Glutamate Supplementation Ameliorated Growth Impairment and Intestinal Injury in High-Soya-Meal-Fed Epinephelus coioides
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Feed and Rearing Management
2.2. Sample Collection
2.3. Nutrient and Amino Acid Analysis
2.4. Serum Component Determination
2.5. Assay for Digestive Enzyme Activity
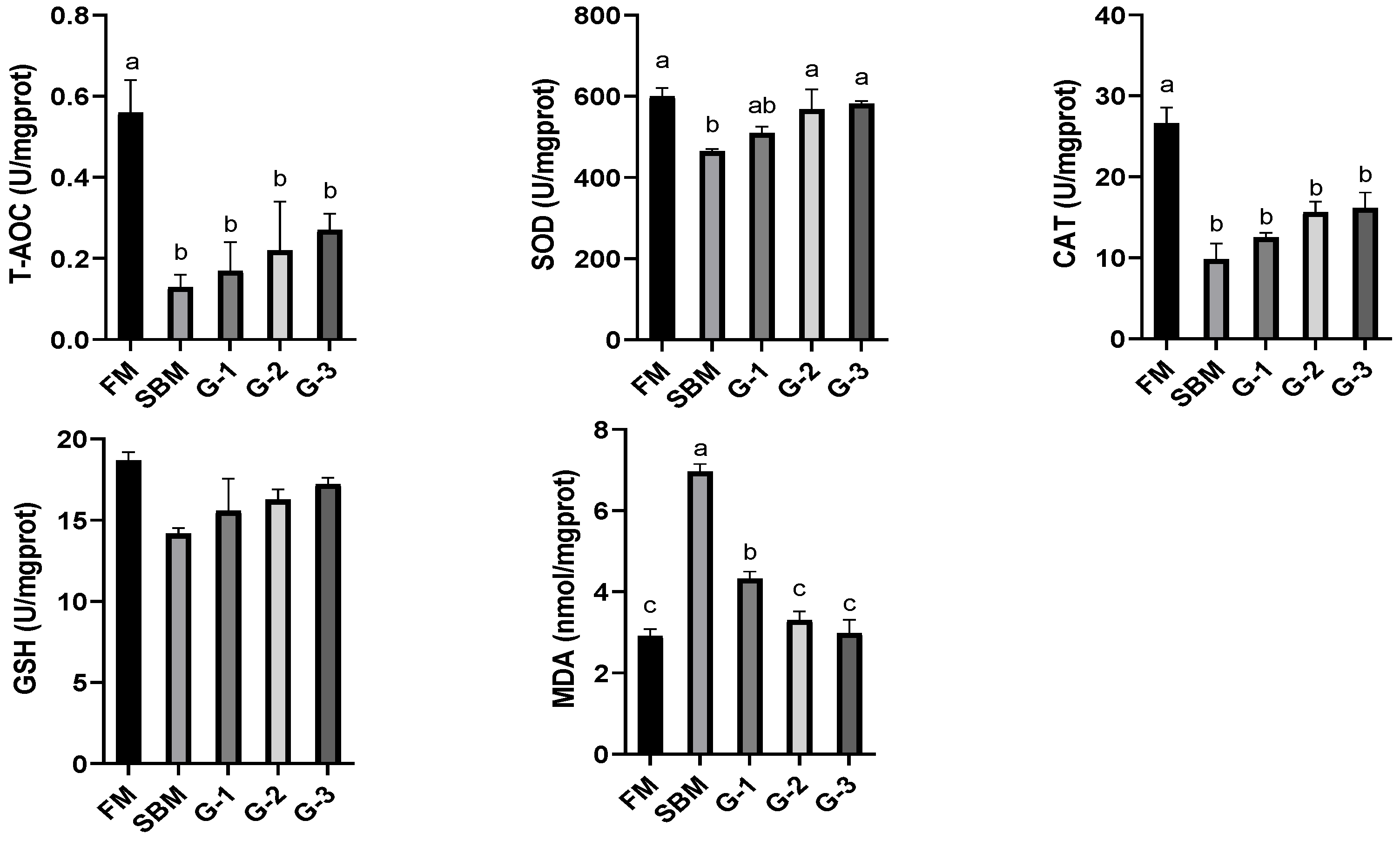
2.6. Assay for Antioxidant Capacity
2.7. Assay for Metabolic Enzyme Activity
2.8. Intestinal Histological Observation
2.9. RNA Extraction and mRNA Level Analysis
2.10. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Body and Muscle Nutrients
3.3. Serum Biochemical Components
3.4. Intestinal Antioxidant Capacity
3.5. Intestinal Activities of Digestive Enzymes
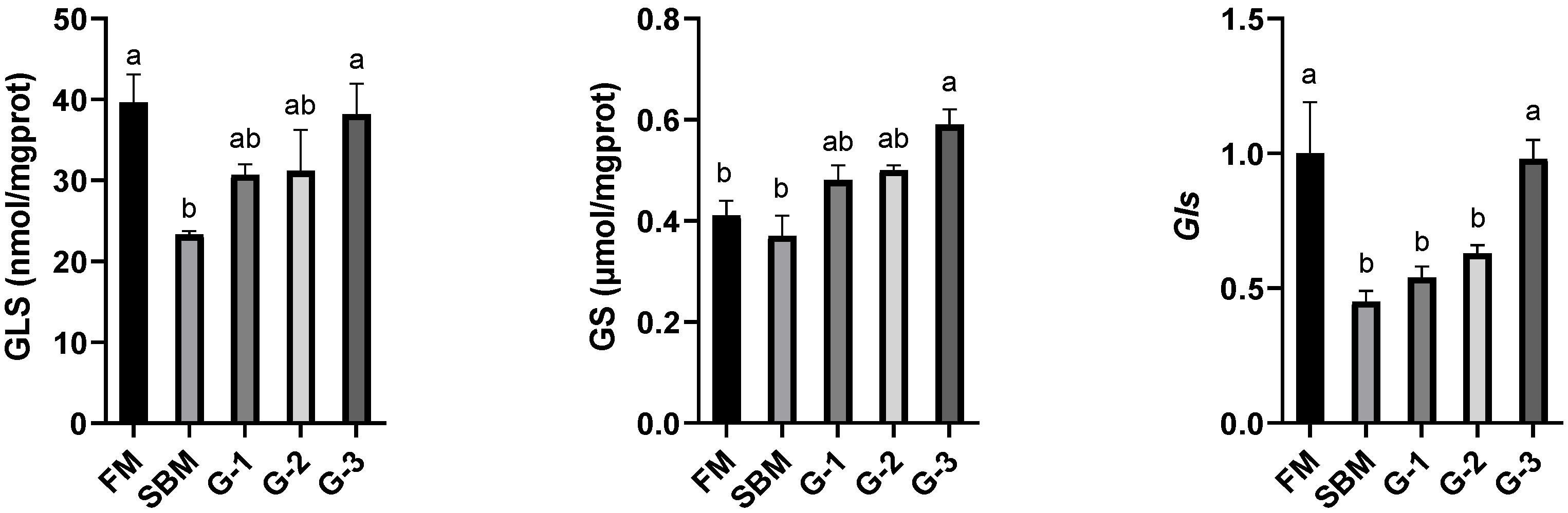
3.6. Intestinal Activities and Gene Expression of Amino Acid Metabolic Enzymes
3.7. Intestinal Histomorphology
3.8. Intestinal Inflammatory Cytokine Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardy, R.W. Utilization of Plant Proteins in Fish Diets: Effects of Global Demand and Supplies of Fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Han, D.; Shan, X.; Zhang, W.; Chen, Y.; Wang, Q.; Li, Z.; Zhang, G.; Xu, P.; Li, J.; Xie, S. A Revisit to Fishmeal Usage and Associated Consequences in Chinese Aquaculture. Rev. Aquac. 2016, 10, 493–507. [Google Scholar] [CrossRef]
- Rajabdeen, J.; Vanjiappan, R.; Rajamohamed, K.; Kondusamy, A.; Moturi, M.; Syama, D.J. Fishmeal Availability in the Scenarios of Climate Change: Inevitability of Fishmeal Replacement in Aquafeeds and Approaches for the Utilization of Plant Protein Sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Ye, J.; Liu, X.; Wang, Z.; Wang, K. Effect of Partial Fish Meal Replacement by Soya Meal on the Growth Performance and Biochemical Indices of Juvenile Japanese Flounder Paralichthys olivaceus. Aquac. Int. 2011, 19, 143–153. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Wang, X.; Ye, J. Growth Performance, Plasma Components, and Intestinal Barrier in Grouper (Epinephelus coioides) Are Altered by Dietary Fish Meal Replacement with Extruded Soya Meal. Aquac. Rep. 2021, 21, 100863. [Google Scholar] [CrossRef]
- Baeverfjord, G.T.; Krogdahl, A. Development and Regression of Soya Meal Induced Enteritis in Atlantic Salmon, Salmo salar L., Distal Intestine: A Comparison with the Intestines of Fasted Fish. J. Fish Dis. 2010, 19, 375–387. [Google Scholar] [CrossRef]
- Gu, M.; Pan, S.; Li, Q.; Qi, Z.; Deng, W.; Bai, N. Protective Effects of Glutamine against Soy Saponins-Induced Enteritis, Tight Junction Disruption, Oxidative Damage and Autophagy in the Intestine of Scophthalmus maximus L. Fish Shellfish Immunol. 2021, 114, 49–57. [Google Scholar] [CrossRef]
- Rimoldi, S.; Finzi, G.; Ceccotti, C.; Girardello, R.; Grimaldi, A.; Ascione, C.; Terova, G. Butyrate and Taurine Exert a Mitigating Effect on the Inflamed Distal Intestine of European Sea Bass Fed with a High Percentage of Soya Meal. Fish. Aquat. Sci. 2016, 19, 40. [Google Scholar] [CrossRef]
