Effects of Tea Polyphenols on Post-Weaning Meat Quality and Antioxidant Status in Lambs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocol
2.2. Sample Collection
2.3. Determination of Meat Quality
2.4. Determination of Antioxidant Indicators
2.5. Determination of Fatty Acid Indexes
2.6. RT-qPCR
2.7. Statistical Analysis
3. Results
3.1. Effect of Tea Polyphenols on the Muscle Quality of Weaned Lambs
3.2. Effects of Tea Polyphenols on the Antioxidant Properties of Weaned Lambs’ Muscles
3.3. Effects of Tea Polyphenols on the Fatty Acids in the Muscles of Weaned Lambs
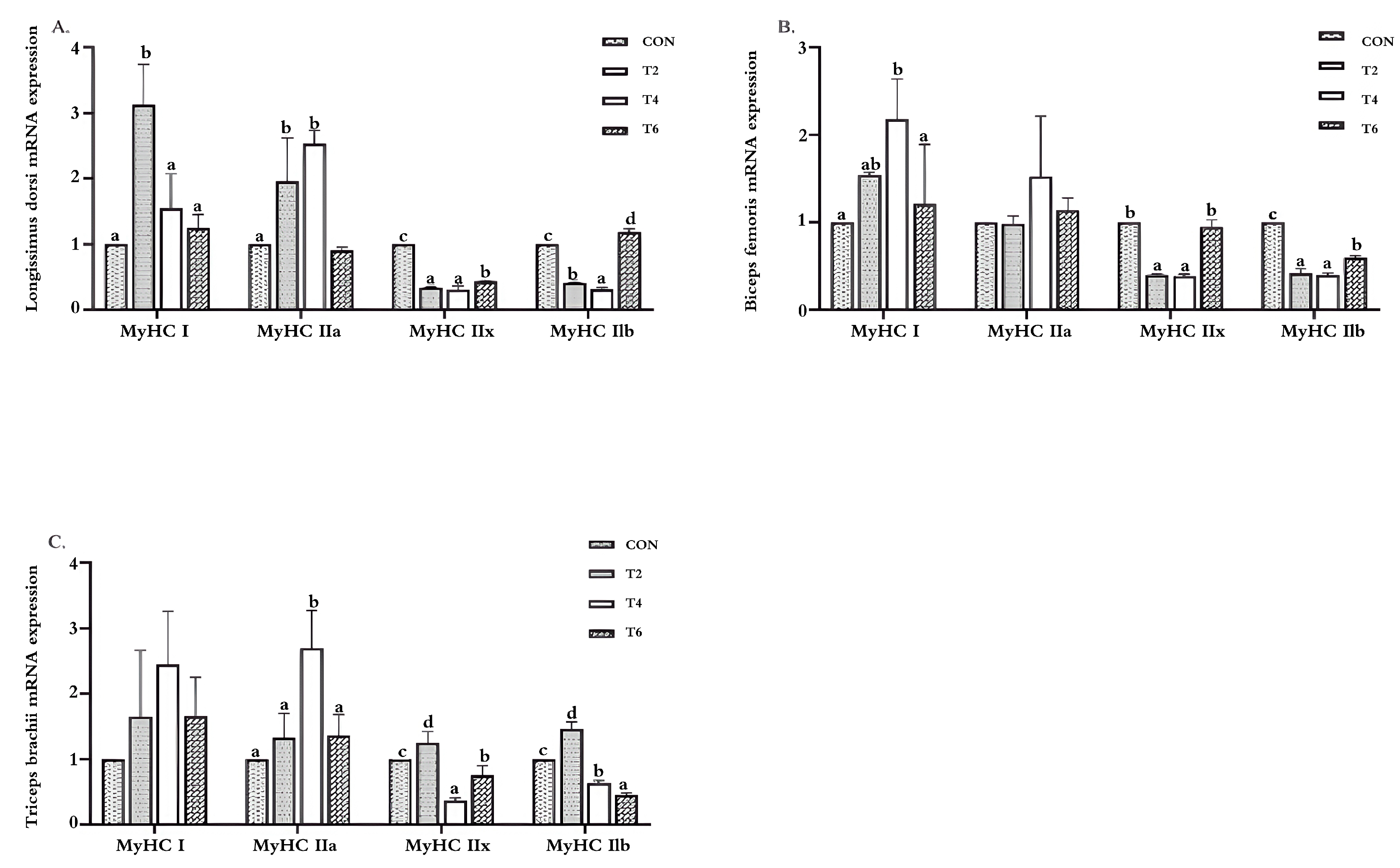
3.4. Effects of Tea Polyphenols on the Relative Expression Level of the MyHC mRNA in Weaned Lamb Muscles
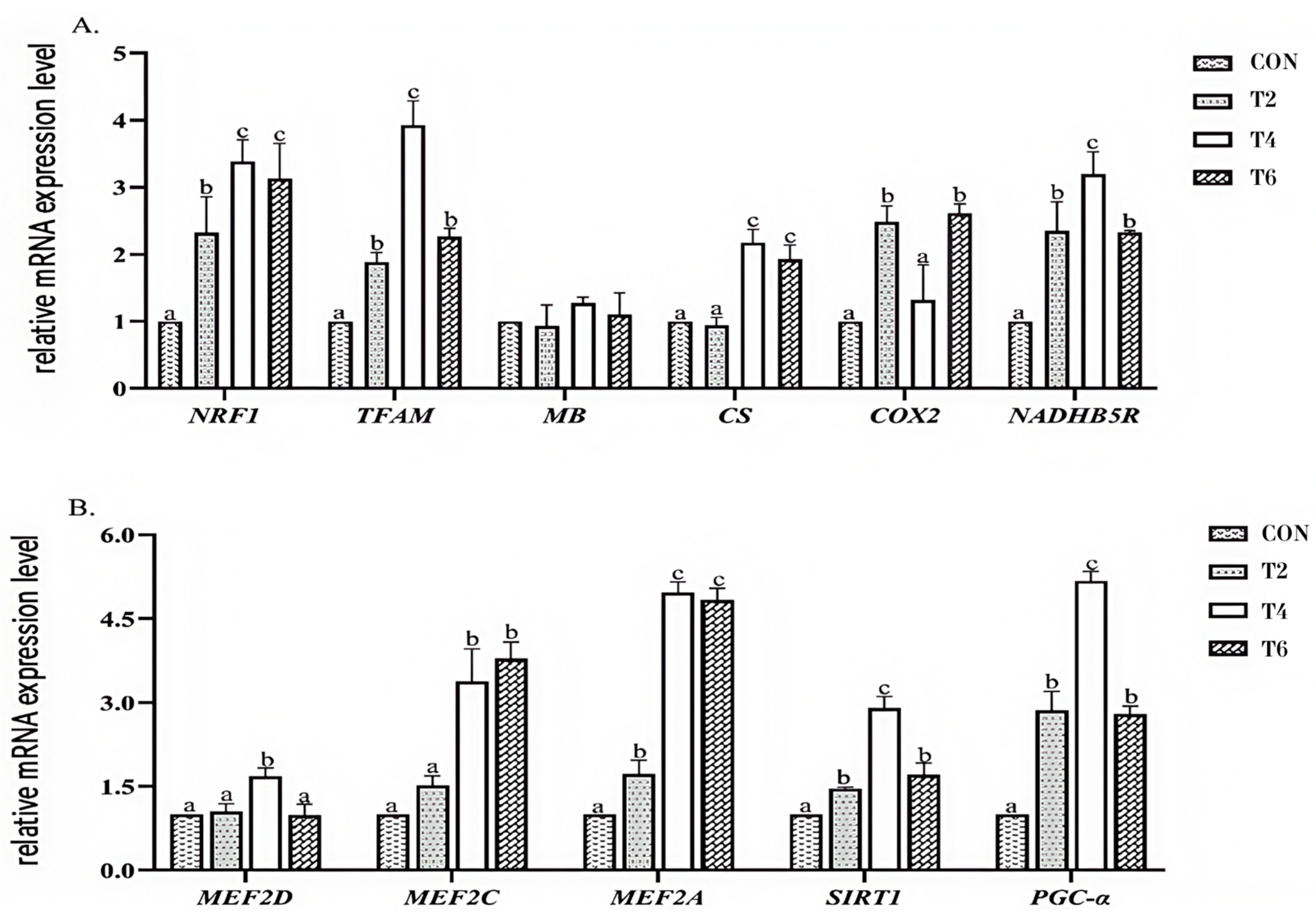
3.5. Effect of Tea Polyphenols on the Expression of Related Genes in the PGC-α Pathway in the Longissimus Dorsi Muscle
4. Discussion
4.1. Effect of Tea Polyphenol Administration on the Muscle Quality of Weaned Lambs
4.2. Effect of Tea Polyphenols on the Antioxidant Activity of Weaned Lamb Muscles
4.3. Effects of Tea Polyphenols on Fatty Acids in the Muscles of Weaned Lambs
4.4. Effects of Tea Polyphenols on the Relative Expression of the MyHC mRNA in the Muscle of Weaned Lambs
4.5. Effects of Tea Polyphenols on the Expression Levels of Genes Related to the PGC-α Pathway in the Longissimus Dorsi Muscle
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wan, X.; Zhan, J.; Ho, C.-T. Focusing on the recent progress of tea polyphenol chemistry and perspectives. Food Sci. Hum. Wellness 2022, 11, 437–444. [Google Scholar] [CrossRef]
- Namal Senanayake, S.P.J. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological Actions and Underlying Mechanisms of Catechin: A Review. Mini-Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef]
