Comparative Transcriptomic Analysis of the Liver and Spleen in Ussuri Catfish (Pseudobagrus ussuriensis) Challenged with Polyriboinosinic Polyribocytidylic Acid (Poly(I:C))
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish Handling and Trial Design
2.3. RNA Extraction, Library Construction, Sequencing
2.4. Quality Control and De Novo Assembly
2.5. Functional Annotation
2.6. Analysis of Differentially Expressed Unigenes (DEGs), Cluster Analysis, and GO and KEGG Enrichment
2.7. Quantitative Real-Time PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Overview of RNA-Seq Data
3.2. Transcriptomic Responses in Liver and Spleen of P. ussuriensis at 3 h Post-Poly(I:C) Stimulation
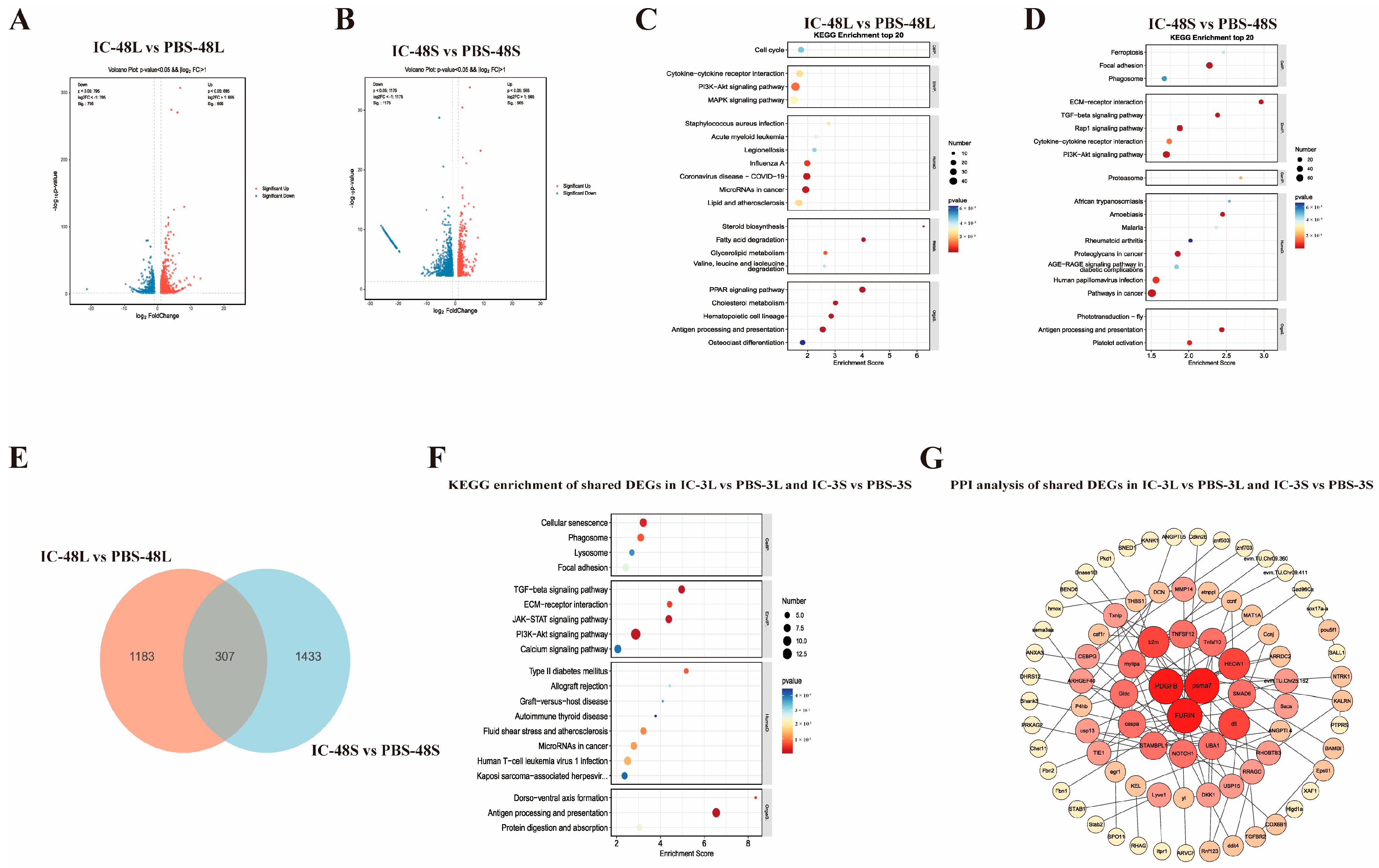
3.3. Transcriptomic Responses in Liver and Spleen of P. ussuriensis at 48 h Post-Poly(I:C) Stimulation
3.4. Temporal Analysis of Shared Immune-Related Genes in Liver Following Poly(I:C) Stimulation
3.5. Temporal Analysis of Shared Immune-Related Genes in Spleen Following Poly(I:C) Stimulation
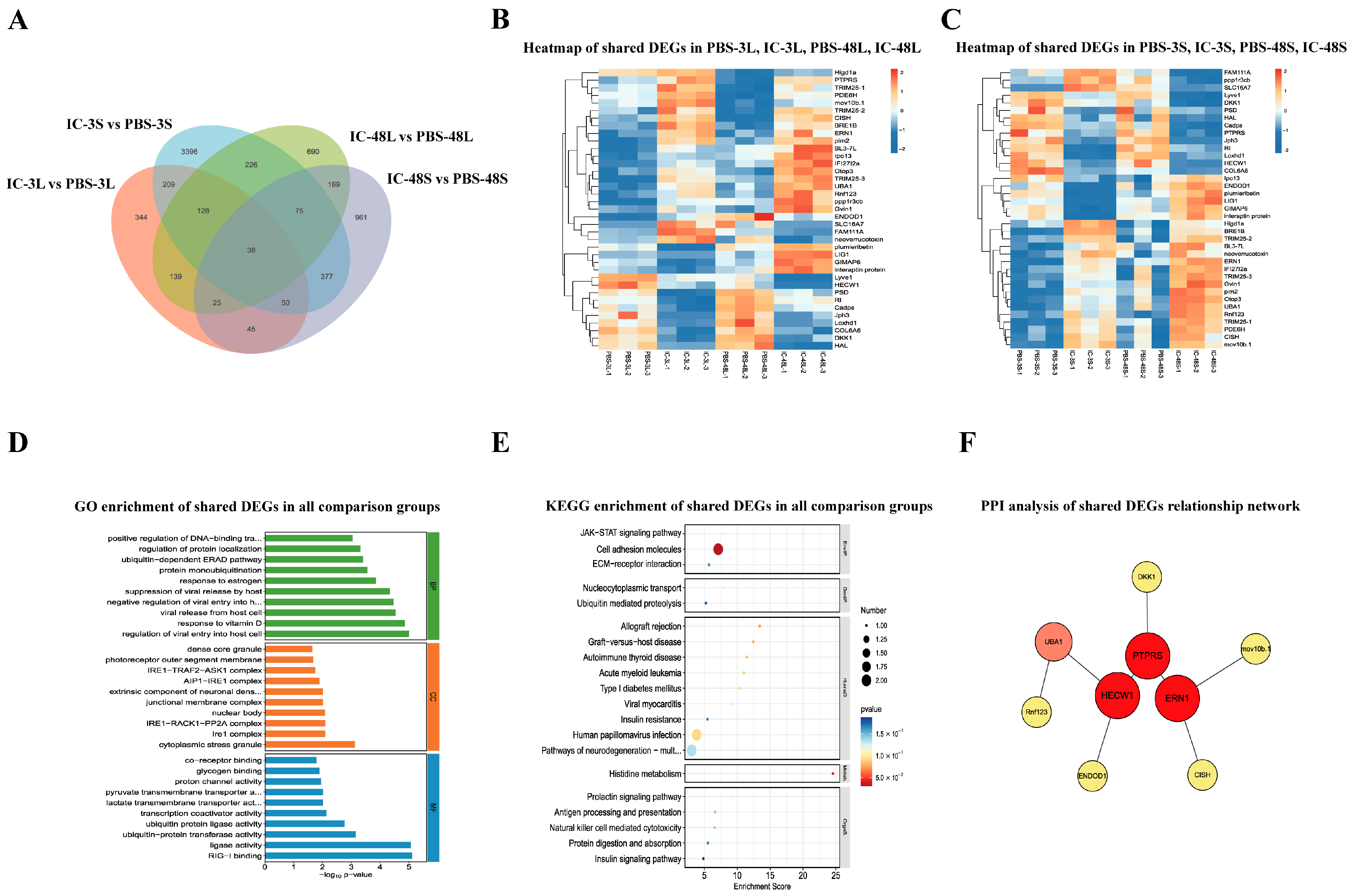
3.6. Analysis of Shared DEGs Among All Comparison Groups
3.7. Validation of Key Genes by qPCR
4. Discussion
4.1. Molecular Mechanisms of Innate Immune Responses and Maintenance of Tissue Homeostasis in the Liver
4.2. Multiple Functioning of the Spleen as a Basis for Structural Regulation and Immune Coordination
4.3. Coherence and Appropriateness of the Immune Response as a Consequence of Co-Activation Key Pathways in the Liver and Spleen
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega-Villaizan, M.D.M.; Chico, V.; Perez, L. Fish Innate Immune Response to Viral Infection-An Overview of Five Major Antiviral Genes. Viruses 2022, 14, 1546. [Google Scholar] [CrossRef]
- Zou, J.; Bird, S.; Secombes, C. Antiviral sensing in teleost fish. Curr. Pharm. Des. 2010, 16, 4185–4193. [Google Scholar] [CrossRef]
- Pietretti, D.; Wiegertjes, G.F. Ligand specificities of Toll-like receptors in fish: Indications from infection studies. Dev. Comp. Immunol. 2014, 43, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhong, H.; Feng, H. Post-translational modifications and regulations of RLR signaling molecules in cytokines-mediated response in fish. Dev. Comp. Immunol. 2023, 141, 104631. [Google Scholar] [CrossRef] [PubMed]
- Biacchesi, S.; Merour, E.; Lamoureux, A.; Bernard, J.; Bremont, M. Both STING and MAVS fish orthologs contribute to the induction of interferon mediated by RIG-I. PLoS ONE 2012, 7, e47737. [Google Scholar] [CrossRef] [PubMed]
