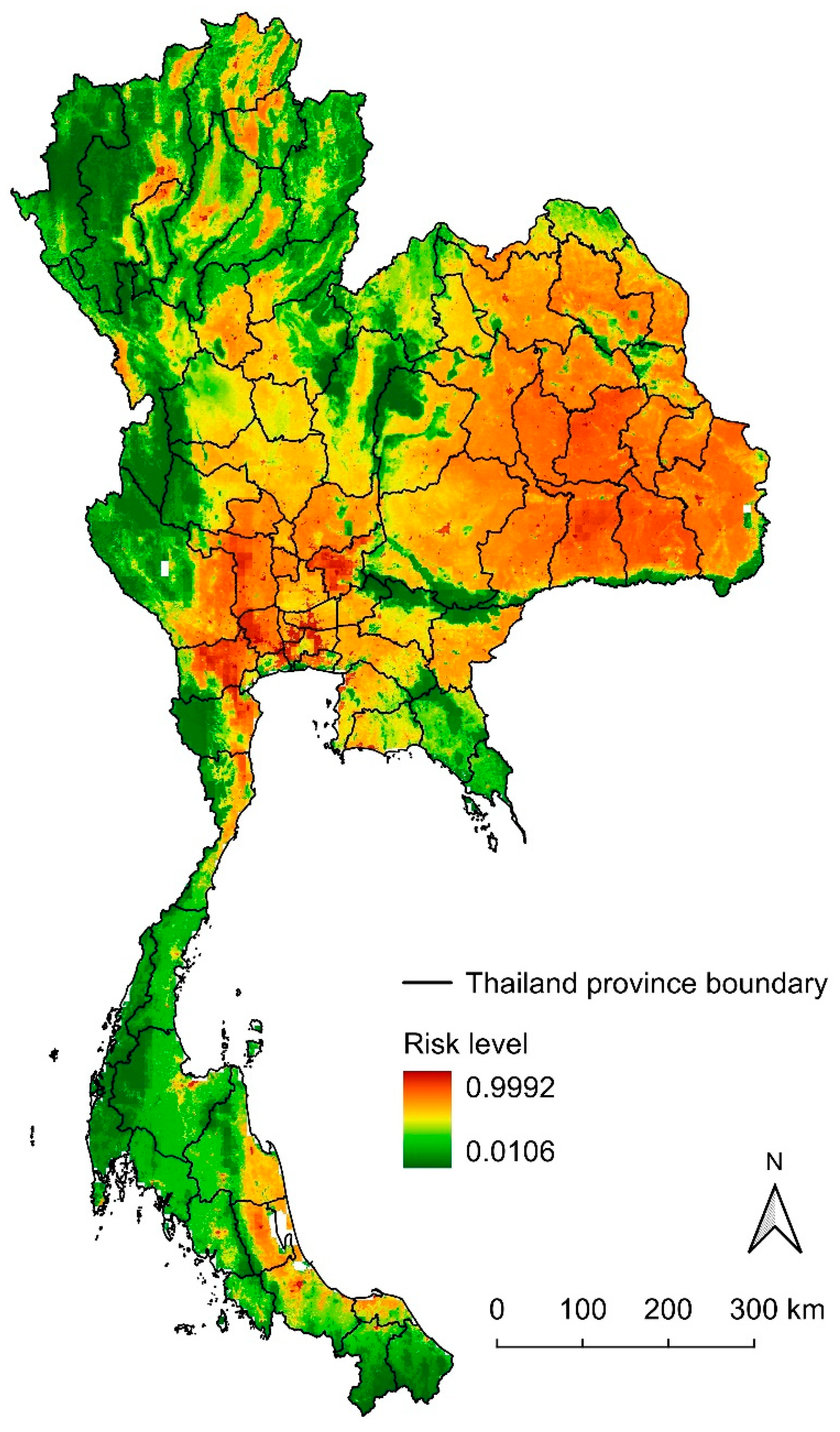
3.4. Potential Risk Map of Lumpy Skin Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LSD | Lumpy skin disease |
| MaxEnt | Maximum-entropy modeling |
| NDVI | Normalized difference vegetation index |
| AUC | Area under the curve |
| TSS | True skill statistic |
| LSDV | Lumpy skin disease virus |
| ENM | Ecological niche modeling |
| DLD | Department of Livestock Development |
| WOAH | World Organisation for Animal Health |
| FAO | Food and Agriculture Organization |
| GEE | The Google Earth Engine |
| CSVs | Comma-separated values |
| TIFF | Tagged image file format |
| ASCII | American Standard Code for Information Interchange |
| ROC | Receiver operating characteristic |
References
- Akther, M.; Akter, S.H.; Sarker, S.; Aleri, J.W.; Annandale, H.; Abraham, S.; Uddin, J.M. Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges. Viruses 2023, 15, 1861. [Google Scholar] [CrossRef]
- Moudgil, G.; Chadha, J.; Khullar, L.; Chhibber, S.; Harjai, K. Lumpy skin disease: Insights into current status and geographical expansion of a transboundary viral disease. Microb. Pathog. 2024, 186, 106485. [Google Scholar] [CrossRef]
- Adamu, K.; Abayneh, T.; Getachew, B.; Mohammed, H.; Deresse, G.; Zekarias, M.; Chala, W.; Gelaye, E. Lumpy skin disease virus isolation, experimental infection, and evaluation of disease development in a calf. Sci. Rep. 2024, 14, 20460. [Google Scholar] [CrossRef]
- Bhoye, S. Lumpy skin disease (LSD) in cattle caused by the lumpy skin disease virus (Neethling virus): A member of the genus capripoxvirus within the family poxviridae. Int. J. Vet. Sci. Anim. Husb. 2022, 7, 25–28. [Google Scholar] [CrossRef]
- Datten, B.; Chaudhary, A.A.; Sharma, S.; Singh, L.; Rawat, K.D.; Ashraf, M.S.; Alneghery, L.M.; Aladwani, M.O.; Rudayni, H.A.; Dayal, D.; et al. An Extensive Examination of the Warning Signs, Symptoms, Diagnosis, Available Therapies, and Prognosis for Lumpy Skin Disease. Viruses 2023, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.J.; Lee, E.-S.; Yoo, H.S. Lumpy skin disease as an emerging infectious disease: An overview and perspectives. J. Vet. Sci. 2023, 24, e42. [Google Scholar] [CrossRef]
- Kutumbetov, L.; Ragatova, A.; Azanbekova, M.; Myrzakhmetova, B.; Aldayarov, N.; Zhugunissov, K.; Abduraimov, Y.; Nissanova, R.; Sarzhigitova, A.; Kemalova, N.; et al. Investigation of the Pathogenesis of Lumpy Skin Disease Virus in Indigenous Cattle in Kazakhstan. Pathogens 2025, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Gari, G.; Bonnet, P.; Roger, F.; Waret-Szkuta, A. Epidemiological aspects and financial impact of lumpy skin disease in Ethiopia. Prev. Vet. Med. 2011, 102, 274–283. [Google Scholar] [CrossRef]
