Distribution of Nasal Myiases Affecting Roe Deer in Spain and Associated Risk Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Area of Study and Sampled Animals
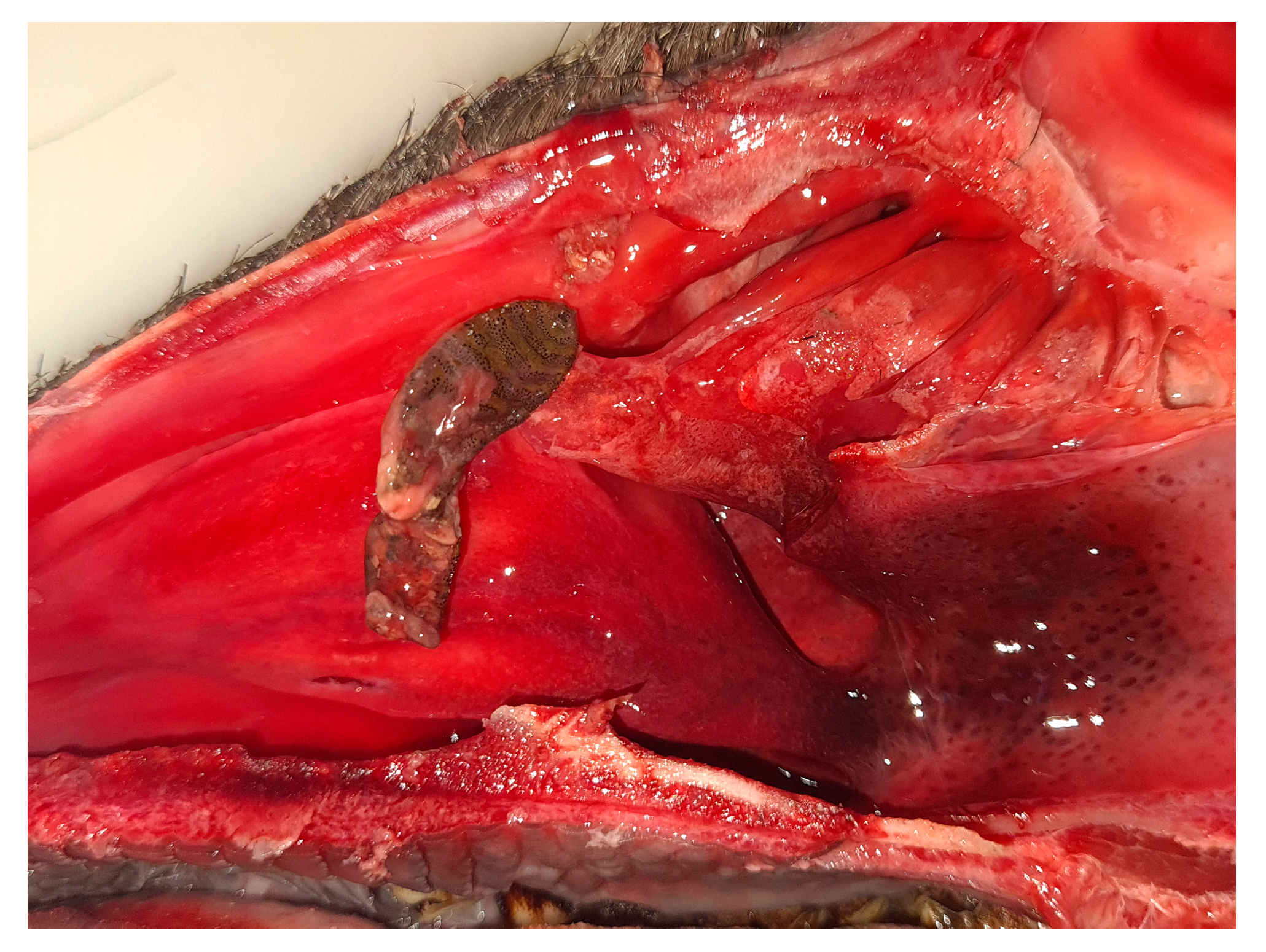
2.2. Larval Collection and Identification
2.3. Roe Deer Data Collection
2.4. Statistical Analysis
2.5. QGis Project
3. Results
3.1. Overall Prevalence and Larval Mean Intensity
3.2. Cephenemyia stimulator Risk Factor Analysis
| Variable | Categories | Positive Animals/Total (%; CI 95%) | Mean Larvae Intensity (SD) |
|---|---|---|---|
| Sex | Female | 374/1070 (35.0%; 32.09–37.90) | 39.9 (58.31) |
| Male | 264/530 (49.8%; 45.47–54.15) | 50.0 (87.58) | |
| Age | Fawn (0–3 mo) | 0/30 (0%; 0–11.57) | 0 |
| Young (>3 mo–2 yr) | 209/489 (42.7%; 38.31–47.26) | 72.9 (108.45) | |
| Adult (>2–6 yr) | 323/838 (38.5%; 35.23–41.93) | 29.1 (36.34) | |
| Old (≥6 yr) | 106/243 (43.6%; 37.29–50.11) | 32.8 (40.23) | |
| Month | January | 69/138 (50%; 41.38–58.62) | 50.1 (96.47) |
| February | 160/630 (25.4%; 22.04–28.99) | 33.5 (40.50) | |
| March | 46/68 (67.7%; 55.21–78.49) | 54.2 (83.92) | |
| April | 83/156 (53,2%; 45.06–61.23) | 42.2 (52.00) | |
| May | 48/116 (41.4%; 32.31–50.90) | 41.2 (44.13) | |
| June | 30/71 (42.3%; 30.61–54.56) | 23.8 (26.56) | |
| July | 37/68 (54.4%; 41.88–66.55) | 37.4 (36.27) | |
| August | 21/40 (52.5%; 36.13–68.49) | 53.7 (61.60) | |
| September | 37/61 (60.7%; 47.31–72.93) | 31.4 (32.50) | |
| October | 55/77 (71.4%; 60.00–81.15) | 82.2 (148.65) | |
| November | 27/81 (33.3%; 23.24–44.68) | 54.2 (91.02) | |
| December | 25/94 (26.6%; 18.00–36.71) | 38.8 (48.20) | |
| Season | Winter | 275/838 (32.8%; 29.64–36.11) | 41.6 (67.25) |
| Spring | 159/340 (46.8%; 41.36–52.22) | 38.3 (46.25) | |
| Summer | 82/154 (53.2%; 45.05–61.32) | 40.1 (43.26) | |
| Autumn | 122/268 (45.5%; 39.45–51.69) | 60.0 (112.57) | |
| Year | 2018 | 7/14 (50%; 23.04–76.97) | 21.4 (10.47) |
| 2019 | 14/26 (53.9%; 33.37–73.41) | 19.9 (24.71) | |
| 2020 | 20/38 (52.6%; 35.82–69.02) | 26.4 (30.00) | |
| 2021 | 61/96 (63.5%; 53.09–73.13) | 54.6 (64.01) | |
| 2022 | 126/262 (48.1%; 41.90–54.32) | 56.2 (72.71) | |
| 2023 | 142/425 (33.4%; 28.94–38.12) | 46.3 (82.29) | |
| 2024 | 215/580 (37.1%; 33.13–41.14) | 38.4 (77.71) | |
| 2025 | 53/159 (33.3%; 26.07–41.23) | 36.4 (36.16) | |
| Autonomous region | Andalucía | 0/10 (0%; 0–30.85) | 0 |
| Aragón | 45/187 (24.1%; 18.13–30.84) | 17.2 (24.07) | |
| Cantabria | 71/125 (56.8%; 47.64–65.63) | 29.8 (34.07) | |