- Plant, K.P.; Harbottle, H.; Thune, R.L. Poly I:C induces an antiviral state against Ictalurid Herpesvirus 1 and Mx1 transcription in the channel catfish (Ictalurus punctatus). Dev. Comp. Immunol. 2005, 29, 627–635. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhao, Y.; Kong, X.; Wu, F.; Zhao, X. Molecular characterization and expression analysis of toll-like receptors 5 and 22 from natural triploid Carassius auratus. Fish Shellfish Immunol. 2017, 64, 1–13. [Google Scholar] [CrossRef]
- Gu, T.; Lu, L.; An, C.; Chen, B.; Wei, W.; Wu, X.; Xu, Q.; Chen, G. MDA5 and LGP2 acts as a key regulator though activating NF-κB and IRF3 in RLRs signaling of mandarinfish. Fish Shellfish Immunol. 2019, 86, 1114–1122. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Chen, Z.; Li, C.; Zhao, X.; Kong, X. Molecular cloning and expression analysis of MyD88 and TRAF6 in Qihe crucian carp Carassius auratus. Fish Shellfish Immunol. 2019, 87, 829–838. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, Y.; Liu, Y.; Zhang, Q.; Wang, Z.; Mei, J.; Wang, D. Transcriptome analysis of largemouth bass (Micropterus salmoides) challenged with LPS and polyI:C. Fish Shellfish Immunol. 2023, 133, 108534. [Google Scholar] [CrossRef]
- Tang, Y.; Lv, X.; Liu, X.; Song, J.; Wu, Y.; Zhou, Q.; Zhu, R. Three IRF4 paralogs act as negative regulators of type I IFN responses in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2022, 131, 537–548. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.; Wu, Y.; Song, J.; Wang, X.; Zhu, R. Molecular characterization of yellow catfish (Pelteobagrus fulvidraco) IRF7 suggests involvement in innate immune response. Dev. Comp. Immunol. 2020, 109, 103700. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, Z.Z.; Zhang, D.Z.; Wang, Z.F.; Zhu, X.Y.; Tang, B.P.; Jiang, S.H.; Zhang, H.B.; Zhou, C.L.; Chai, X.Y.; et al. Transcriptome analysis of yellow catfish (Pelteobagrus fulvidraco) liver challenged with polyriboinosinic polyribocytidylic acid (poly I:C). Fish Shellfish Immunol. 2017, 68, 395–403. [Google Scholar] [CrossRef]