- Khatri, G.; Rai, A.; Aashish; Shahzaib; Hyder, S.; Priya; Hasan, M.M. Epidemic of lumpy skin disease in Pakistan. Vet. Med. Sci. 2023, 9, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.T.; Truong, A.D.; Dang, A.K.; Ly, D.V.; Nguyen, C.T.; Chu, N.T.; Hoang, T.V.; Nguyen, H.T.; Nguyen, V.T.; Dang, H.V. Lumpy skin disease outbreaks in vietnam, 2020. Transbound. Emerg. Dis. 2021, 68, 977–980. [Google Scholar] [CrossRef]
- Wilhelm, L.; Ward, M.P. The Spread of Lumpy Skin Disease Virus across Southeast Asia: Insights from Surveillance. Transbound. Emerg. Dis. 2023, 2023, 3972359. [Google Scholar] [CrossRef]
- Maw, M.T.; Khin, M.M.; Hadrill, D.; Meki, I.K.; Settypalli, T.B.K.; Kyin, M.M.; Myint, W.W.; Thein, W.Z.; Aye, O.; Palamara, E. First report of lumpy skin disease in Myanmar and molecular analysis of the field virus isolates. Microorganisms 2022, 10, 897. [Google Scholar] [CrossRef]
- Porco, A.; Chea, S.; Sours, S.; Nou, V.; Groenenberg, M.; Agger, C.; Tum, S.; Chhuon, V.; Sorn, S.; Hong, C.; et al. Case report: Lumpy skin disease in an endangered wild banteng (Bos javanicus) and initiation of a vaccination campaign in domestic livestock in Cambodia. Front. Vet. Sci. 2023, 10, 1228505. [Google Scholar] [CrossRef]
- Arjkumpa, O.; Suwannaboon, M.; Boonrod, M.; Punyawan, I.; Liangchaisiri, S.; Laobannue, P.; Lapchareonwong, C.; Sansri, C.; Kuatako, N.; Panyasomboonying, P.; et al. The First Lumpy Skin Disease Outbreak in Thailand (2021): Epidemiological Features and Spatio-Temporal Analysis. Front. Vet. Sci. 2022, 8, 799065. [Google Scholar] [CrossRef]
- Maulana, K.Y.; Na-Lampang, K.; Arjkumpa, O.; Buamithup, N.; Intawong, K.; Punyapornwithaya, V. Geographical Distribution, Spatial Directional Trends, and Spatio-Temporal Clusters of the First Rapid and Widespread Lumpy Skin Disease Outbreaks in Thailand. Transbound. Emerg. Dis. 2025, 2025, 4900775. [Google Scholar] [CrossRef]
- Maulana, K.Y.; Srisawang, S.; Li, Z.; Li, W.; Modethed, W.; Punyapornwithaya, V. Spatial epidemiology of lumpy skin disease: Unraveling patterns in dairy farm clusters with short interfarm proximity. Anim. Dis. 2025, 5, 23. [Google Scholar] [CrossRef]
- Modethed, W.; Singhla, T.; Boonsri, K.; Pringproa, K.; Sthitmatee, N.; Vinitchaikul, P.; Sansamur, C.; Kreausukon, K.; Punyapornwithaya, V. Identifying the patterns and sizes of the first lumpy skin disease outbreak clusters in Northern Thailand with a high degree of dairy farm aggregation using spatio-temporal models. PLoS ONE 2023, 18, e0291692. [Google Scholar] [CrossRef]
- Punyapornwithaya, V.; Seesupa, S.; Phuykhamsingha, S.; Arjkumpa, O.; Sansamur, C.; Jarassaeng, C. Spatio-temporal patterns of lumpy skin disease outbreaks in dairy farms in northeastern Thailand. Front. Vet. Sci. 2022, 9, 957306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, S.; Lu, H.; Niu, B. Spatio-temporal analysis and risk modeling of foot-and-mouth disease outbreaks in China. Prev. Vet. Med. 2024, 224, 106120. [Google Scholar] [CrossRef]