- Petcu, C.D.; Mihai, O.D.; Tăpăloagă, D.; Gheorghe-Irimia, R.-A.; Pogurschi, E.N.; Militaru, M.; Borda, C.; Ghimpețeanu, O.-M. Effects of Plant-Based Antioxidants in Animal Diets and Meat Products: A Review. Foods 2023, 12, 1334. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, L.; Xu, Y.; He, X.; Gan, S.; Yin, F. The Effects of Tea Polyphenols in Feed on the Immunity, Antioxidant Capacity, and Gut Microbiota of Weaned Goat Kids. Animals 2025, 15, 467. [Google Scholar] [CrossRef] [PubMed]
- Mahlake, S.K.; Mnisi, C.M.; Kumanda, C.; Mthiyane, D.M.N.; Montso, P.K. Green Tea (Camellia sinensis) Products as Alternatives to Antibiotics in Poultry Nutrition: A Review. Antibiotics 2022, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, C.; Wu, J.; Cui, Y.; Zhang, M.; Bi, C.; Shan, A.; Dou, X. EGCG improve meat quality, restore lipid metabolism disorder and regulate intestinal flora in high-fat fed broilers. Poult. Sci. 2025, 104, 104875. [Google Scholar] [CrossRef] [PubMed]
- Oanh, N.C.; Thu, C.T.T.; Hong, N.T.; Giang, N.T.P.; Hornick, J.L.; Dang, P.K. Growth performance, meat quality, and blood characteristics of finisher crossbred pigs fed diets supplemented with different levels of green tea (Camellia sinensis) by-products. Vet. World 2023, 16, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Han, L.; Li, J.; Yu, Q.; CaI, Z. Effects of Tea Polyphenols on Mitochondrial Apoptosis and Meat Tenderness in Post-mortem Yak Meat. Trans. Chin. Soc. Agric. Mach. 2019, 50, 352–359. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, F.; Wang, J.; Wu, P.; Qiu, X.; He, X.; Xiao, Y.; Gan, S. Effect of tea polyphenols on intestinal barrier and immune function in weaned lambs. Front. Vet. Sci. 2024, 11, 1361507. [Google Scholar] [CrossRef]
- Xie, C.; Niu, S.; Tian, W. Tea Polyphenols Relieve the Fluoride-Induced Oxidative Stress in the Intestinal Porcine Epithelial Cell Model. Toxics 2025, 13, 83. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef] [PubMed]
- GB/T 6435-2014:2014; Determination of Moisture in Feedstuffs. Standardization Administration of the People’s Republic of China: Beijing, China, 2014.