- Tan, P.; Wu, X.; Zhu, W.; Lou, B.; Chen, R.; Wang, L. Effect of Tributyrin Supplementation in High-soya Bean Meal Diet on Growth Performance, Body Composition, Intestine Morphology and Microbiota of Juvenile Yellow Drum (Nibea albiflora). Aquac. Res. 2020, 51, 2004–2019. [Google Scholar] [CrossRef]
- Volatiana, J.A.; Wang, L.; Neveen, G.; Tong, S.L.; Zhang, G.W.; Shao, Q.J. Tributyrin-Supplemented High-Soya Bean Meal Diets of Juvenile Black Sea Bream, Acanthopagrus schlegelii: Study on Growth Performance and Intestinal Morphology and Structure. Aquac. Res. 2020, 51, 135. [Google Scholar] [CrossRef]
- Wu, N.; Xu, X.; Wang, B.; Li, X.-M.; Cheng, Y.-Y.; Li, M.; Xia, X.-Q.; Zhang, Y.-A. Anti-Foodborne Enteritis Effect of Galantamine Potentially via Acetylcholine Anti-Inflammatory Pathway in Fish. Fish Shellfish Immunol. 2020, 97, 204–215. [Google Scholar] [CrossRef]
- Xie, J.; Li, M.; Ye, W.; Shan, J.; Zhao, X.; Duan, Y.; Liu, Y.; Unger, B.H.; Cheng, Y.; Zhang, W.; et al. Sinomenine Hydrochloride Ameliorates Fish Foodborne Enteritis via α7nAchR-Mediated Anti-Inflammatory Effect Whilst Altering Microbiota Composition. Front. Immunol. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, Z.; Zheng, J.; Dai, J.; Ou, W.; Xu, W.; Ai, Q.; Zhang, W.; Niu, J.; Mai, K.; et al. Citric Acid Mitigates Soya Meal Induced Inflammatory Response and Tight Junction Disruption by Altering TLR Signal Transduction in the Intestine of Turbot, Scophthalmus maximus L. Fish Shellfish Immunol. 2019, 92, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Qin, Y.; Song, T.; Wang, K.; Ye, J. Dietary Sodium Butyrate Administration Alleviates High Soya Meal-Induced Growth Retardation and Enteritis of Orange-Spotted Groupers (Epinephelus coioides). Front. Mar. Sci. 2022, 9, 1–17. [Google Scholar] [CrossRef]
- Song, T.; Qin, Y.; Ke, L.; Wang, X.; Wang, K.; Sun, Y.; Ye, J. Dietary Lactoferrin Supplementation Improves Growth Performance and Intestinal Health of Juvenile Orange-Spotted Groupers (Epinephelus coioides). Metabolites 2022, 12, 915. [Google Scholar] [CrossRef]
- Reeds, P. Intestinal Glutamate Metabolism. J. Nutr. 2000, 130, 978S. [Google Scholar] [CrossRef]
- Yoshida, C.; Maekawa, M.; Bannai, M.; Yamamoto, T. Glutamate Promotes Nucleotide Synthesis in the Gut and Improves Availability of Soya Meal Feed in Rainbow Trout. Springerplus 2016, 5, 1021. [Google Scholar] [CrossRef]
- Fang, L.Y.; Xu, Y.S.; Liu, T.J.; Li, H.Q.; Liu, C.; Li, H.; Zhai, X.L.; Xur, Y.; Shen, Z.W.; Chen, Y.J.; et al. Dietary Glutamic Acid, Glutamine and Monosodium Glutamate on Feeding, Growth, Gastrointestinal and Liver Function of Mandarin Fish (Siniperca chuatsi). Acta Hydrobiol. Sin 2024, 48, 592–599. [Google Scholar] [CrossRef]
- Caballero-Solares, A.; Viegas, I.; Salgado, M.C.; Siles, A.M.; Sáez, A.; Metón, I.; Baanante, I.V.; Fernández, F. Diets Supplemented with Glutamate or Glutamine Improve Protein Retention and Modulate Gene Expression of Key Enzymes of Hepatic Metabolism in Gilthead Seabream (Sparus aurata) Juveniles. Aquaculture 2015, 444, 79–87. [Google Scholar] [CrossRef]