- Tesfaye, S.; Regassa, F.; Beyene, G.; Leta, S.; Paeshuyse, J. Spatiotemporal analysis and forecasting of lumpy skin disease outbreaks in Ethiopia based on retrospective outbreak reports. Front. Vet. Sci. 2024, 11, 1277007. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E. Ecological Niche Modeling: An Introduction for Veterinarians and Epidemiologists. Front. Vet. Sci. 2020, 7, 519059. [Google Scholar] [CrossRef]
- Meshgi, B.; Majidi-Rad, M.; Hanafi-Bojd, A.A.; Fathi, S. Ecological niche modeling for predicting the habitat suitability of fascioliasis based on maximum entropy model in southern Caspian Sea littoral, Iran. Acta Trop. 2019, 198, 105079. [Google Scholar] [CrossRef]
- Smeraldo, S.; Di Febbraro, M.; Bosso, L.; Flaquer, C.; Guixé, D.; Lisón, F.; Meschede, A.; Juste, J.; Prüger, J.; Puig-Montserrat, X.; et al. Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: Lessons from bats. Biodivers. Conserv. 2018, 27, 2425–2441. [Google Scholar] [CrossRef]
- Ardestani, E.G.; Mokhtari, A. Modeling the lumpy skin disease risk probability in central Zagros Mountains of Iran. Prev. Vet. Med. 2020, 176, 104887. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.; Korennoy, F.; Alvarez, J.; Picasso-Risso, C.; Perez, A.; VanderWaal, K. Mapping changes in the spatiotemporal distribution of lumpy skin disease virus. Transbound. Emerg. Dis. 2019, 66, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; An, Q.; Sun, Z.; Gao, X.; Wang, H. Risk Factors and Spatiotemporal Distribution of Lumpy Skin Disease Occurrence in the Asian Continent during 2012–2022: An Ecological Niche Model. Transbound. Emerg. Dis. 2023, 2023, 6207149. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ma, J. Spatial distribution and risk areas of foot and mouth disease in mainland China. Prev. Vet. Med. 2021, 189, 105311. [Google Scholar] [CrossRef]
- Podshibyakin, D.; Padilo, L.; Agoltsov, V.; Chernykh, O.; Popova, O.; Mutalif, K.; Solotova, N. Analysis of environmental factors influencing lumpy skin disease outbreak seasonality and assessment of its spread risk in the Saratovskaya oblast of Russia. Vet. World 2024, 17, 630. [Google Scholar] [CrossRef]
- Zheng, J.-H.; Gao, X.; Liu, B.-Y.; Xiao, J.-H.; Wang, H.-B. Study on the potential distribution of the vector of lumpy skin disease-Stomoxys calcitrans in China using a Maxent model. Zhongguo Yufang Shouyi Xuebao/Chin. J. Prev. Vet. Med. 2021, 43, 482–488. [Google Scholar]
- The Government Public Relations Department. Thailand Geography. 2023. Available online: https://thailand.go.th/issue-focus-detail/009_141 (accessed on 15 August 2025).