- GB/T 6432-2018:2018; Determination of Crude Protein in Feeds—Kjeldahl Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 6438-2007:2007; Animal Feeding Stuffs—Determination of Crude Ash. Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- Vogel, K.P.; Pedersen, J.F.; Masterson, S.D.; Toy, J.J. Evaluation of a filter bag system for NDF, ADF, and IVDMD forage analysis. Crop Sci. 1999, 39, 276–279. [Google Scholar] [CrossRef]
- GB/T 6436-2018:2018; Determination of Calcium in Feeds. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 6437-2018:2018; Determination of Phosphorus in Feeds—Spectrophotometry. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Fernández-Barroso, M.; Silió, L.; Rodríguez, C.; Palma-Granados, P.; López, A.; Caraballo, C.; Sánchez-Esquiliche, F.; Gómez-Carballar, F.; García-Casco, J.M.; Muñoz, M. Genetic parameter estimation and gene association analyses for meat quality traits in open-air free-range Iberian pigs. J. Anim. Breed. Genet. 2020, 137, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska-Kapituła, M.; Żmijewski, T. The influence of age and gender on the quality of raw and roasted wild boars (Sus scrofa) meat. Meat Sci. 2021, 181, 108600. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Warner, R.D.; Rosenvold, K. Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: A review. Anim. Prod. Sci. 2014, 54, 375–395. [Google Scholar] [CrossRef]
- Dai, Z.; Han, L.; Li, Z.; Gu, M.; Xiao, Z.; Lu, F. Combination of Chitosan, Tea Polyphenols, and Nisin on the Bacterial Inhibition and Quality Maintenance of Plant-Based Meat. Foods 2022, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Yajing, Z.; Shaoping, Z.; Ailing, C.; Tingting, M.; Shan, D.; Xiaofang, Z.; Yanhua, H.; Jing, F. Effects of Dietary Tea Polyphenols for Parental-Pigeon on Growth Performance, Slaughter Performance, Meat Quality and Muscle Antioxidant Capacity of Squab. China Poult. 2021, 43, 41. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Tan, C.Y.; Han, X.F.; Tang, S.X.; Tan, Z.L.; Zeng, B. Effect of dietary tea catechins supplementation in goats on the quality of meat kept under refrigeration. Small Rumin. Res. 2009, 87, 122–125. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Q.D.; Zeng, W.C. Effect of tea polyphenols on the tenderness of yak meat. J. Food Process. Preserv. 2020, 44, e14433. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem.-Biol. Interact. 1998, 111–112, 1–14. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, X.; Yuan, J. Substituting ethoxyquin with tea polyphenols and propyl gallate enhanced feed oxidative stability, broiler hepatic antioxidant capacity and gut health. Poult. Sci. 2024, 103, 104368. [Google Scholar] [CrossRef]
- Sha, L.; Liu, S. Effect of tea polyphenols on the inhibition of heterocyclic aromatic amines in grilled mutton patties. J. Food Process. Preserv. 2022, 46, e16811. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Petrotos, K.; Goulas, P.; Kouretas, D. Effects of dietary grape pomace supplementation on performance, carcass traits and meat quality of lambs. In Vivo 2018, 32, 807–812. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Priyashantha, H.; Vidanarachchi, J.K.; Kiani, A.; Holman, B.W.B. Effects of Nutritional Factors on Fat Content, Fatty Acid Composition, and Sensorial Properties of Meat and Milk from Domesticated Ruminants: An Overview. Animals 2024, 14, 840. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Mapiye, C.; Aldai, N.; Turner, T.; Aalhus, J.; Rolland, D.; Kramer, J.; Dugan, M. The labile lipid fraction of meat: From perceived disease and waste to health and opportunity. Meat Sci. 2012, 92, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Scerra, M.; Caparra, P.; Foti, F.; Galofaro, V.; Sinatra, M.; Scerra, V. Influence of ewe feeding systems on fatty acid composition of suckling lambs. Meat Sci. 2007, 76, 390–394. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Qu, Q.; Wang, Q.; Liu, S.; Shi, D.; Ling, C. Effect of tea polyphenol on growth performance and fatty acid content of breast muscle in broilers under heat stress. China Poult. 2015, 37, 30–32. (In Chinese) [Google Scholar] [CrossRef]