- Dong, B.; Wu, L.; Wang, Y.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; Xie, S.; Liu, Z.; Jin, J. Glutamate Improves Flesh Quality and Muscle Growth of Triploid Crucian Carp. Aquac. Rep. 2023, 33, 101832. [Google Scholar] [CrossRef]
- Matias, A.C.; Viegas, A.R.; Couto, A.; Lourenço-Marques, C.; Aragão, C.; Castanho, S.; Gamboa, M.; Candeias-Mendes, A.; Soares, F.; Modesto, T.; et al. Effect of Dietary L-Glutamine Supplementation on the Intestinal Physiology and Growth during Solea senegalensis Larval Development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2024, 272, 110961. [Google Scholar] [CrossRef]
- Qiyou, X.; Qing, Z.; Hong, X.; Changan, W.; Dajiang, S. Dietary Glutamine Supplementation Improves Growth Performance and Intestinal Digestion/Absorption Ability in Young Hybrid Sturgeon (Acipenser schrenckii ♀× Huso dauricus ♂). J. Appl. Ichthyol. 2011, 27, 721–726. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, J.-X.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Zhou, X.-Q. Effect of Dietary Glutamine on Growth Performance, Non-Specific Immunity, Expression of Cytokine Genes, Phosphorylation of Target of Rapamycin (TOR), and Anti-Oxidative System in Spleen and Head Kidney of Jian Carp (Cyprinus carpio Var. Jian). Fish Physiol. Biochem. 2015, 41, 635–649. [Google Scholar] [CrossRef]
- Ding, Z.; Li, W.; Huang, J.; Yi, B.; Xu, Y. Dietary Alanyl-Glutamine and Vitamin E Supplements Could Considerably Promote the Expression of GPx and PPARα Genes, Antioxidation, Feed Utilization, Growth, and Improve Composition of Juvenile Cobia. Aquaculture 2017, 470, 95–102. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, C.; Zhao, Z.; Luo, L.; Du, X.; Xu, Q. Effect of N-Carbamoylglutamate Supplementation on the Growth Performance, Antioxidant Status and Immune Response of Mirror Carp (Cyprinus carpio) Fed an Arginine-Deficient Diet. Fish Shellfish Immunol. 2019, 84, 280–289. [Google Scholar] [CrossRef]
- Carvalho, P.L.P.F.; Xavier, W.D.S.; Guimarães, M.G.; Rodrigues, E.J.D.; Furuya, W.M.; Yamamoto, F.Y.; Pezzato, L.E.; Gatlin, D.M.; Barros, M.M. Dietary Glutamine Improves Growth and Intestinal Morphology of Juvenile GIFT Tilapia (Oreochromis niloticus) but Has Limited Effects on Innate Immunity and Antioxidant Capacity. Aquaculture 2023, 563, 738976. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Rufino-Palomares, E.; Ordóñez-Grande, B.; Gallardo, M.A.; Oliva-Teles, A.; Peres, H. Dietary Glutamine Supplementation Effects on Amino Acid Metabolism, Intestinal Nutrient Absorption Capacity and Antioxidant Response of Gilthead Sea Bream (Sparus aurata) Juveniles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 191, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Belghit, I.; Philip, A.J.P.; Maas, R.M.; Lock, E.-J.; Eding, E.H.; Espe, M.; Schrama, J.W. Impact of Dietary Glutamate and Glycine on Growth and Nutrient Utilization in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2023, 568, 739311. [Google Scholar] [CrossRef]