- Tainchum, K.; Sukonthabhirom, S.; Duvallet, G.; Akratanakul, P.; Muenworn, V.; Chareonviriyaphap, T. Population structure of Stomoxys calcitrans (Diptera: Muscidae) from nine regions of Thailand. J. Econ. Entomol. 2010, 103, 1012–1018. [Google Scholar] [CrossRef]
- Thepparat, A.; Kamata, N.; Siriyasatien, P.; Prempree, W.; Dasuntad, K.; Chittsamart, B.; Sanguansub, S. Seasonal Abundance and Diversity of Culicoides Biting Midges in Livestock Sheds in Kanchanaburi Province, Thailand. Insects 2024, 15, 701. [Google Scholar] [CrossRef]
- Suwankitwat, N.; Songkasupa, T.; Boonpornprasert, P.; Sripipattanakul, P.; Theerawatanasirikul, S.; Deemagarn, T.; Suwannaboon, M.; Arjkumpa, O.; Buamithup, N.; Hongsawat, A. Rapid spread and genetic characterisation of a recently emerged recombinant lumpy skin disease virus in Thailand. Vet. Sci. 2022, 9, 542. [Google Scholar] [CrossRef]
- Gomontean, B.; Vaisusuk, K.; Chatan, W.; Wongpakam, K.; Sankul, P.; Lachanthuek, L.; Mintara, R.; Thanee, I.; Pramual, P. Diversity, abundance and host blood meal analysis of Culicoides Latreille (Diptera: Ceratopogonidae) from cattle pens in different land use types from Thailand. Insects 2023, 14, 574. [Google Scholar] [CrossRef]
- Li, H.; Pan, H.; Xu, L.; Li, S.; Li, S.; Chen, S.; Man, C.; Du, L.; Chen, Q.; Xiao, J.; et al. Predicting Risk Areas of Classical Scrapie in China Based on Environmental Suitability. Transbound. Emerg. Dis. 2023, 2023, 2826256. [Google Scholar] [CrossRef]
- Ma, J.; Gao, X.; Liu, B.; Chen, H.; Xiao, J.; Wang, H. Peste des petits ruminants in China: Spatial risk analysis. Transbound. Emerg. Dis. 2019, 66, 1784–1788. [Google Scholar] [CrossRef]
- Xie, C.; Tian, E.; Jim, C.Y.; Liu, D.; Hu, Z. Effects of climate-change scenarios on the distribution patterns of Castanea henryi. Ecol. Evol. 2022, 12, e9597. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, L.; Jiao, Y.; Li, S.; Yang, Y.; Lan, F.; Chen, S.; Man, C.; Du, L.; Chen, Q.; et al. Risk Assessment of Global Animal Melioidosis Under Current and Future Climate Scenarios. Animals 2025, 15, 455. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wen, F.; Lu, L.; Nie, C.; Sun, Z.; Liu, R.; Huang, W.; Ye, H. Analysis of Spatiotemporal Variation in Habitat Suitability for Oedaleus decorus asiaticus Bei-Bienko on the Mongolian Plateau Using Maxent and Multi-Source Remote Sensing Data. Insects 2023, 14, 492. [Google Scholar] [CrossRef]
- Padalia, H.; Srivastava, V.; Kushwaha, S.P.S. Modeling potential invasion range of alien invasive species, Hyptis suaveolens (L.) Poit. in India: Comparison of MaxEnt and GARP. Ecol. Inform. 2014, 22, 36–43. [Google Scholar] [CrossRef]
- Bosso, L.; Russo, D.; Di Febbraro, M.; Cristinzio, G.; Zoina, A. Potential distribution of Xylella fastidiosa in Italy: A maximum entropy model. Phytopathol. Mediterr. 2016, 55, 62–72. [Google Scholar]