- Youngnoi, N.; Yamkasem, J.; Khemthong, M.; Setthawong, P.; Nedumpun, T.; Surachetpong, W.; Lertwanakarn, T. Granulocyte tropism and lymphocyte depletion highlight the immunopathogenesis of tilapia lake virus infection in Nile tilapia. Fish Shellfish Immunol. 2025, 163, 110410. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Lu, L.F.; Xiong, F.; Wang, X.L.; Jiang, J.Y.; Zhang, C.; Li, Z.C.; Han, K.J.; Li, S. Zebrafish CERKL Enhances Host TBK1 Stability and Simultaneously Degrades Viral Protein via Ubiquitination Modulation. J. Immunol. 2022, 208, 2196–2206. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, M.X. TBK1 Isoform Inhibits Grass Carp Reovirus Infection by Targeting the Degradation of Viral Nonstructural Proteins NS80 and NS38. J. Immunol. 2023, 210, 191–203. [Google Scholar] [CrossRef]
- Liu, Q.N.; Xin, Z.Z.; Chai, X.Y.; Jiang, S.H.; Li, C.F.; Zhang, D.Z.; Zhou, C.L.; Tang, B.P. Identification of differentially expressed genes in the spleens of polyriboinosinic polyribocytidylic acid (poly I:C)-stimulated yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol. 2016, 56, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Li, D.; Lian, F.; Yang, S.; Wu, J.; Liu, H.; Bu, G.; Meng, F.; Cao, X.; et al. Transcriptome profiling of spleen provides insights into the antiviral mechanism in Schizothorax prenanti after poly (I:C) challenge. Fish Shellfish Immunol. 2017, 62, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Dettleff, P.; Moen, T.; Santi, N.; Martinez, V. Transcriptomic analysis of spleen infected with infectious salmon anemia virus reveals distinct pattern of viral replication on resistant and susceptible Atlantic salmon (Salmo salar). Fish. Shellfish. Immunol. 2017, 61, 187–193. [Google Scholar] [CrossRef]
- Guo, X.; Wang, W.; Zheng, Q.; Qin, Q.; Huang, Y.; Huang, X. Comparative transcriptomic analysis reveals different host cell responses to Singapore grouper iridovirus and red-spotted grouper nervous necrosis virus. Fish. Shellfish. Immunol. 2022, 128, 136–147. [Google Scholar] [CrossRef]
- Mu, Y.; Li, M.; Ding, F.; Ding, Y.; Ao, J.; Hu, S.; Chen, X. De novo characterization of the spleen transcriptome of the large yellow croaker (Pseudosciaena crocea) and analysis of the immune relevant genes and pathways involved in the antiviral response. PLoS ONE 2014, 9, e97471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Cao, Y.; Gu, L.; Li, T.; Liu, Y.; Song, J.; Wang, W.; Wang, X.; Li, B.; et al. Transcriptome Analysis of Brain and Skin Reveals Immune Responses to Acute Hypoxia and Reoxygenation in Pseudobagrus ussuriensis. Animals 2024, 14, 246. [Google Scholar] [CrossRef]