- Tan, C.; Zhong, R.; Tan, Z.; Han, X.; Tang, S.; Xiao, W.; Sun, Z.; Wang, M. Dietary inclusion of tea catechins changes fatty acid composition of muscle in goats. Lipids 2011, 46, 239–247. [Google Scholar] [CrossRef]
- Zierath, J.R.; Hawley, J.A. Skeletal muscle fiber type: Influence on contractile and metabolic properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef]
- Mo, M.; Zhang, Z.; Wang, X.; Shen, W.; Zhang, L.; Lin, S. Molecular mechanisms underlying the impact of muscle fiber types on meat quality in livestock and poultry. Front. Vet. Sci. 2023, 10, 1284551. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Xue, Y.; Huang, Z.; Chen, X.; Jia, G.; Zhao, H.; Liu, G. Naringin induces skeletal muscle fiber type transformation via AMPK/PGC-1α signaling pathway in mice and C2C12 myotubes. Nutr. Res. 2021, 92, 99–108. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Ordway, G.A.; Garry, D.J. Myoglobin: An essential hemoprotein in striated muscle. J. Exp. Biol. 2004, 207 Pt 20, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, G.; Ji, Z.; Chao, T.; Liu, Z.; Wang, X.; Liu, G.; Wu, C.; Wang, J. Three slow skeletal muscle troponin genes in small-tailed Han sheep (Ovis aries): Molecular cloning, characterization and expression analysis. Mol. Biol. Rep. 2016, 43, 999–1010. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Ismail, I.; Joo, S.T. The Relationship between Muscle Fiber Composition and Pork Taste-traits Assessed by Electronic Tongue System. Korean J. Food Sci. Anim. Resour. 2018, 38, 1305–1314. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.I.; Oh, H.T.; Kim, H.K.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Catechins activate muscle stem cells by Myf5 induction and stimulate muscle regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Cheng, X.; Cui, Y.; Xia, Q.; Yan, X.; Zhang, M.; Lan, G.; Liu, J.; Shan, T.; Huang, Y. Resveratrol regulates skeletal muscle fibers switching through the AdipoR1-AMPK-PGC-1α pathway. Food Funct. 2019, 10, 3334–3343. [Google Scholar] [CrossRef]
- Patrizio, F.; Ditroilo, M.; Felici, F.; Duranti, G.; De Vito, G.; Sabatini, S.; Sacchetti, M.; Bazzucchi, I. The acute effect of Quercetin on muscle performance following a single resistance training session. Eur. J. Appl. Physiol. 2018, 118, 1021–1031. [Google Scholar] [CrossRef]
- Kong, S.; Cai, B.; Nie, Q. PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol. Genet. Genom. 2022, 297, 621–633. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine Regulation of the Fasting Response by PPARα-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Feng, J.; Wang, M.; Wufuer, R.; Liu, K.; Zhang, Z.; Zhang, Y. Nrf1 is an indispensable redox-determining factor for mitochondrial homeostasis by integrating multi-hierarchical regulatory networks. Redox Biol. 2022, 57, 102470. [Google Scholar] [CrossRef]
- Ramanathan, R.; Mancini, R.A. Effects of pyruvate on bovine heart mitochondria-mediated metmyoglobin reduction. Meat Sci. 2010, 86, 738–741. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A.; Mancini, R.A.; Seyfert, M.; Hunt, M.C. Postmortem oxygen consumption by mitochondria and its effects on myoglobin form and stability. J. Agric. Food Chem. 2005, 53, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Rahmel, T.; Marko, B.; Nowak, H.; Bergmann, L.; Thon, P.; Rump, K.; Kreimendahl, S.; Rassow, J.; Peters, J.; Singer, M.; et al. Mitochondrial dysfunction in sepsis is associated with diminished intramitochondrial TFAM despite its increased cellular expression. Sci. Rep. 2020, 10, 21029. [Google Scholar] [CrossRef] [PubMed]