| Castilla y León | 220/520 (42.31%; 38.02–46.68) | 39.5 (43.59) | |
| Castilla-La Mancha | 4/314 (1.27%; 0.35–3.23) | 11.5 (18.43) | |
| Cataluña | 37/93 (39.8%; 29.78–50.46) | 16.7 (17.07) | |
| Comunidad de Madrid | 0/1 (0%; 0–97.50) | 0 | |
| Comunidad Foral Navarra | 9/14 (64.3%; 35.14–87.24) | 20.1 (24.76) | |
| Comunidad Valenciana | 0/15 (0%; 0–21.80) | 0 | |
| Extremadura | 1/2 (50%; 1.26–98.74) | 16.0 (0) | |
| Galicia | 148/195 (75.9%; 69.27–81.72) | 79.1 (122.72) | |
| La Rioja | 42/51 (82.4%; 69.13–91.60) | 32.2 (28.90) | |
| País Vasco | 52/63 (82.5%; 70.90–90.95) | 44.2 (66.74) | |
| Principado de Asturias | 9/10 (90%; 55.50–99.75) | 36.0 (33.70) | |
| Bio-region | Atlantic | 260/368 (70.7%; 65.71–75.26) | 59.2 (99.49) |
| Northern Plateau | 331/734 (45.1%; 41.45–48.78) | 33.4 (41.18) | |
| South Central | 1/31 (3.2%; 0.08–16.70) | 16.0 (0) | |
| Interior Mountains | 29/383 (7.6%; 5.13–10.70) | 46.9 (46.56) | |
| South and East coast | 17/84 (20.2%; 12.25–30.41) | 17.3 (20.73) | |
| Land cover | Agriculture | 158/538 (29.4%; 25.55–33.42) | 33.3 (41.10) |
| Broadleaf | 128/355 (36.1%; 31.06–41.29) | 44.3 (55.28) | |
| Coniferous | 68/153 (44.4%; 36.42–52.69) | 42.7 (47.82) | |
| Mix forested areas | 147/214 (68.7%; 62.02–74.84) | 53.4 (92.70) | |
| Shrubs | 137/340 (40.3%; 35.04–45.72) | 47.0 (94.24) | |
| Roe deer abundance | 0–0.3 | 1/20 (5%; 0.13–24.87) | 5.0 (0) |
| 0.3–0.7 | 77/235 (32.8%; 26.80–39.17) | 43.7 (111.10) | |
| 0.7–1.4 | 213/684 (31.1%; 27.68–34.76) | 33.9 (40.82) | |
| 1.4–2.6 | 186/407 (45.7%; 40.78–50.68) | 55.7 (90.18) | |
| 2.6–9.8 | 161/254 (63.4%; 57.14–69.32) | 44.5 (53.51) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Fuentes, E.; Markina, F.A. Distribución actual del corzo (Capreolus capreolus) en España. In Proceedings of the XV Reunión de Ungulados Silvestres Ibéricos, Jaén, Spain, 23–24 November 2024. [Google Scholar]
- Colwell, D.D.; Hall, M.J.R.; Scholl, P.J. The Oestrid Flies: Biology, Host Parasite Relationships, Impact and Management; CABI Publishing: Wallingford, UK, 2006; p. 359. [Google Scholar]
- Zumpt, F. Myiasis in Man and Animals in the Old World; Butterworth & Co., Ltd.: London, UK, 1965; pp. 205–219. [Google Scholar]
- Pajares Bernaldo de Quirós, G. Estudio Sobre la Infestación Por Larvas de Cephenemyia stimulator (Diptera: Oestridae) en Corzos (Capreolus capreolus) del Norte de España. Ph.D. Thesis, Universidad de Santiago de Compostela, Lugo, Spain, 2016. [Google Scholar]