- Zhu, C.; Pan, Z.; Chang, G.; Wu, N.; Liu, T.J.A. Transcriptomic insights into immune responses to ulcerative syndrome in Pseudobagrus ussuriensis. Aquaculture 2021, 537, 736504. [Google Scholar] [CrossRef]
- Bu, X.; Lian, X.; Wang, Y.; Luo, C.; Tao, S.; Liao, Y.; Yang, J.; Chen, A.; Yang, Y. Dietary yeast culture modulates immune response related to TLR2-MyD88-NF-kbeta signaling pathway, antioxidant capability and disease resistance against Aeromonas hydrophila for Ussuri catfish (Pseudobagrus ussuriensis). Fish Shellfish Immunol. 2019, 84, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, H.; Pan, Z.; Cheng, L.; Sun, Y.; Wang, H.; Chang, G.; Wu, N.; Ding, H.; Zhao, H.; et al. Insights into chromosomal evolution and sex determination of Pseudobagrus ussuriensis (Bagridae, Siluriformes) based on a chromosome-level genome. DNA Res. 2022, 29, dsac028. [Google Scholar] [CrossRef]
- Su, H.; Su, J. Cyprinid viral diseases and vaccine development. Fish Shellfish Immunol. 2018, 83, 84–95. [Google Scholar] [CrossRef]
- Xie, M.; Zhou, Y.; Gong, Y.; Liu, M.; Zhen, P.; Li, Z.; Zhou, L.; Gui, J.; Wang, Z. Growth Superiority and Genetic Characterization of the Hybrid from Female Ussuri Catfish (Pseudobagrus ussuriensis) and Male Longsnout Catfish (Leiocassis longirostris). Animals 2024, 14, 3617. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, L.; Zhao, J.; Liu, M.; Wang, K.; Zhou, Q.; Cao, Y.; Hu, R.; Wang, W.; Liu, Q. Comprehensive multi-omics and biochemical analysis to elucidate the molecular response mechanisms of gill and kidney tissues under acute salinity stress in Pseudobagras ussuriensis. BMC Genom. 2025, 26, 590. [Google Scholar] [CrossRef]
- Xu, A.; Han, F.; Zhang, Y.; Zhou, T.; Gao, T. Comparative Transcriptomic Analyses Revealed the Effects of Poly (I:C) on the Liver and Spleen of Argyrosomus japonicus. Int. J. Mol. Sci. 2022, 23, 9801. [Google Scholar] [CrossRef]
- Chu, Q.; Gao, Y.; Xu, G.; Wu, C.; Xu, T. Transcriptome comparative analysis revealed poly(I:C) activated RIG-I/MDA5-mediated signaling pathway in miiuy croaker. Fish Shellfish Immunol. 2015, 47, 168–174. [Google Scholar] [CrossRef]
- Wang, L.; Guan, T.; Gu, J.; Zhu, C.; Pan, Z.; Wang, H.; Li, J. Comparative transcriptome analysis of gonads in male and female Pseudobagrus ussuriensis (Bagridae, Siluriformes). Comp. Biochem. Physiol. Part. D Genom. Proteom. 2023, 47, 101105. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Wang, S.; Lv, Y.; Deng, H.; Chang, K.; Zhou, P.; Hu, C. Grass carp (Ctenopharyngodon idella) TNK1 modulates JAK-STAT signaling through phosphorylating STAT1. Dev. Comp. Immunol. 