- Yanping, B.; Yanru, H.; Lin, S.; Bing, S.; Lu, D.; Lihua, Z.; Ruijun, L.; Gerile, A.; Ye, J. Effects of lactobacillus induced mitochondrial biogenesis on muscle fiber properties and meat quality of sheep. Trans. Chin. Soc. Agric. Eng. 2021, 37. [Google Scholar] [CrossRef]
| Ingredients (%) | Content |
|---|---|
| Pennisetum | 50.00 |
| Corn | 29.00 |
| Soybean meal | 10.00 |
| Bran | 7.50 |
| Premix 1 | 2.00 |
| CaHPO4 | 0.50 |
| NaHCO3 | 0.50 |
| NaCl | 0.50 |
| Total | 100.00 |
| Nutrient levels | |
| Dry matter (%) [15] | 90.80 |
| Metabolic energy (MJ/Kg) 2 | 10.43 |
| Crude protein [16] | 14.69 |
| Crude fat [17] | 2.84 |
| Acid detergent fiber [18] | 26.23 |
| Neutral detergent fiber [18] | 39.9 |
| Ca [19] | 0.54 |
| P [20] | 1.10 |
| Gene symbol | Product Length, bp | Accession No. | Nucleotide Sequence of Primers (5’→3’) |
|---|---|---|---|
| MyHCI | 139 | XM_004010325.3 | F: CTTCCCCATATATACAGCCCCC R: CTTGGCTTTCAAACGCGCC |
| MyHCIIα | 106 | XM_012122422.2 | F: AGCTCCAAGGTAAGTGGGAA R: GCCTGGAAGTGAGACGGTTC |
| MyHCIIx | 139 | XM_004012706.4 | F: TTAAGAAAGAGGGAGGCGAC R: CAAAGACCCTGCCTTGGAGAT |
| MyHCIIb | 182 | XM_027974884.1 | F: GCCCTTGGAATGAGGCTGAC R: ATGCGCTCCTTTCGGACTT |
| β-actin | 181 | XM_001009784.1 | F: GCAAATGCTTCTAGGCGGAC R: GGCCATCCCAGCCTCATAAC |
| COX2 | 151 | 1485859 | F: AAGACGCAGCATCACCCATTA R: TCTTGTGOGTCTATGGTGCT |
| CS | 142 | 102168803 | F: TCCGTGGCCCAATGTAGATG R: GGGAGAATCCTGGCTCTGAC |
| MB | 292 | 100860833 | F: TTCCCAAATGCTTGACAGGGA R: GAGTTGGGTTTCAGGCCACT |
| MEF2A | 173 | 102180703 | F: AGGGGGAGACTTTGTAGGCA R: CCTGGGGCTGAGGAAAACAT |
| MEF2C | 158 | 102175290 | F: TTAGCCCAGGCTATGGAGGT R: CCCTGAACCACTACTCAGCA |
| MEF2D | 104 | 102174260 | F: ACTGTGCTCATGAACGGTCT R: CGGCTGACAGTCCTCTTGTA |
| NADHB5R | 210 | 102175455 | F: GGAGGGAACACGTGTGCTTA R: TGTAATCTTGATCCAGCACTCAG |
| NRF1 | 167 | 102180186 | F: CTGCGCTGTCCGATATCCTG R: GCATCTGGGAACCTATGCCC |
| PGC-1α | 170 | 100861288 | F: ATGGGGCGATCTTGAACGTG R: GGGCTACTCAGTCATGCCAA |
| SIRT1 | 155 | 102180824 | F: ATTGGCTGGCAGGAGATACA R: GTGGTCCAACCCACATGACT |
| TFAM | 134 | 102187195 | F: ATGGTTTTAGTCCCAAGTGCC R: AAGCCAACCTGGTTCCAAGA |
| GAPDH | 171 | 100860872 | F: TGAAGGGGTCATTGATGGCA R: TGAAAGAGGGTGAATGGGCA |
| Groups | ||||||
|---|---|---|---|---|---|---|
| Item | CON | TP2 | TP4 | TP6 | SEM | p-Value |
| Longissimus dorsi | ||||||
| pH0h | 5.61 | 5.85 | 5.94 | 5.90 | 0.13 | 0.064 |
| pH24h | 5.68 | 5.89 | 5.81 | 5.93 | 0.14 | 0.299 |
| Water loss rate (%) | 15.40 | 27.30 | 23.20 | 26.21 | 0.06 | 0.218 |
| Cooked meat rate (%) | 68.95 | 69.03 | 71.82 | 62.35 | 0.03 | 0.091 |
| Shear force/N | 28.68 | 28.79 | 28.81 | 33.21 | 3.95 | 0.569 |
| Biceps femoris | ||||||
| pH0h | 5.94 | 6.16 | 5.72 | 5.97 | 0.16 | 0.081 |
| pH24h | 5.54 | 5.70 | 5.68 | 5.44 | 0.11 | 0.059 |
| Water loss rate (%) | 40.30 | 42.15 | 38.65 | 42.85 | 0.05 | 0.799 |
| Cooked meat rate (%) | 61.28 a | 63.13 ab | 73.10 bc | 76.35 c | 0.05 | 0.019 |
| Shear force/N | 26.61 | 24.73 | 22.30 | 29.10 | 3.57 | 0.285 |
| Arm triceps | ||||||
| pH0h | 5.91 a | 5.91 a | 6.12 ab | 6.25 b | 0.14 | 0.043 |
| pH24h | 5.55 a | 5.57 a | 5.60 ab | 5.71 b | 0.06 | 0.033 |
| Water loss rate (%) | 20.30 | 26.98 | 24.58 | 27.10 | 0.04 | 0.291 |
| Cooked meat rate (%) | 64.05 | 62.60 | 66.08 | 60.18 | 0.03 | 0.215 |
| Shear force/N | 29.35 | 24.45 | 21.35 | 27.00 | 3.30 | 0.105 |