- Gebrewahid, Y.; Abrehe, S.; Meresa, E.; Eyasu, G.; Abay, K.; Gebreab, G.; Kidanemariam, K.; Adissu, G.; Abreha, G.; Darcha, G. Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Process. 2020, 9, 6. [Google Scholar] [CrossRef]
- Mouton, A.M.; De Baets, B.; Goethals, P.L. Ecological relevance of performance criteria for species distribution models. Ecol. Model. 2010, 221, 1995–2002. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Issimov, A.; Taylor, D.B.; Shalmenov, M.; Nurgaliyev, B.; Zhubantayev, I.; Abekeshev, N.; Kushaliyev, K.; Kereyev, A.; Kutumbetov, L.; Zhanabayev, A.; et al. Retention of lumpy skin disease virus in Stomoxys spp (Stomoxys calcitrans, Stomoxys sitiens, Stomoxys indica) following intrathoracic inoculation, Diptera: Muscidae. PLoS ONE 2021, 16, e0238210. [Google Scholar] [CrossRef]
- Paslaru, A.I.; Verhulst, N.O.; Maurer, L.M.; Brendle, A.; Pauli, N.; Vögtlin, A.; Renzullo, S.; Ruedin, Y.; Hoffmann, B.; Torgerson, P.R.; et al. Potential mechanical transmission of Lumpy skin disease virus (LSDV) by the stable fly (Stomoxys calcitrans) through regurgitation and defecation. Curr. Res. Insect Sci. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Duval, P.; Antonelli, P.; Aschan-Leygonie, C.; Valiente Moro, C. Impact of Human Activities on Disease-Spreading Mosquitoes in Urban Areas. J. Urban Health 2023, 100, 591–611. [Google Scholar] [CrossRef]
- Bunmee, T.; Chaiwang, N.; Kaewkot, C.; Jaturasitha, S. Current situation and future prospects for beef production in Thailand—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 968–975. [Google Scholar] [CrossRef]
- Issimov, A.; Kushaliyev, K.; Abekeshev, N.; Molla, W.; Rametov, N.; Bayantassova, S.; Zhanabayev, A.; Paritova, A.; Shalmenov, M.; Ussenbayev, A.; et al. Risk factors associated with lumpy skin disease in cattle in West Kazakhstan. Prev. Vet. Med. 2022, 207, 105660. [Google Scholar] [CrossRef]
- An, Q.; Li, Y.-P.; Sun, Z.; Gao, X.; Wang, H.-B. Global Risk Assessment of the Occurrence of Bovine Lumpy Skin Disease: Based on an Ecological Niche Model. Transbound. Emerg. Dis. 2023, 2023, 2349173. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016, 6, 29002. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, M.; Magallanes, S.; Mora-Rubio, C.; Bravo-Barriga, D.; de Lope, F.; Marzal, A. Landscape and climatic factors shaping mosquito abundance and species composition in southern Spain: A machine learning approach to the study of vector ecology. Ecol. Inform. 2024, 84, 102860. [Google Scholar] [CrossRef]
- Kenyeres, Z.; Sáringer-Kenyeres, M. Predictability of the distribution of human-biting mosquitoes using large-scale habitat patterns. Hydrobiologia 2022, 849, 1041–1052. [Google Scholar] [CrossRef]