2021, 116, 103951. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Liao, Z.; Huo, X.; Yang, C.; Zhang, Y.; Su, J. IFN1 Enhances Thrombocyte Phagocytosis through IFN Receptor Complex-JAK/STAT-Complement C3.3-CR1 Pathway and Facilitates Antibacterial Immune Regulation in Teleost. J. Immunol. 2023, 210, 1043–1058. [Google Scholar] [CrossRef]
- Song, Y.J.; Zhang, J.; Xu, Z.; Nie, P.; Chang, M.X. Liver X Receptor LXRalpha Promotes Grass Carp Reovirus Infection by Attenuating IRF3-CBP Interaction and Inhibiting RLR Antiviral Signaling. J. Immunol. 2023, 211, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, L.; Yang, S.; Cao, Y.; Song, X.; Xiao, J.; Feng, H. Black carp PRMT6 inhibits TBK1-IRF3/7 signaling during the antiviral innate immune activation. Fish Shellfish Immunol. 2019, 93, 108–115. [Google Scholar] [CrossRef]
- Tan, S.; Chen, Y.; Wang, J.; Li, Z.; Li, J.; Liu, H.; Yu, J.; Yue, R.; Xiao, J.; Wu, H.; et al. Black carp OTUD1 negatively regulates antiviral innate immunity via deubiquitination and degradation of IRF3/7. Fish Shellfish Immunol. 2025, 163, 110407. [Google Scholar] [CrossRef]
- Qian, B.; Xue, L.; Qi, X.; Bai, Y.; Wu, Y. Gene networks and toxicity/detoxification pathways in juvenile largemouth bass (Micropterus salmoides) liver induced by acute lead stress. Genomics 2020, 112, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Wang, K.; Qin, C.; He, Y.; Luo, L.; Lin, S.; Chen, Y. Transcriptome Profiling Unveils the Mechanisms of Inflammation, Apoptosis, and Fibrosis in the Liver of Juvenile Largemouth Bass Micropterus salmoides Fed High-Starch Diets. Animals 2024, 14, 3394. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Liu, H.; Zhang, H.; Zhang, W.; Li, M.; Huang, Y.; Yao, J.; Huang, X.; Geng, Y.; Chen, D.; et al. High Starch in Diet Leads to Disruption of Hepatic Glycogen Metabolism and Liver Fibrosis in Largemouth Bass (Micropterus salmoides), Which is Mediated by the PI3K/Akt Signaling Pathway. Front. Physiol. 2022, 13, 880513. [Google Scholar] [CrossRef]
- Yue, Y.X.; Huang, S.S.; Weng, Y.Z.; Lu, Y.; Jia, B.B.; Yang, Z.X. Nobiletin Regulates Polyinosinic-polycytidylic Acid-induced Inflammation in Macrophages Partially via the PPAR-gamma Signaling Pathway. Curr. Pharm. Des. 2024, 30, 2937–2946. [Google Scholar] [CrossRef]