- Angulo-Valadez, C.E.; Scholl, P.J.; Cepeda-Palacios, R.; Jacquiet, P.; Dorchies, P. Nasal bots—A fascinating world! Vet. Parasitol. 2010, 174, 19–25. [Google Scholar] [CrossRef]
- Cacard, B. Mortality in Roe Deer (Capreolus capreolus) in France. Ph.D. Thesis, Ecole Nationale Vétérinaire d’Alfort, Paris, France, 2004. [Google Scholar]
- McMahon, D.C. Bot Fly Larvae (Cephenemyia spp., Oestridae) in Mule Deer (Odocoileus hemionus) from Utah. J. Wildl. Dis. 1989, 25, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, L.E.; López-Beceiro, A.M. Cefenemiosis en el Corzo; Colección de manuales prácticos; Mutuasport: Madrid, Spain, 2023; pp. 101–102. [Google Scholar]
- Ahaduzzaman, M. The global and regional prevalence of oestrosis in sheep and goats: A systematic review of articles and meta-analysis. Parasit. Vectors. 2019, 12, 346. [Google Scholar] [CrossRef]
- Capelle, K.J. The occurrence of Oestrus ovis L. (Diptera: Oestridae) in the bighorn sheep from Wyoming and Montana. J. Parasitol. 1966, 52, 618–621. [Google Scholar] [CrossRef]
- Sánchez, A.; Caparrós, N.; Ostrowski, S.; Sarasa, M.; Pérez, J.M. Oestrosis in Asiatic Ibex (Capra sibirica): A case report and molecular characterization of larvae. Vet. Parasitol. 2017, 236, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calabuig, N.; Remesar, S.; Varas, G.; García-Dios, D.; Saldaña, A.; Díaz, P.; Díez-Baños, P.; Morrondo, P.; Panadero, R. First report of Oestrus ovis infesting roe deer (Capreolus capreolus) in an area with high sympatry between wild and domestic ruminants. In Proceedings of the Annual Meeting of the European Veterinary Parasitology College, Paris, France, 29–30 June 2023. [Google Scholar]
- Zanzani, S.; Cozzi, L.; Olivieri, E.; Gazzonis, A.; Manfredi, M.T. Oestrus ovis L. (Diptera: Oestridae) induced nasal myiasis in a dog from Northern Italy. Case Rep. Vet. Med. 2016, 1, 5205416. [Google Scholar]
- Sante, L.; Hernández-Porto, M.; Tinguaro, V.; Lecuona, M. Oftalmomiasis y miasis nasal por Oestrus ovis en paciente residente en las Islas Canarias con características epidemiológicas poco frecuentes. Enferm. Infecc. Microbiol. Clin. 2017, 35, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Tabuenca-del Barrio, L.; Mozo-Cuadrado, M.; Zubicoa-Eneriz, A.; Plaza-Ramos, P. Ocular external myiasis. A series of cases due to larvae Oestrus ovis in Navarra, Spain. Arch. Soc. Esp. Oftalmol. 2018, 93, 567–570. [Google Scholar] [CrossRef]