| Groups | ||||||
|---|---|---|---|---|---|---|
| Item | CON | TP2 | TP4 | TP6 | SEM | p-Value |
| Longissimus dorsi | ||||||
| T-SOD (U/mgprot) | 24.44 a | 36.44 b | 36.53 b | 36.70 b | 3.06 | 0.001 |
| T-AOC (mmol/gprot) | 1.01 | 1.04 | 1.00 | 1.02 | 0.01 | 0.131 |
| MDA (nmol/mgprot) | 1.28 | 1.12 | 1.11 | 1.17 | 0.09 | 0.283 |
| GSH-Px (U/gprot) | 19.82 a | 25.19 ab | 23.73 ab | 28.93 b | 2.57 | 0.017 |
| H2O2 (mmol/gprot) | 6.00 b | 5.93 b | 5.34 ab | 4.88 a | 0.32 | 0.006 |
| CAT (U/mgprot) | 9.66 a | 13.97 a | 33.79 b | 23.22 ab | 6.53 | 0.026 |
| Biceps femoris | ||||||
| T-SOD (U/mgprot) | 29.66 a | 30.23 a | 30.37 a | 33.59 b | 1.04 | 0.005 |
| T-AOC (mmol/gprot) | 0.97 a | 0.97 a | 1.04 b | 1.04 b | 0.01 | <0.001 |
| MDA (nmol/mgprot) | 1.24 c | 1.10 bc | 0.75 a | 1.04 b | 0.08 | <0.001 |
| GSH-Px (U/gprot) | 21.20 a | 25.27 b | 28.68 b | 25.36 b | 1.61 | 0.002 |
| H2O2 (mmol/gprot) | 7.04 c | 5.42 b | 3.11 a | 5.52 b | 0.29 | <0.001 |
| CAT (U/mgprot) | 16.65 a | 22.27 a | 71.19 b | 26.49 a | 5.49 | <0.001 |
| Arm triceps | ||||||
| T-SOD (U/mgprot) | 44.91 a | 50.68 a | 47.34 a | 60.44 b | 3.93 | 0.004 |
| T-AOC (mmol/gprot) | 0.98 | 0.99 | 1.02 | 1.01 | 0.01 | 0.59 |
| MDA (nmol/mgprot) | 1.17 | 0.91 | 0.96 | 1.04 | 0.31 | 0.847 |
| GSH-Px (U/gprot) | 34.32 a | 39.33 ab | 41.10 b | 43.92 b | 2.58 | 0.011 |
| H2O2 (mmol/gprot) | 12.03 c | 7.44 ab | 9.94 bc | 6.70 a | 1.31 | 0.002 |
| CAT (U/mgprot) | 29.88 a | 67.79 b | 60.31 ab | 81.57 b | 15.11 | 0.047 |
| Groups | ||||||
|---|---|---|---|---|---|---|
| Item | CON | TP2 | TP4 | TP6 | SEM | p-Value |
| Decanoic acid C10-0 | 1.36 | 1.75 | 1.38 | 2.12 | 0.70 | 0.670 |
| Lignoceric acid C24-0 | 5.06 | 5.03 | 4.92 | 4.98 | 0.07 | 0.271 |
| Hexanoic acid C6-0 | 0.17 | 0.82 | 0.17 | 0.16 | 0.51 | 0.516 |
| Octanoic acid C8-0 | 0.42 | 0.49 | 0.44 | 0.54 | 0.09 | 0.564 |
| Nonanoic acid C9-0 | 0.29 | 0.35 | 0.34 | 0.31 | 0.09 | 0.889 |
| Myristoleic acid C14-1 | 2.14 | 2.87 | 2.31 | 3.89 | 0.85 | 0.243 |
| Palmitelaidic Acid C16-1T | 4.61 | 4.97 | 6.65 | 8.04 | 1.66 | 0.219 |
| Linolelaidic acid C18-2n6t | 3.01 | 2.67 | 3.38 | 4.48 | 0.65 | 0.102 |
| C19:1(Cis-10) acid | 5.89 | 5.35 | 5.97 | 6.53 | 0.37 | 0.072 |
| Henicosanoic acid C21-0 | 2.11 | 2.11 | 2.06 | 2.08 | 0.04 | 0.372 |
| Cis-4,7,10,13,16,19-docosahexaenoic acid | 10.68 | 13.27 | 7.15 | 20.71 | 4.62 | 0.089 |
| All-cis-7,10,13,16,19-docosapentaenoic acid | 28.47 | 29.04 | 25.95 | 37.37 | 4.80 | 0.174 |
| Tricosanoic acid C23-0 | 4.28 | 4.29 | 4.20 | 4.24 | 0.06 | 0.436 |
| Palmitic acid C16-0 | 490.26 | 457.31 | 541.78 | 704.28 | 95.75 | 0.123 |
| Cis-9-palmitoleic acid C16-1 | 12.21 a | 11.77 a | 15.22 a | 25.75 b | 3.82 | 0.021 |
| Heptadecanoic acid C17-0 | 11.16 | 10.37 | 13.04 | 14.80 | 2.82 | 0.444 |
| Stearic acid C18-0 | 482.31 | 390.01 | 515.24 | 615.11 | 79.09 | 0.112 |
| Oleic acid C18-1n9c | 310.04 | 229.04 | 414.63 | 681.48 | 146.23 | 0.064 |
| Elaidic acid C18-1n9t | 32.87 a | 30.48 a | 44.55 ab | 67.89 b | 11.57 | 0.042 |
| Linoleic acid C18-2n6c | 136.10 a | 123.26 a | 178.87 a | 321.44 b | 59.66 | 0.037 |
| A-Linolenic acid C18-3n3 | 5.20 a | 4.62 a | 7.13 ab | 13.34 b | 2.74 | 0.045 |
| Gamma linolenic acid C18-3n6 | 5.54 a | 5.64 a | 5.91 a | 7.24 b | 0.54 | 0.047 |
| Nonadecanoic acidC19-0 | 2.80 | 2.75 | 2.72 | 2.80 | 0.11 | 0.861 |
| Arachidonic acid C20-0 | 3.28 | 2.80 | 2.80 | 3.11 | 0.43 | 0.631 |
| Cis-11-eicosenoiccid C20-1(cis-11) | 4.48 | 3.08 | 3.90 | 4.52 | 0.79 | 0.298 |
| Cis-11,14-eicosadienoic acid C20-2 | 3.89 | 3.35 | 3.84 | 4.30 | 0.33 | 0.107 |
| Cis-8,11,14-eicosatrienoic acid C20-3n6 | 11.71 | 12.70 | 11.94 | 17.12 | 2.50 | 0.184 |
| Arachidonic acid C20-4n6 | 160.89 | 165.69 | 164.90 | 239.82 | 43.00 | 0.272 |