- Arimoto, K.I.; Miyauchi, S.; Troutman, T.D.; Zhang, Y.; Liu, M.; Stoner, S.A.; Davis, A.G.; Fan, J.B.; Huang, Y.J.; Yan, M.; et al. Expansion of interferon inducible gene pool via USP18 inhibition promotes cancer cell pyroptosis. Nat. Commun. 2023, 14, 251. [Google Scholar] [CrossRef]
- Lopez-Polo, V.; Maus, M.; Zacharioudakis, E.; Lafarga, M.; Attolini, C.S.; Marques, F.D.M.; Kovatcheva, M.; Gavathiotis, E.; Serrano, M. Release of mitochondrial dsRNA into the cytosol is a key driver of the inflammatory phenotype of senescent cells. Nat. Commun. 2024, 15, 7378. [Google Scholar] [CrossRef]
- Chen, S.; Ye, J.; Lin, Y.; Chen, W.; Huang, S.; Yang, Q.; Qian, H.; Gao, S.; Hua, C. Crucial Roles of RSAD2/viperin in Immunomodulation, Mitochondrial Metabolism and Autoimmune Diseases. Inflammation 2025, 48, 520–540. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.S.; Vishwakarma, S.; Trimbake, D.; Gurav, Y.K.; Potdar, V.A.; Mokashi, N.D.; Patsute, S.D.; Kaushal, H.; Choudhary, M.L.; Tilekar, B.N.; et al. Pro-inflammatory CXCL-10, TNF-alpha, IL-1beta, and IL-6: Biomarkers of SARS-CoV-2 infection. Arch. Virol. 2021, 166, 3301–3310. [Google Scholar] [CrossRef]
- Hodzic, A.; Gesslbauer, B.; Bochkov, V.; Oskolkova, O.V. Cooperative induction of CXCL chemokines by inflammatory cytokines and oxidized phospholipids. Immunology 2024, 173, 286–295. [Google Scholar] [CrossRef]
- Ma, J.; Wu, Y.; Ma, L.; Yang, X.; Zhang, T.; Song, G.; Li, T.; Gao, K.; Shen, X.; Lin, J.; et al. A blueprint for tumor-infiltrating B cells across human cancers. Science 2024, 384, eadj4857. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, D.; Churov, A.; Fu, R. Research Progress on NK Cell Receptors and Their Signaling Pathways. Mediat. Inflamm. 2020, 2020, 6437057. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-beta signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Xu, J.; Zhang, Z.; Yu, R. ITGA1 Promotes Glioma Cell Proliferation and Affects Immune Cell Infiltration in Low-Grade Glioma. Mediators Inflamm. 2024, 2024, 6147483. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Duan, J.; Wang, L.; Xiao, S.; Li, L.; Yan, X.; Yao, W.; Wu, L.; Zhang, S.; Zhang, Y.; et al. PTK2 promotes cancer stem cell traits in hepatocellular carcinoma by activating Wnt/beta-catenin signaling. Cancer Lett. 2019, 450, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Noshita, S.; Kubo, Y.; Kajiwara, K.; Okuzaki, D.; Nada, S.; Okada, M. A TGF-beta-responsive enhancer regulates SRC expression and epithelial-mesenchymal transition-associated cell migration. J. Cell Sci. 2023, 136, jcs261001. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Grandis, J.R.; Bauman, J.E. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral. Oncol. 2016, 56, 84–92. [Google Scholar] [CrossRef]