- Burrin, D.G.; Stoll, B. Metabolic Fate and Function of Dietary Glutamate in the Gut. Am. J. Clin. Nutr. 2009, 90, 850S–856S. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yin, L.; Li, J.Y.; Li, Q.; Shi, D.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, Y. Glutamate Attenuates Lipopolysaccharide-Induced Oxidative Damage and mRNA Expression Changes of Tight Junction and Defensin Proteins, Inflammatory and Apoptosis Response Signaling Molecules in the Intestine of Fish. Fish Shellfish Immunol. 2017, 70, 473–484. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, B.; Yu, C.; Li, J.; Zhang, L.; Sun, H.; Gao, F.; Zhou, G. L-Glutamate Supplementation Improves Small Intestinal Architecture and Enhances the Expressions of Jejunal Mucosa Amino Acid Receptors and Transporters in Weaning Piglets. PLoS ONE 2014, 9, e111950. [Google Scholar] [CrossRef]
- Li, S.; Guo, Q.; Li, S.; Zheng, H.; Chi, S.; Xu, Z.; Wang, Q. Glutamine Protects against LPS-Induced Inflammation via Adjusted NODs Signaling and Enhanced Immunoglobulins Secretion in Rainbow Trout Leukocytes. Dev. Comp. Immunol. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and Metabolism of Glutamate and Glutamine in Fish. Amino Acids 2020, 52, 671–691. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Qi, M.; Li, J.; Tan, B. Glutamine, Glutamate, and Aspartate Differently Modulate Energy Homeostasis of Small Intestine under Normal or Low Energy Status in Piglets. Anim. Nutr. 2021, 8, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Hissen, K.L.; He, W.; Wu, G.; Criscitiello, M.F. Dietary L-Glutamate Modulates Intestinal Mucosal Immunity of Juvenile Hybrid Striped Bass (Morone saxatilis ♀ × Morone chrysops ♂). Front. Immunol. 2025, 16, 1575644. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Zou, J.; Chen, Z.; Zheng, F.; Xu, Z.; Lin, Y.-H.; Wang, Q. Dietary Glutamine Inclusion Regulates Immune and Antioxidant System, as Well as Programmed Cell Death in Fish to Protect against Flavobacterium Columnare Infection. Antioxidants 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H.; Ai, Q. Effects of Dietary Glutamine on Survival, Growth Performance, Activities of Digestive Enzyme, Antioxidant Status and Hypoxia Stress Resistance of Half-Smooth Tongue Sole (Cynoglossus semilaevis Günther) Post Larvae. Aquaculture 2015, 446, 48–56. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Duan, J.; Huang, P.; Yin, J. Low-Protein Diets Supplemented with Glutamic Acid or Aspartic Acid Ameliorates Intestinal Damage in Weaned Piglets Challenged with Hydrogen Peroxide. Anim. Nutr. 2021, 7, 356–364. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Viana, M.T.; Mata-Sotres, J.A.; Campos, A.; Pohlenz, C.; Lazo, J.P. Dietary Glutamine Enhances Growth Performance and Gut Integrity of Totoaba Macdonaldi Juveniles Fed Low Fishmeal Diets but Has Limited Synergetic Effects in Combination with a Prebiotic. Aquaculture 2023, 576, 739834. [Google Scholar] [CrossRef]
- He, Y.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Chi, S.; Tan, B. Glutamine Improves Growth and Intestinal Health in Juvenile Hybrid Groupers Fed High-Dose Glycinin. Fish Shellfish Immunol. 2023, 141, 109003. [Google Scholar] [CrossRef]