- Dudziński, W. Studies on Cephenemyia stimulator (Clark, 1815) (Diptera, Oestridae), the parasite of the European Roe Deer, Capreolus capreolus (L.). I. Biology. Acta Parasitol. Pol. 1970, 18, 555–572. [Google Scholar]
- Barth, D.; Kudlich, H.; Schaich, K. Ocurrence and significance os nasal bot infestation in roe bucks. In Wildlife Diseases; Springer: Berlin/Heidelberg, Germany, 1976; pp. 609–613. [Google Scholar]
- Sugár, L. The occurrence of nasal throat bot flies (Oestridae) in wild ruminants in Hungary. Parasitol. Hung. 1974, 7, 181–189. [Google Scholar]
- Notario, A.; Castresana, L. Contribution to the knowledge of Cephenemyia stimulator Clark, 1815 (Diptera, Oestridae) in Spain. Folia Venatoria 2001, 30–31, 325–326. [Google Scholar]
- Pajares, G. Apuntes de Biología. Primera cita en España de Cephenemyia stimulator en corzos. Bol. ACE 2009, 11, 36. [Google Scholar]
- Fidalgo, L.E.; López-Beceiro, A.; Pérez-Jiménez, J.M.; Martínez-Carrasco, C. Bases Epidemiológicas para el Control de Cephenemyia stimulator en Corzos en España; Universidade de Santiago de Compostela & Fundación FEDENCA: Madrid, Spain, 2013. [Google Scholar]
- Arias, M.S.; Pajares, G.; Paz-Silva, A.; Díez-Baños, N.; Suárez, J.L.; Díez-Baños, P.; Sánchez-Andrade, R.; Morrondo, P. Antigen characterization from second instars of oestrid bot flies for the detection of anti-Cephenemyia stimulator antibodies by ELISA in roe deer (Capreolus capreolus). Med. Vet. Entomol. 2014, 28, 83–89. [Google Scholar] [CrossRef]
- Martínez-Calabuig, N.; Panadero, R.; Varas, G.; Remesar, R.; López, C.M.; Saldaña, A.; Díaz, P.; Díez-Baños, P.; Morrondo, P.; García-Dios, D. Prevalence of nasopharyngeal myiasis in roe deer (Capreolus capreolus) from an area with high sympatry between wild and domestic ungulates in Central Spain. Eur. J. Wildl. Res. 2024, 70, 60. [Google Scholar] [CrossRef]
- Martínez-Calabuig, N.; Vieira-Pinto, M.; Saldaña, A.; Panadero, R.; Aranha, J. Prevalence and Spatial Distribution of Cephenemyia stimulator in Roe Deer (Capreolus capreolus) from the North of Spain and Portugal. Insects 2025, 16, 274. [Google Scholar] [CrossRef]
- Markina, F.A. El corzo en Álava: Pasado, presente y futuro. Bol. ACE 2017, 15, 48. [Google Scholar]
- Nores, C. El corzo ibérico, entre el pasado y el futuro. Bol. ACE 2017, 15, 100–101. [Google Scholar]
- Extensión Superficial de las Comunidades Autónomas y Provincias, por Zonas Altimétricas. Fondo Documental del Instituto Nacional de Estadística. Anuario 1994. Available online: https://www.ine.es/inebaseweb/pdfDispacher.do?td=154090&L=0 (accessed on 4 July 2025).