- Lencer, W.I.; DeLuca, H.; Grey, M.J.; Cho, J.A. Innate immunity at mucosal surfaces: The IRE1-RIDD-RIG-I pathway. Trends Immunol. 2015, 36, 401–409. [Google Scholar] [CrossRef]
- Lu, C.; Ning, G.; Si, P.; Zhang, C.; Liu, W.; Ge, W.; Cui, K.; Zhang, R.; Ge, S. E3 ubiquitin ligase HECW1 promotes the metastasis of non-small cell lung cancer cells through mediating the ubiquitination of Smad4. Biochem. Cell Biol. 2021, 99, 675–681. [Google Scholar] [CrossRef]




| Software | Version | Parameters | Function |
|---|---|---|---|
| DESeq | 1.34.1 | qvalue(pvalue)<0.05, |log2FoldChange|>1 | Non-biological repeated difference analysis |
| htseq-count | 0.11.2 | -s reverse | Gene quantification |
| hisat2 | 2.1.0 | --rna-strandness rf --fr | genomic alignment |
| DESeq2 | 1.22.2 | qvalue(pvalue)<0.05, |log2FoldChange|>1 | Biological duplication and paired difference analysis |
| fastp | 0.20.1 | --length_required 50 | Original read base quality control |
| fastqc | v0.11.9 | default | Raw read quality assessment |
| RseQC | 4.0.0 | default | RNA quality control |
| samtools | 1.9 | mpileup -uRf -d 1000000 | sam and bam file analysis |
| Gene Name | Sequences (5′-3′) |
|---|---|
| Ptprs | F: TAAAGGGCTATCGGGTT |
| R: GCTCTGGATGGTGGTGA | |
| Dkk1 | F: CTGCTTACTGTCCCGTG |
| R: GTAGCGTTTGCCTGATG | |
| Ern1 | F: ACCGAGACATCAAACCG |
| R: AACATTCCTGCCACCTG | |
| Endod1 | F: GGAGAGCGAGAGACCAG |
| R: GGAGCACCAGAGAGAGG | |
| Cish | F: CAGGACGAAGAGTGTGA |
| R: CAAGATGCTGTAGGGAT | |
| Uba1 | F: CTGCTATCGCCACAACC |
| R: GAATACTCTTTCCCCGC | |
| β-actin | F: AGAGCGTAACCCTCGTAG R: CTGCTTTGCGGCTGAATA |
| Sample | RawReads (M) | RawBases (G) | CleanReads (M) | CleanBases (G) | ValidBases (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|---|
| IC-3L-1 | 24.87 | 7.24 | 23.99 | 6.99 | 96.48 | 96.41 | 44.61 |
| IC-3L-2 | 24.76 | 7.24 | 23.99 | 7.01 | 96.86 | 96.07 | 46.11 |
| IC-3L-3 | 24.53 | 7.2 | 23.89 | 7.01 | 97.4 | 96.06 | 46.25 |
| IC-3S-1 | 47.85 | 7.06 | 46.74 | 6.90 | 97.67 | 94.73 | 43.99 |
| IC-3S-2 | 48.06 | 7.09 | 46.96 | 6.93 | 97.69 | 94.82 | 43.87 |
| IC-3S-3 | 48.83 | 7.21 | 47.57 | 7.03 | 97.41 | 94.42 | 44.29 |
| IC-48L-1 | 21.99 | 6.37 | 21.03 | 6.09 | 95.65 | 96.09 | 44.05 |