- Cheng, Z.; Gatlin, D.M.; Buentello, A. Dietary Supplementation of Arginine and/or Glutamine Influences Growth Performance, Immune Responses and Intestinal Morphology of Hybrid Striped Bass (Morone chrysops × Morone saxatilis). Aquaculture 2012, 362, 39–43. [Google Scholar] [CrossRef]
- Gu, M.; Bai, N.; Xu, B.; Xu, X.; Jia, Q.; Zhang, Z. Protective Effect of Glutamine and Arginine against Soya Meal-Induced Enteritis in the Juvenile Turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2017, 70, 95–105. [Google Scholar] [CrossRef]
- Yue, H.; Wu, J.; Fu, P.; Ruan, R.; Ye, H.; Hu, B.; Chen, X.; Li, C. Effect of Glutamine Supplementation against Soya Meal-Induced Growth Retardation, Hepatic Metabolomics and Transcriptome Alterations in Hybrid Sturgeon Acipenser baerii ♀ × A. schrenckii ♂. Aquac. Rep. 2022, 24, 101158. [Google Scholar] [CrossRef]
- Shapawi, R.; Abdullah, F.C.; Senoo, S.; Mustafa, S. Nutrition, Growth and Resilience of Tiger Grouper (Epinephelus Fuscoguttatus)×giant Grouper (Epinephelus lanceolatus) Hybrid—A Review. Rev. Aquac. 2019, 11, 1285–1295. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture. China Fishery Statistics Yearbook. 2024; China Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Wang, Y.R.; Wang, L.; Zhang, C.X.; Song, K. Effects of Substituting Fishmeal with Soya Meal on Growth Performance and Intestinal Morphology in Orange-Spotted Grouper (Epinephelus coioides). Aquac. Rep. 2017, 5, 52–57. [Google Scholar] [CrossRef]
- Qin, Y.; He, L.; Wang, Y.; Li, D.; Chen, W.; Ye, J. Growth Performance, Fatty Acid Composition, and Lipid Metabolism Are Altered in Groupers (Epinephelus coioides) by Dietary Fish Oil Replacement with Palm Oil. Anim. Nutr. 2022, 534, 736281. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Qian, X.; Feng, H.; Yi, K.; Ye, J. Growth and Metabolic Responses of Grouper Juveniles (Epinephelus coioides) Fed Diets Containing Varying Levels of Leucine. Aquaculture 2021, 534, 736281. [Google Scholar] [CrossRef]
- Hanaki, K.-I.; Ike, F.; Kajita, A.; Yasuno, W.; Yanagiba, M.; Goto, M.; Sakai, K.; Ami, Y.; Kyuwa, S. A Broadly Reactive One-Step SYBR Green I Real-Time RT-PCR Assay for Rapid Detection of Murine Norovirus. PLoS ONE 2014, 9, e98108. [Google Scholar] [CrossRef]
- Schmittgen, T.D. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wang, M.; Li, E.; Huang, Y.; Liu, W.; Wang, S.; Li, W.; Chen, L.; Wang, X. Dietary Supplementation with Glutamate Enhanced Antioxidant Capacity, Ammonia Detoxification and Ion Regulation Ability in Nile Tilapia (Oreochromis niloticus) Exposed to Acute Alkalinity Stress. Aquaculture 2025, 594, 741360. [Google Scholar] [CrossRef]
- Cheng, Z.; Buentello, A.; Gatlin, D.M. Effects of Dietary Arginine and Glutamine on Growth Performance, Immune Responses and Intestinal Structure of Red Drum, Sciaenops ocellatus. Aquaculture 2011, 319, 247–252. [Google Scholar] [CrossRef]