- Centenera, R. Capturas y Abundancia Del Corzo en España; Asociación del Corzo Espanol: Madrid, Spain, 2023; Unpublished manuscript. [Google Scholar]
- Høye, T.T. Age determination in roe deer: A new approach to tooth wear evaluated on known age in individuals. Acta Theriol. 2006, 51, 205–214. [Google Scholar] [CrossRef]
- Spanish Wildlife Disease Surveillance Scheme (Internal Report to the Spanish Ministry of Agriculture, MARM and Spatial Aggregation of Wildlife. 2008. Available online: https://www.mapa.gob.es/dam/mapa/contenido/ganaderia/temas/sanidad-animal-e-higiene-ganadera/sanidad-animal/enfermedades/fauna-silvestre/pvfs2025final.pdf (accessed on 4 July 2025).
- Muñoz, P.M.; Boadella, M.; Arnal, M.; de Miguel, M.J.; Revilla, M.; Martínez, D.; Vicente, J.; Acevedo, P.; Oleaga, Á.; Ruiz-Fons, F.; et al. Spatial distribution and risk factors of Brucellosis in Iberian wild ungulates. BMC Infect. Dis. 2010, 10, 46. [Google Scholar] [CrossRef]
- CORINE Land Cover 2018. Available online: https://land.copernicus.eu/en/products/corine-land-cover/clc2018 (accessed on 4 July 2025).
- Fernández-López, J.; Blanco-Aguiar, J.A.; Vicente, J.; Acevedo, P. Can we model distribution of population abundance from wildlife–vehicles collision data? Ecography 2022, 2022, e06113. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 13 June 2025).
- Király, I.; Egri, B. Epidemiological characteristics of Cephenemyia stimulator (Clark, 1815) larval infestation in european roe deer (Capreolus capreolus) in Hungary. Acta Zool. Acad. Sci. Hung. 2007, 53, 271–279. [Google Scholar]
- Pinnyey, S.; Majzinger, I.; Barta, T.; Huber, J. Examination of the Nasal Botfly (Cephenemyia stimulator, Clark, 1815) in the Roe Deer (Capreolus capreolus, Linnaeus 1758), in Hungary. Sci. Pap. Anim. Sci. Biotechnol. 2017, 50, 143–146. [Google Scholar]
- Kornaś, S.; Kowal, J.; Wajdzik, M.; Nosal, P.; Wojtaszek, M.; Basiaga, M. Cephenemyia stimulator (Diptera) infection in roe deer (Capreolus capreolus) from Kraków area, southern Poland. Ann. Parasitol. 2016, 62, 115–118. [Google Scholar]
- Flis, M.; Rataj, B.; Grela, E.R. Occurrence of Cephenemyia stimulator Larvae in Male Roe Deer (Capreolus capreolus L.) in The Lublin Upland, Poland, and Their Impact on Particular Animal Health Indicators. J. Vet. Res. 2021, 65, 287–292. [Google Scholar] [CrossRef]
- Salaba, O.; Vadlejch, J.; Petrtyl, M.; Valek, P.; Kudrnacova, M.; Jankovska, I.; Bartak, M.; Sulakova, H.; Langrova, I. Cephenemyia stimulator and Hypoderma diana infection of roe deer in the Czech Republic over an 8-year period. Parasitol. Res. 2013, 112, 1661–1666. [Google Scholar] [CrossRef]
- Kusak, J.S.; Spicic, V.; Slijepcevic, S.; Bosnic, R.R.; Janje, S.; Duvnjak, M.; Sindicic, D.; Majnaric, Z.; Cvetnic, D.; Huber, D. Health Status of red deer and roe deer in Gorski Kotar, Croatia. Veterinarski Archiv. 2012, 82, 59–73. [Google Scholar]
- Hoekman, E.D. Dutch Roe Deer (Capreolus capreolus), Review of Cases Presented at the Dutch Wildlife Health Centre. Ph.D. Thesis, Utrecht Veterinary Faculty, Utrecht, The Netherlands, 2013. [Google Scholar]