| Eicosapentaenoic acid C20-5n3 | 27.01 | 26.41 | 26.84 | 38.04 | 5.90 | 0.221 |
| Saturated fatty acids (SFAs) | 1024.02 | 905.63 | 1110.93 | 1387.1 | 175.58 | 0.114 |
| Unsaturated fatty acids (UFAs) | 828.72 | 752.44 | 1020 | 1619.26 | 288.59 | 0.062 |
| Monounsaturated fatty acids (MUFAs) | 364.27 a | 281.80 a | 485.64 b | 789.59 c | 161.73 | 0.049 |
| Polyunsaturated fatty acids (PUFAs) | 345.05 a | 338.90 a | 390.80 a | 640.59 b | 103.91 | 0.049 |
| n6/n3 | 7.02 | 6.59 | 8.49 | 7.91 | 1.03 | 0.311 |
| n-3 Polyunsaturated fatty acids (n-3 PUFAs) | 42.96 a | 44.30 a | 41.12 a | 72.09 b | 10.66 | 0.048 |
| n-6 Polyunsaturated fatty acids (n-6 PUFAs) | 302.54 a | 294.59 a | 349.68 a | 568.50 b | 94.82 | 0.050 |
| Groups | ||||||
|---|---|---|---|---|---|---|
| Item | CON | TP2 | TP4 | TP6 | SEM | p-Value |
| Decanoic acid C10-0 | 1.57 | 1.13 | 1.01 | 1.32 | 0.44 | 0.615 |
| Lignoceric acid C24-0 | 5.04 | 4.95 | 5.01 | 5.02 | 0.12 | 0.894 |
| Hexanoic acid C6-0 | 0.19 | 0.14 | 0.08 | 0.11 | 0.07 | 0.526 |
| Octanoic acid C8-0 | 0.47 | 0.39 | 0.41 | 0.43 | 0.04 | 0.227 |
| Nonanoic acid C9-0 | 0.20 | 0.22 | 0.26 | 0.23 | 0.04 | 0.503 |
| Myristoleic acid C14-1 | 2.81 | 2.95 | 2.23 | 2.69 | 0.86 | 0.851 |
| Palmitelaidic Acid C16-1T | 6.10 | 9.63 | 5.82 | 7.17 | 2.24 | 0.371 |
| Linolelaidic acid C18-2n6t | 3.11 | 3.36 | 3.49 | 3.21 | 0.30 | 0.635 |
| C19:1(Cis-10) acid | 6.11 | 5.82 | 6.01 | 5.79 | 0.36 | 0.782 |
| Henicosanoic acid C21-0 | 2.11 | 2.07 | 2.09 | 2.09 | 0.05 | 0.849 |
| Cis-4,7,10,13,16,19-docosahexaenoic acid | 8.91 | 9.52 | 7.71 | 12.23 | 3.46 | 0.626 |
| All-cis-7,10,13,16,19-docosapentaenoic acid | 33.72 | 30.98 | 28.43 | 32.03 | 7.75 | 0.918 |
| Tricosanoic acid C23-0 | 4.31 | 4.24 | 4.28 | 4.29 | 0.10 | 0.907 |
| Palmitic acid C16-0 | 482.01 | 477.27 | 488.70 | 477.08 | 47.98 | 0.994 |
| Cis-9-palmitoleic acid C16-1 | 15.04 | 16.05 | 13.80 | 14.16 | 3.18 | 0.894 |
| Heptadecanoic acid C17-0 | 11.29 | 11.07 | 11.54 | 10.10 | 1.32 | 0.975 |
| Stearic acid C18-0 | 549.43 | 460.22 | 483.37 | 445.46 | 51.22 | 0.902 |
| Oleic acid C18-1n9c | 357.41 | 347.92 | 313.87 | 328.69 | 74.45 | 0.935 |
| Elaidic acid C18-1n9t | 44.79 | 64.99 | 46.43 | 48.87 | 8.91 | 0.168 |
| Linoleic acid C18-2n6c | 170.61 | 286.40 | 200.58 | 181.52 | 43.56 | 0.101 |
| A-Linolenic acid C18-3n3 | 7.03 | 8.21 | 6.47 | 6.89 | 1.63 | 0.745 |
| Gamma linolenic acid C18-3n6 | 5.63 | 6.47 | 5.89 | 6.00 | 0.39 | 0.262 |
| Nonadecanoic acidC19-0 | 2.77 | 2.74 | 2.77 | 2.73 | 0.09 | 0.963 |
| Arachidonic acid C20-0 | 2.73 | 2.50 | 2.59 | 2.54 | 0.28 | 0.845 |
| Cis-11-eicosenoiccid C20-1(cis-11) | 3.88 | 3.63 | 3.72 | 3.12 | 0.47 | 0.442 |
| Cis-11,14-eicosadienoic acid C20-2 | 3.97 | 4.23 | 4.09 | 3.73 | 0.28 | 0.400 |
| Cis-8,11,14-eicosatrienoic acid C20-3n6 | 12.28 | 12.86 | 10.98 | 12.04 | 2.52 | 0.897 |
| Arachidonic acid C20-4n6 | 178.49 | 194.53 | 142.56 | 139.84 | 48.34 | 0.620 |
| Eicosapentaenoic acid C20-5n3 | 26.50 | 23.63 | 24.57 | 33.08 | 6.56 | 0.507 |
| Saturated fatty acids (SFAs) | 996.28 | 991.05 | 1024.77 | 976.17 | 102.10 | 0.969 |
| Unsaturated fatty acids (UFAs) | 973.51 | 1144.11 | 896.58 | 912.55 | 226.64 | 0.693 |
| Monounsaturated fatty acids (MUFAs) | 428.60 | 443.44 | 384.02 | 403.53 | 86.94 | 0.904 |
| Polyunsaturated fatty acids (PUFAs) | 397.17 | 528.76 | 387.77 | 379.56 | 100.12 | 0.443 |
| n6/n3 | 8.34 ab | 11.65 c | 8.98 b | 6.61 a | 0.89 | 0.003 |
| n-3 Polyunsaturated fatty acids (n-3 PUFAs) | 42.45 | 41.36 | 38.74 | 52.20 | 11.11 | 0.655 |
| n-6 Polyunsaturated fatty acids (n-6 PUFAs) | 354.73 | 487.40 | 349.03 | 327.36 | 91.67 | 0.351 |
| Groups | ||||||
|---|---|---|---|---|---|---|
| Item | CON | TP2 | TP4 | TP6 | SEM | p-Value |