| IC-48L-2 | 23.48 | 6.83 | 22.58 | 6.56 | 96.15 | 96.42 | 43.7 |
| IC-48L-3 | 20.69 | 6.02 | 19.93 | 5.8 | 96.31 | 95.85 | 44.68 |
| IC-48S-1 | 24.92 | 7.24 | 23.96 | 6.96 | 96.14 | 95.38 | 45.2 |
| IC-48S-2 | 21.75 | 6.48 | 21.55 | 6.41 | 99.05 | 95.61 | 43.19 |
| IC-48S-3 | 22.18 | 6.43 | 21.26 | 6.16 | 95.82 | 96.84 | 44.56 |
| PBS-3L-1 | 24.76 | 7.19 | 23.77 | 6.9 | 96.02 | 95.98 | 44.58 |
| PBS-3L-2 | 24.4 | 7.13 | 23.64 | 6.91 | 96.87 | 96.22 | 45.63 |
| PBS-3L-3 | 24.72 | 7.23 | 23.97 | 7.02 | 96.98 | 95.78 | 45.65 |
| PBS-3S-1 | 25.27 | 7.34 | 24.24 | 7.05 | 95.94 | 96.36 | 44.81 |
| PBS-3S-2 | 25.1 | 7.24 | 23.94 | 6.9 | 95.35 | 96.41 | 40.65 |
| PBS-3S-3 | 20.99 | 6.08 | 20.09 | 5.82 | 95.7 | 96.22 | 44.19 |
| PBS-48L-1 | 23.13 | 6.76 | 22.42 | 6.55 | 96.92 | 96.07 | 44 |
| PBS-48L-2 | 24.1 | 7.03 | 23.3 | 6.8 | 96.69 | 96.01 | 44.44 |
| PBS-48L-3 | 22.57 | 6.53 | 21.55 | 6.24 | 95.51 | 95.96 | 43.75 |
| PBS-48S-1 | 23.94 | 6.95 | 22.98 | 6.68 | 96.01 | 96.21 | 42.74 |
| PBS-48S-2 | 25.4 | 7.34 | 24.16 | 6.98 | 95.14 | 96.93 | 43.08 |
| PBS-48S-3 | 24.99 | 7.26 | 24.02 | 6.98 | 96.13 | 96.01 | 43.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, K.; Lu, L.; Miao, H.; Gu, L.; Dou, Z.; Liu, Q. Comparative Transcriptomic Analysis of the Liver and Spleen in Ussuri Catfish (Pseudobagrus ussuriensis) Challenged with Polyriboinosinic Polyribocytidylic Acid (Poly(I:C)). Animals 2025, 15, 2454. https://doi.org/10.3390/ani15162454
Liu Y, Wang K, Lu L, Miao H, Gu L, Dou Z, Liu Q. Comparative Transcriptomic Analysis of the Liver and Spleen in Ussuri Catfish (Pseudobagrus ussuriensis) Challenged with Polyriboinosinic Polyribocytidylic Acid (Poly(I:C)). Animals. 2025; 15(16):2454. https://doi.org/10.3390/ani15162454
Chicago/Turabian StyleLiu, Yu, Ke Wang, Lingyun Lu, Huanhuan Miao, Libo Gu, Zhipeng Dou, and Qing Liu. 2025. "Comparative Transcriptomic Analysis of the Liver and Spleen in Ussuri Catfish (Pseudobagrus ussuriensis) Challenged with Polyriboinosinic Polyribocytidylic Acid (Poly(I:C))" Animals 15, no. 16: 2454. https://doi.org/10.3390/ani15162454
APA StyleLiu, Y., Wang, K., Lu, L., Miao, H., Gu, L., Dou, Z., & Liu, Q. (2025). Comparative Transcriptomic Analysis of the Liver and Spleen in Ussuri Catfish (Pseudobagrus ussuriensis) Challenged with Polyriboinosinic Polyribocytidylic Acid (Poly(I:C)). Animals, 15(16), 2454. https://doi.org/10.3390/ani15162454