- Curlík, J.; Letková, V.; Ciberej, J.; Lazar, P.; Goldová, M.; Koˇcišová, A.; Košuthová, L.; Trávniˇcek, M.; Bhide, M.; Lazar, G.; et al. The occurrence of the genera Hypoderma, Cephenemyia and Pharyngomyia in deer in the Slovak Republic. Folia Vet. 2004, 48, 92–94. [Google Scholar]
- Jogisalu, I. Roe Deer Nose Botfly Larvae and Helminths Impact on the European Roe Deer (Capreolus capreolus). Ph.D. Thesis, Tartu University, Tartu, Republic of Estonia, 2010. [Google Scholar]
- Pajares, G.; Arias, M.S.; Pérez-Creo, A.; Prieto, A.; Callejo, A.; Díez-Baños, P.; Morrondo, P. Epidemiología de la cefenemiosis en corzos del noroeste de España. Bol. ACE 2017, 15, 129–134. [Google Scholar]
- Morrondo, M.P.; Pérez-Creo, A.; Prieto, A.; Cabanelas, E.; Díaz-Cao, J.M.; Arias, M.S.; Panadero, R. Prevalence and distribution of infectious and parasitic agents in roe deer from Spain and their possible role as reservoirs. Ital. J. Anim. Sci. 2016, 16, 266–274. [Google Scholar] [CrossRef]
- Arias, M.S.; Pajares, G.; Díez-Baños, N.; Pérez-Creo, A.; Prieto, A.; Díez-Baños, P.; Morrondo, P. Distribución de la infestación por Cephenemyia stimulator en corzos en España mediante inmunodiagnóstico. Bol. ACE 2017, 15, 141–145. [Google Scholar]
- Morellet, N.; Van Moorter, B.; Cargnelutti, B.; Angibault, J.M.; Lourtet, B.; Merlet, J.; Ladet, S.; Hewison, A.J.M. Landscape composition influences roe deer habitat selection at both home range and landscape scales. Landscape Ecol. 2011, 26, 999–1010. [Google Scholar] [CrossRef]
- El Lobo Ibérico (Canis lupus signatus) en España: Poblaciones y Efectos en la Ganadería. Available online: https://fundacionartemisan.com/wp-content/uploads/2023/09/informe-situacion-lobo-espana.pdf (accessed on 23 July 2025).
- Alcaide, M.; Reina, D.; Sánchez-López, J.; Frontera, E.; Navarrete, I. Seroprevalence of Oestrus ovis (Diptera, Oestridae) infestation and associated risk factors in ovine livestock from southwestern Spain. J. Med. Entomol. 2005, 42, 327. [Google Scholar] [CrossRef]
- Maublanc, M.L.; Bideau, E.; Picot, D.; Rames, J.L.; Dubois, M.; Ferté, H.; Gerard, J.F. Demographic crash associated with high parasite load in an experimental roe deer (Capreolus capreolus) population. Eur. J. Wildl. Res. 2009, 55, 621–625. [Google Scholar] [CrossRef]
- Arias, M.S.; Pajares, G.; Díez-Baños, N.; Pérez-Creo, A.; Prieto, A.; Díez-Baños, P.; Morrondo, P. Cephenemyiosis, an emergent myiasis in roe deer (Capreolus capreolus) from northwestern Spain. Parasitol. Res. 2016, 115, 4605–4610. [Google Scholar] [CrossRef] [PubMed]
- Morrondo, P.; Pajares, G.; Arias, M.S.; Martínez-Calabuig, N.; Remesar, S.; García-Dios, D.; Díaz, P.; López, C.M.; Panadero, R.; Díez-Baños, P. An update on Cephenemyiosis in the European Roe deer: Emergent Myiasis in Spain. Animals 2021, 11, 3382. [Google Scholar] [CrossRef]