| Decanoic acid C10-0 | 1.45 | 1.72 | 1.31 | 2.13 | 0.59 | 0.554 |
| Lignoceric acid C24-0 | 5.04 | 4.98 | 5.06 | 5.05 | 0.09 | 0.771 |
| Hexanoic acid C6-0 | 0.11 | 0.11 | 0.14 | 0.15 | 0.05 | 0.840 |
| Octanoic acid C8-0 | 0.53 | 0.39 | 0.40 | 0.46 | 0.08 | 0.362 |
| Nonanoic acid C9-0 | 0.26 | 0.21 | 0.23 | 0.30 | 0.05 | 0.328 |
| Myristoleic acid C14-1 | 2.57 | 2.84 | 2.44 | 3.32 | 0.75 | 0.666 |
| Palmitelaidic Acid C16-1T | 7.57 | 6.70 | 6.58 | 7.98 | 1.97 | 0.868 |
| Linolelaidic acid C18-2n6t | 4.93 | 4.38 | 4.21 | 4.16 | 0.68 | 0.667 |
| C19:1(Cis-10) acid | 6.47 | 6.04 | 5.70 | 5.87 | 0.37 | 0.265 |
| Henicosanoic acid C21-0 | 2.12 | 2.10 | 2.11 | 2.10 | 0.04 | 0.973 |
| Cis-4,7,10,13,16,19-docosahexaenoic acid | 11.29 | 13.07 | 7.61 | 11.71 | 3.75 | 0.539 |
| All-cis-7,10,13,16,19-docosapentaenoic acid | 39.09 | 37.02 | 29.07 | 27.35 | 6.59 | 0.277 |
| Tricosanoic acid C23-0 | 4.30 | 4.25 | 4.32 | 4.31 | 0.07 | 0.786 |
| Palmitic acid C16-0 | 663.36 | 471.04 | 515.80 | 531.32 | 92.36 | 0.263 |
| Cis-9-palmitoleic acid C16-1 | 18.78 | 14.13 | 15.80 | 18.69 | 4.68 | 0.708 |
| Heptadecanoic acid C17-0 | 15.64 b | 11.96 a | 11.38 a | 11.64 a | 1.33 | 0.038 |
| Stearic acid C18-0 | 635.21 | 470.78 | 499.38 | 485.00 | 85.29 | 0.266 |
| Oleic acid C18-1n9c | 581.03 | 424.06 | 463.53 | 431.03 | 96.56 | 0.391 |
| Elaidic acid C18-1n9t | 70.23 | 62.06 | 40.72 | 61.52 | 16.30 | 0.372 |
| Linoleic acid C18-2n6c | 326.74 | 311.04 | 247.49 | 239.51 | 79.31 | 0.622 |
| A-Linolenic acid C18-3n3 | 10.97 | 10.93 | 9.36 | 8.23 | 2.30 | 0.599 |
| Gamma linolenic acid C18-3n6 | 6.20 | 5.87 | 6.20 | 6.25 | 0.48 | 0.847 |
| Nonadecanoic acidC19-0 | 2.96 | 2.83 | 2.81 | 2.77 | 0.10 | 0.278 |
| Arachidonic acid C20-0 | 3.07 | 2.73 | 2.61 | 2.51 | 0.31 | 0.358 |
| Cis-11-eicosenoiccid C20-1(cis-11) | 4.83 b | 3.86 ab | 3.63 a | 3.33 a | 0.45 | 0.048 |
| Cis-11,14-eicosadienoic acid C20-2 | 4.65 | 4.36 | 3.72 | 3.78 | 0.42 | 0.154 |
| Cis-8,11,14-eicosatrienoic acid C20-3n6 | 16.23 | 13.69 | 13.05 | 12.63 | 3.17 | 0.679 |
| Arachidonic acid C20-4n6 | 236.47 | 144.44 | 187.07 | 130.79 | 46.59 | 0.180 |
| Eicosapentaenoic acid C20-5n3 | 31.63 | 29.54 | 26.11 | 32.05 | 6.90 | 0.818 |
| Saturated fatty acids (SFAs) | 1360.36 | 999.00 | 1066.20 | 1080.25 | 184.10 | 0.285 |
| Unsaturated fatty acids (UFAs) | 1484.58 | 1174.28 | 1181.73 | 1076.77 | 262.57 | 0.480 |
| Monounsaturated fatty acids | 682.55 | 512.27 | 531.01 | 525.62 | 109.60 | 0.415 |
| Polyunsaturated fatty acids (PUFAs) | 623.30 | 514.89 | 483.84 | 428.54 | 134.00 | 0.553 |
| n6/n3 | 10.55 | 9.13 | 10.22 | 7.15 | 1.49 | 0.178 |
| n-3 Polyunsaturated fatty acids (n-3 PUFAs) | 53.89 | 53.54 | 43.08 | 51.99 | 12.14 | 0.789 |
| n-6 Polyunsaturated fatty acids (n-6 PUFAs) | 569.41 | 461.35 | 440.76 | 376.55 | 123.75 | 0.509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Wang, J.; Ma, J.; Du, C.; Yin, F. Effects of Tea Polyphenols on Post-Weaning Meat Quality and Antioxidant Status in Lambs. Animals 2025, 15, 2414. https://doi.org/10.3390/ani15162414
Bai Y, Wang J, Ma J, Du C, Yin F. Effects of Tea Polyphenols on Post-Weaning Meat Quality and Antioxidant Status in Lambs. Animals. 2025; 15(16):2414. https://doi.org/10.3390/ani15162414
Chicago/Turabian StyleBai, Yuxin, Jialin Wang, Jian Ma, Chunmei Du, and Fuquan Yin. 2025. "Effects of Tea Polyphenols on Post-Weaning Meat Quality and Antioxidant Status in Lambs" Animals 15, no. 16: 2414. https://doi.org/10.3390/ani15162414
APA StyleBai, Y., Wang, J., Ma, J., Du, C., & Yin, F. (2025). Effects of Tea Polyphenols on Post-Weaning Meat Quality and Antioxidant Status in Lambs. Animals, 15(16), 2414. https://doi.org/10.3390/ani15162414