- Palomino Ramos, A.R.; Campelo, D.A.V.; Carneiro, C.L.D.S.; Zuanon, J.A.S.; Da Matta, S.L.P.; Furuya, W.M.; Salaro, A.L. Optimal Dietary L-Glutamine Level Improves Growth Performance and Intestinal Histomorphometry of Juvenile Giant Trahira (Hoplias lacerdae), a Neotropical Carnivorous Fish Species. Aquaculture 2022, 547, 737469. [Google Scholar] [CrossRef]
- Cai, Y.; He, L.; Cao, S.; Zeng, P.; Xu, L.; Luo, Y.; Tang, X.; Wang, Q.; Liu, Z.; He, Z.; et al. Insights into Dietary Different Co-Forms of Lysine and Glutamate on Growth Performance, Muscle Development, Antioxidation and Related Gene Expressions in Juvenile Grass Carp (Ctenopharyngodon idellus). Mar. Biotechnol. 2024, 26, 74–91. [Google Scholar] [CrossRef]
- Qu, F.; Liu, Z.; Hu, Y.; Zhao, Q.; Zhou, Y.; Liu, Z.; Zhong, L.; Lu, S.; Li, J. Effects of Dietary Glutamine Supplementation on Growth Performance, Antioxidant Status and Intestinal Function in Juvenile Grass Carp (Ctenopharyngodon idella). Aquac. Nutr. 2019, 25, 609–621. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Liang, X.; Lou, B.; Zhu, J. Effects of Glutamate on Growth Performance, Gut Digestion and Antioxidant Capacity in Juvenile Little Yellow Croaker. Fishes 2025, 10, 188. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, J.; Liu, H.; Zhang, H.; Shan, H.; Zong, J.; Cao, Q.; Jiang, J. Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass. Fishes 2025, 10, 151. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Zhou, X.-Q.; Zeng, X.-Y.; Feng, L.; Liu, Y.; Jiang, W.-D.; Li, S.-H.; Li, D.-B.; Wu, X.-Q.; et al. Effects of Dietary Glutamate Supplementation on Growth Performance, Digestive Enzyme Activities and Antioxidant Capacity in Intestine of Grass Carp (Ctenopharyngodon idella). Aquac. Nutr. 2015, 21, 935–941. [Google Scholar] [CrossRef]
- Yan, L.; Qiu-Zhou, X. Dietary Glutamine Supplementation Improves Structure and Function of Intestine of Juvenile Jian Carp (Cyprinus carpio Var. Jian). Aquaculture 2006, 256, 389–394. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.-R.; Li, Q.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, J.; Zhou, X.-Q.; Jiang, J. Effect of Dietary L-Glutamate Levels on Growth, Digestive and Absorptive Capability, and Intestinal Physical Barrier Function in Jian Carp (Cyprinus carpio Var. Jian). Anim. Nutr. 2020, 6, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Jiang, Q.Y.; Zhang, T.; Yin, Y.L.; Li, F.N.; Deng, J.P.; Wu, G.Y.; Kong, X.F. Dietary Supplementation with Arginine and Glutamic Acid Modifies Growth Performance, Carcass Traits, and Meat Quality in Growing-Finishing Pigs. J. Anim. Sci. 2017, 95, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Knabe, D.A.; Tekwe, C.D.; Dahanayaka, S.; Ficken, M.D.; Fielder, S.E.; Eide, S.J.; Lovering, S.L.; Wu, G. Dietary Supplementation with Monosodium Glutamate Is Safe and Improves Growth Performance in Postweaning Pigs. Amino Acids 2013, 44, 911–923. [Google Scholar] [CrossRef]