- Ahmed, T.; Hyder, M.Z.; Liaqat, I.; Scholz, M. Climatic Conditions: Conventional and Nanotechnology-Based Methods for the Control of Mosquito Vectors Causing Human Health Issues. Int. J. Environ. Res. Public Health 2019, 16, 3165. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Tripathi, B.N. Pathogenicity and virulence of lumpy skin disease virus: A comprehensive update. Virulence 2025, 16, 2495108. [Google Scholar] [CrossRef]
- EFSA. Lumpy skin disease II. Data collection and analysis. EFSA J. 2018, 16, e05176. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef]
- Mulatu, E.; Feyisa, A. Review: Lumpy skin disease. J. Vet. Sci. Technol. 2018, 9, 1000535. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.; Babiuk, S.; Klement, E. Lumpy Skin Disease; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Ali, H.; Ali, A.A.; Atta, M.S.; Cepica, A. Common, Emerging, Vector-Borne and Infrequent Abortogenic Virus Infections of Cattle. Transbound. Emerg. Dis. 2012, 59, 11–25. [Google Scholar] [CrossRef]
- Halvorsen, R.; Mazzoni, S.; Dirksen, J.W.; Næsset, E.; Gobakken, T.; Ohlson, M. How important are choice of model selection method and spatial autocorrelation of presence data for distribution modelling by MaxEnt? Ecol. Model. 2016, 328, 108–118. [Google Scholar] [CrossRef]
- Buaban, S.; Puangdee, S.; Duangjinda, M.; Boonkum, W. Estimation of genetic parameters and trends for production traits of dairy cattle in Thailand using a multiple-trait multiple-lactation test day model. Asian-Australas. J. Anim. Sci. 2020, 33, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Arjkumpa, O.; Wachoom, W.; Puyati, B.; Jindajang, S.; Suwannaboon, M.; Premashthira, S.; Prarakamawongsa, T.; Dejyong, T.; Sansamur, C.; Salvador, R.; et al. Analysis of factors associated with the first lumpy skin disease outbreaks in naïve cattle herds in different regions of Thailand. Front. Vet. Sci. 2024, 11, 1338713. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Praveen, J.; Nameer, P.O. Contrasting occupancy models with presence-only models: Does accounting for detection lead to better predictions? Ecol. Model. 2022, 472, 110105. [Google Scholar] [CrossRef]
- Mercier, A.; Arsevska, E.; Bournez, L.; Bronner, A.; Calavas, D.; Cauchard, J.; Falala, S.; Caufour, P.; Tisseuil, C.; Lefrançois, T. Spread rate of lumpy skin disease in the Balkans, 2015–2016. Transbound. Emerg. Dis. 2018, 65, 240–243. [Google Scholar] [CrossRef]



| Variable | Description | Source | Included |
|---|---|---|---|
| Bio 1 | Annual mean temperature | WorldClim | No |
| Bio 2 | Mean diurnal range | WorldClim | Yes |
| Bio 3 | Isothermality | WorldClim | No |
| Bio 4 | Temperature seasonality | WorldClim | No |
| Bio 5 | Max temperature of the warmest month | WorldClim | No |