- Panadero, R.; Dacal, V.; López, C.; Vázquez, L.; Cienfuegos, S.; Díaz, P.; Morrondo, P.; Díez-Baños, P. Immunomodulatory effect of Hypoderma lineatum antigens: In vitro effect on bovine lymphocyte proliferation and cytokine production. Parasite Immunol. 2009, 31, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, R.; Habela, M.A. First report of Cephenemyia stimulator (Diptera, Oestridae) parasitizing Roe deer (Capreolus capreolus) in Extremadura (Spain). Galemys Span. J. Mammal. 2013, 25, 29–34. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.; Díez-Baños, N.; Hidalgo, M.R.; González-Hidalgo, S.; Carreno, R. Nasopharyngeal Botflies Oestrus ovis and Cephenemyia stimulator in Roe Deer (Capreolus capreolus) from Northern Spain. J. Wildl. Dis. 2024, 60, 956–959. [Google Scholar] [CrossRef]




| Factor | Estimate | Z Value | Probability | OR | CI 95% |
|---|---|---|---|---|---|
| Month February 1 | - | - | - | - | - |
| Month January | 0.602 | 2.283 | 0.022 | 1.83 | 1.09–3.07 |
| Month March | 0.308 | 0.763 | 0.446 | 1.36 | 0.62–3.02 |
| Month April | 0.660 | 2.462 | 0.014 | 1.94 | 1.15–3.28 |
| Month May | 0.111 | 0.364 | 0.716 | 1.12 | 0.61–2.03 |
| Month June | 1.279 | 3.290 | 0.001 | 3.59 | 1.69–7.83 |
| Month July | 1.589 | 3.200 | 0.001 | 4.90 | 1.94–13.87 |
| Month August | 0.902 | 1.942 | 0.052 | 2.46 | 0.99–6.20 |
| Month September | 0.988 | 2.666 | 0.008 | 2.69 | 1.31–5.61 |
| Month October | 0.725 | 2.036 | 0.042 | 2.06 | 1.03–4.19 |
| Month November | 0.509 | 1.368 | 0.171 | 1.66 | 0.80–3.44 |
| Month December | 0.367 | 0.998 | 0.318 | 1.44 | 0.70–2.97 |
| Year 2023 1 | - | - | - | - | - |
| Year 2018 | −2.021 | −2.513 | 0.012 | 0.13 | 0.03–0.70 |
| Year 2019 | −1.572 | −2.629 | 0.009 | 0.21 | 0.06–0.68 |
| Year 2020 | −1.089 | −1.949 | 0.051 | 0.34 | 0.11–1.02 |
| Year 2021 | 0.188 | 0.550 | 0.582 | 1.21 | 0.62–2.38 |
| Year 2022 | 0.763 | 3.201 | 0.001 | 2.14 | 1.35–3.43 |
| Year 2024 | 0.449 | 2.221 | 0.026 | 1.57 | 1.06–2.33 |
| Year 2025 | 0.836 | 2.875 | 0.004 | 2.31 | 1.31–4.09 |
| AR Castilla-La Mancha 1 | - | - | - | - | - |
| AR Andalucía | −14.230 | −0.012 | 0.990 | 6.61 × | −Inf – Inf |
| AR Aragón | 1.400 | 1.911 | 0.056 | 4.05 | 1.02–18.69 |
| AR Cantabria | 3.512 | 3.420 | <0.001 | 33.51 | 4.46–262.10 |
| AR Castilla y León | 3.156 | 4.999 | <0.001 | 23.47 | 7.37–91.96 |
| AR Cataluña | 2.683 | 3.481 | <0.001 | 14.62 | 3.41–72.41 |
| AR Comunidad de Madrid | −13.766 | −0.003 | 0.997 | 1.05 × | - – Inf |
| AR Comunidad Foral de Navarra | 3.885 | 4.418 | <0.001 | 48.66 | 9.16–298.51 |
| AR Comunidad Valenciana | −13.243 | −0.015 | 0.988 | 1.77 × | −Inf – Inf |
| AR Extremadura | 20.274 | 0.017 | 0.987 | 6.38 × | −Inf – - |
| AR Galicia | 5.084 | 4.690 | <0.001 | 161.35 | 19.41–1416.05 |
| AR La Rioja | 5.122 | 6.783 | <0.001 | 167.66 | 40.95–817.08 |
| AR País Vasco | 4.926 | 5.994 | <0.001 | 137.80 | 29.79–772.16 |
| AR Principado de Asturias | 4.246 | 2.843 | 0.004 | 69.76 | 4.55–2174.45 |
| Bio-region Interior Mountain + Age adult 1 | - | - | - | - | - |
| Bio-region Atlantic + Age fawn | −19.196 | −0.029 | 0.977 | 4.60 × | −Inf – Inf |
| Bio-region Atlantic + Age young | 1.277 | 1.355 | 0.175 | 3.59 | 0.57–23.77 |
| Bio-region Atlantic + Age adult | 1.504 | 1.600 | 0.110 | 4.50 | 0.72–29.71 |
| Bio-region Atlantic + Age old | 2.172 | 2.055 | 0.040 | 8.77 | 1.14–74.37 |
| Bio-region Northern Plateau + Age fawn | −16.916 | −0.004 | 0.997 | 4.50 | - – Inf |