- Puntel, R.L.; Roos, D.H.; Grotto, D.; Garcia, S.C.; Nogueira, C.W.; Batista Teixeira Rocha, J. Antioxidant Properties of Krebs Cycle Intermediates against Malonate Pro-Oxidant Activity in Vitro: A Comparative Study Using the Colorimetric Method and HPLC Analysis to Determine Malondialdehyde in Rat Brain Homogenates. Life Sci. 2007, 81, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Mourente, G.; Bell, J.G.; Tocher, D.R. Does Dietary Tocopherol Level Affect Fatty Acid Metabolism in Fish? Fish Physiol. Biochem. 2007, 33, 269–280. [Google Scholar] [CrossRef]
- Wahid, S.T.; Lee, S.S.; Kim, I.H. The Impact of Glycine and Glutamate, as Components of Glutathione Precursors, on the Productivity, Digestive Performance and Blood Profile of Weaning Pigs. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M. Urea and Glutamine Synthesis: Environmental Influences on Nitrogen Excretion. Fish Physiol. 2001, 20, 239–277. [Google Scholar] [CrossRef]
- Young, V.R.; Ajami, A.M. Glutamate: An Amino Acid of Particular Distinction. J. Nutr. 2000, 130, 892S–900S. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Hu, H.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. The Protective Role of Glutamine on Enteropathy Induced by High Dose of Soya Meal in Turbot, Scophthalmus maximus L. Aquaculture 2018, 497, 10. [Google Scholar] [CrossRef]
- Hasebe, M.; Suzuki, H.; Mori, E.; Furukawa, J.; Kobayashi, K.; Ueda, Y. Glutamate in Enteral Nutrition: Can Glutamate Replace Glutamine in Supplementation to Enteral Nutrition in Burned Rats? J. Parenter. Enteral. Nutr. 1999, 23, S78–S82. [Google Scholar] [CrossRef]




| Ingredients (g/kg) | Diets | ||||
|---|---|---|---|---|---|
| FM | SBM | G1 | G2 | G3 | |
| Fish meal | 520.0 | 220.0 | 220.0 | 220.0 | 220.0 |
| Casein | 131.0 | 120.0 | 120.0 | 120.0 | 120.0 |
| Gelatin | 33.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Soya meal | 0 | 460.0 | 460.0 | 460.0 | 460.0 |
| Soya oil | 42.6 | 42.6 | 42.6 | 42.6 | 42.6 |
| Fish oil | 8.2 | 30.8 | 30.8 | 30.8 | 30.8 |
| Soy lecithin | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Added glutamate | 0 | 0 | 10.0 | 20.0 | 30.0 |
| Corn starch | 206.8 | 38.3 | 28.3 | 18.3 | 8.3 |
| Sodium alginate | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Ca(H2PO4)2 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Choline chloride | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Stay-C 35% | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Premix | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Nutrient level | |||||
| Dry matter (g/kg) | 905.8 | 907.8 | 903.1 | 905.5 | 905.8 |
| Crude protein (g/kg) | 484.0 | 482.9 | 493.2 | 491.0 | 501.9 |
| Crude lipid (g/kg) | 114.1 | 120.6 | 116.7 | 117.9 | 120.5 |
| Ash (g/kg) | 97.5 | 79.3 | 80.6 | 80.9 | 80.9 |
| Total glutamate (g/kg) | 61.2 | 73.1 | 82.2 | 93.1 | 104.5 |
| Gross energy (MJ/kg) | 21.17 | 21.44 | 21.32 | 21.33 | 21.27 |
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | E (%) | Accession No. |
|---|---|---|---|---|
| IL-8 | AAGTTTGCCTTGACCCCGAA | TGAAGCAGATCTCTCCCGGT | 94 | FJ9130641 |
| IL-1β | GCAACTCCACCGACTGATGA | ACCAGGCTGTTATTGACCCG | 116 | EF582837.1 |
| IL-10 | GTCCACCAGCATGACTCCTC | AGGGAAACCCTCCACGAATC | 99 | KJ741852.1 |
| TGF-β1 | GCTTACGTGGGTGCAAACAG | ACCATCTCTAGGTCCAGCGT | 102 | GQ503351.1 |