| Bio 6 | Min temperature of the coldest month | WorldClim | No |
| Bio 7 | Temperature annual range | WorldClim | No |
| Bio 8 | Mean temperature of the wettest quarter | WorldClim | No |
| Bio 9 | Mean temperature of the driest quarter | WorldClim | No |
| Bio 10 | Mean temperature of the warmest quarter | WorldClim | No |
| Bio 11 | Mean temperature of the coldest quarter | WorldClim | No |
| Bio 12 | Annual precipitation | WorldClim | Yes |
| Bio 13 | Precipitation of the wettest month | WorldClim | No |
| Bio 14 | Precipitation of the driest month | WorldClim | Yes |
| Bio 15 | Precipitation seasonality | WorldClim | No |
| Bio 16 | Precipitation of the wettest quarter | WorldClim | Yes |
| Bio 17 | Precipitation of the driest quarter | WorldClim | No |
| Bio 18 | Precipitation of the warmest quarter | WorldClim | No |
| Bio 19 | Precipitation of the coldest quarter | WorldClim | No |
| Cattle density | The density of cattle | FAO | Yes |
| NDVI | Vegetation density and greenness | MODIS MOD13A1 | Yes |
| Land cover type 1 1 | IGBP global vegetation classification scheme | MODIS MCD12Q1 | Yes |
| Variable | Description | Variable Contribution (%) |
|---|---|---|
| Bio 2 | Mean diurnal range | 1.6 |
| Bio 12 | Annual precipitation | 0.6 |
| Bio 14 | Precipitation of the driest month | 1 |
| Bio 16 | Precipitation of the wettest quarter | 4 |
| Cattle density | The density of cattle | 25 |
| NDVI | Vegetation density and greenness | 2.8 |
| Land cover type 1 | IGBP global vegetation classification scheme | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maulana, K.Y.; Siriyakhun, S.; Na-Lampang, K.; Intawong, K.; Olana, K.O.A.; Li, W.; Tamprateep, M.; Punyapornwithaya, V. Spatial Risk Distribution of Lumpy Skin Disease in Thailand Based on Maximum-Entropy Modeling. Animals 2025, 15, 2456. https://doi.org/10.3390/ani15162456
Maulana KY, Siriyakhun S, Na-Lampang K, Intawong K, Olana KOA, Li W, Tamprateep M, Punyapornwithaya V. Spatial Risk Distribution of Lumpy Skin Disease in Thailand Based on Maximum-Entropy Modeling. Animals. 2025; 15(16):2456. https://doi.org/10.3390/ani15162456
Chicago/Turabian StyleMaulana, Kusnul Yuli, Supitchaya Siriyakhun, Kannika Na-Lampang, Kannikar Intawong, Kenny Oriel A. Olana, Wengui Li, Maytawee Tamprateep, and Veerasak Punyapornwithaya. 2025. "Spatial Risk Distribution of Lumpy Skin Disease in Thailand Based on Maximum-Entropy Modeling" Animals 15, no. 16: 2456. https://doi.org/10.3390/ani15162456
APA StyleMaulana, K. Y., Siriyakhun, S., Na-Lampang, K., Intawong, K., Olana, K. O. A., Li, W., Tamprateep, M., & Punyapornwithaya, V. (2025). Spatial Risk Distribution of Lumpy Skin Disease in Thailand Based on Maximum-Entropy Modeling. Animals, 15(16), 2456. https://doi.org/10.3390/ani15162456