| Bio-region Northern Plateau + Age Young | 1.378 | 2.902 | 0.003 | 3.97 | 1.59–10.32 |
| Bio-region Northern Plateau + Age adult | 1.591 | 3.449 | <0.001 | 4.91 | 2.02–12.45 |
| Bio-region Northern Plateau + Age old | 1.670 | 3.386 | <0.001 | 5.31 | 2.05–1.43 |
| Bio-region South Central + Age young | −15.881 | −0.013 | 0.989 | 1.27 × | - – Inf |
| Bio-region South Central + Age adult | −15.666 | −0.020 | 0.984 | 1.57 × | −Inf – Inf |
| Bio-region South Central + Age old | −14.007 | −0.004 | 0.997 | 8.26 × | - – Inf |
| Bio-region Interior Mountains + Age young | −0.471 | −0.762 | 0.446 | 2.30 | 0.18–2.04 |
| Bio-region Interior Mountains + Age old | 0.787 | 1.378 | 0.168 | 2.20 | 0.71–6.84 |
| Bio-region South and East Coast + Age young | −0.158 | −0.148 | 0.883 | 3.14 | 0.08–6.15 |
| Bio-region South and East Coast + Age adult | 0.993 | 1.347 | 0.178 | 2.70 | 0.64–11.53 |
| Bio-region South and East Coast + Age old | −13.908 | −0.008 | 0.993 | 9.11 × | - – Inf |
| Land cover Agriculture 1 | - | - | - | - | - |
| Land cover Broadleaf | 0.848 | 3.101 | 0.002 | 2.34 | 1.37–4.01 |
| Land cover Coniferous | 1.236 | 4.261 | <0.001 | 3.44 | 1.96–6.11 |
| Land cover Mix forested areas | 1.400 | 4.368 | <0.001 | 4.06 | 2.18–7.65 |
| Land cover Shrubs | 1.307 | 5.291 | <0.001 | 3.69 | 2.29–6.04 |
| Abundance (0–0.3) 1 | - | - | - | - | - |
| Abundance (0.3–0.7) | 2.339 | 1.759 | 0.079 | 10.37 | 1.00–262.51 |
| Abundance (0.7–1.4) | 2.932 | 2.201 | 0.028 | 18.76 | 1.81–476.57 |
| Abundance (1.4–2.6) | 3.447 | 2.561 | 0.010 | 31.41 | 2.94–811.72 |
| Abundance (2.6–9.8) | 3.872 | 2.879 | 0.004 | 48.04 | 4.51–1239.53 |
| Factor | F Value | Df | p (>F) |
|---|---|---|---|
| Sex | 11.446 | 1 | <0.001 |
| Age | 42.504 | 2 | <0.001 |
| Year | 4.706 | 7 | <0.001 |
| AR | 7.674 | 10 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Calabuig, N.; López, C.M.; Saldaña, A.; Aranha, J.; Remesar, S.; Vieira-Pinto, M.; García-Dios, D.; Díaz, P.; Fernández-González, C.; Díez-Baños, P.; et al. Distribution of Nasal Myiases Affecting Roe Deer in Spain and Associated Risk Factors. Animals 2025, 15, 2396. https://doi.org/10.3390/ani15162396
Martínez-Calabuig N, López CM, Saldaña A, Aranha J, Remesar S, Vieira-Pinto M, García-Dios D, Díaz P, Fernández-González C, Díez-Baños P, et al. Distribution of Nasal Myiases Affecting Roe Deer in Spain and Associated Risk Factors. Animals. 2025; 15(16):2396. https://doi.org/10.3390/ani15162396
Chicago/Turabian StyleMartínez-Calabuig, Néstor, Ceferino M. López, Ana Saldaña, José Aranha, Susana Remesar, Madalena Vieira-Pinto, David García-Dios, Pablo Díaz, Carlota Fernández-González, Pablo Díez-Baños, and et al. 2025. "Distribution of Nasal Myiases Affecting Roe Deer in Spain and Associated Risk Factors" Animals 15, no. 16: 2396. https://doi.org/10.3390/ani15162396
APA StyleMartínez-Calabuig, N., López, C. M., Saldaña, A., Aranha, J., Remesar, S., Vieira-Pinto, M., García-Dios, D., Díaz, P., Fernández-González, C., Díez-Baños, P., Morrondo, P., & Panadero, R. (2025). Distribution of Nasal Myiases Affecting Roe Deer in Spain and Associated Risk Factors. Animals, 15(16), 2396. https://doi.org/10.3390/ani15162396