| IL-12 | CCAGATTGCACAGCTCAGGA | CCGGACACAGATGGCCTTAG | 115 | KC662465.1 |
| TNF-a | GGATCTGGCGCTACTCAGAC | CGCCCAGATAAATGGCGTTG | 117 | FJ009049.1 |
| Gls | CCCGAACATACGACCAGAGG | CATGATGCGCACTTAGCCAC | 111 | CL1126.Cotig86_All |
| β-actin | GATCTGGCATCACACCTTCT | CATCTTCTCCCTGTTGGCTT | 104 | AY510710.2 |
| Parameters | Diets | ||||
|---|---|---|---|---|---|
| FM | SBM | G-1 | G-2 | G-3 | |
| IABW (g) | 15.10 ± 0.02 | 15.11 ± 0.01 | 15.11 ± 0.04 | 15.10 ± 0.01 | 15.09 ± 0.03 |
| FABW (g) | 81.93 ± 1.32 a | 65.87 ± 0.63 b | 68.00 ± 4.59 b | 75.25 ± 4.06 ab | 77.16 ± 1.58 ab |
| WG (%) | 442.72 ± 8.81 a | 335.95 ± 3.91 b | 349.81 ± 29.38 b | 398.42 ± 27.02 ab | 411.43 ± 11.36 ab |
| SGR (%/d) | 3.02 ± 0.03 a | 2.63 ± 0.02 b | 2.68 ± 0.12 b | 2.86 ± 0.10 ab | 2.91 ± 0.04 ab |
| FCR | 0.95 ± 0.01 | 1.19 ± 0.09 | 1.13 ± 0.10 | 1.05 ± 0.03 | 1.00 ± 0.05 |
| FI (%/d) | 2.34 ± 0.02 | 2.66 ± 0.19 | 2.55 ± 0.17 | 2.50 ± 0.10 | 2.41 ± 0.12 |
| HSI (%) | 1.95 ± 0.16 | 1.33 ± 0.19 | 1.52 ± 0.33 | 1.27 ± 0.07 | 1.63 ± 0.03 |
| CF (%) | 2.89 ± 0.05 | 2.67 ± 0.03 | 2.77 ± 0.06 | 2.83 ± 0.07 | 2.86 ± 0.08 |
| SR (%) | 98.89 ± 1.11 | 96.67 ± 1.93 | 98.89 ± 1.11 | 98.89 ± 1.11 | 98.89 ± 1.11 |
| Parameters (%) | Diets | |||||
|---|---|---|---|---|---|---|
| FM | SBM | G-1 | G-2 | G-3 | ||
| Whole body | Moisture | 69.46 ± 0.23 | 69.12 ± 0.01 | 69.89 ± 0.42 | 69.94 ± 0.32 | 69.23 ± 0.36 |
| Crude protein | 16.55 ± 0.19 | 17.17 ± 0.14 | 17.10 ± 0.37 | 16.28 ± 0.35 | 17.16 ± 0.18 | |
| Crude lipid | 7.45 ± 0.09 | 7.23 ± 0.15 | 7.33 ± 0.29 | 7.36 ± 0.53 | 7.67 ± 0.27 | |
| Ash | 4.54 ± 0.12 | 4.41 ± 0.03 | 4.32 ± 0.05 | 4.35 ± 0.09 | 4.40 ± 0.12 | |
| Muscle | Moisture | 75.65 ± 0.72 | 74.52 ± 0.26 | 74.90 ± 0.24 | 75.00 ± 0.33 | 75.54 ± 0.55 |
| Crude protein | 19.38 ± 0.81 | 20.57 ± 0.04 | 20.70 ± 0.12 | 20.27 ± 0.06 | 19.67 ± 0.39 | |
| Crude lipid | 2.97 ± 0.46 | 2.65 ± 0.36 | 2.24 ± 0.23 | 2.33 ± 0.48 | 2.30 ± 0.20 | |
| Ash | 1.24 ± 0.06 | 1.36 ± 0.01 | 1.32 ± 0.01 | 1.30 ± 0.00 | 1.22 ± 0.06 | |
| Parameters | Diets | |||||
|---|---|---|---|---|---|---|
| FM | SBM | G-1 | G-2 | G-3 | ||
| PI | HMF (μm) | 724.63 ± 21.20 a | 434.34 ± 18.26 b | 434.80 ± 18.03 b | 537.18 ± 85.36 b | 585.92 ± 16.44 b |
| MT (μm) | 243.39 ± 23.53 | 159.31 ± 8.54 | 166.12 ± 4.94 | 176.07 ± 27.11 | 218.76 ± 29.34 | |
| MI | HMF (μm) | 587.18 ± 35.55 | 388.78 ± 79.70 | 394.87 ± 39.67 | 445.47 ± 16.89 | 513.48 ± 47.04 |
| MT (μm) | 209.82 ± 19.10 | 128.23 ± 46.37 | 129.03 ± 13.36 | 170.58 ± 4.61 | 192.14 ± 21.55 | |
| DI | HMF (μm) | 508.90 ± 30.65 | 447.21 ± 11.21 | 481.29 ± 63.97 | 484.80 ± 86.20 | 500.20 ± 95.76 |
| MT (μm) | 217.13 ± 37.45 | 154.86 ± 19.38 | 211.38 ± 40.56 | 211.65 ± 30.54 | 212.95 ± 37.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Xiao, R.; Huo, C.; Wang, K.; Ye, J. Glutamate Supplementation Ameliorated Growth Impairment and Intestinal Injury in High-Soya-Meal-Fed Epinephelus coioides. Animals 2025, 15, 2392. https://doi.org/10.3390/ani15162392
Wang A, Xiao R, Huo C, Wang K, Ye J. Glutamate Supplementation Ameliorated Growth Impairment and Intestinal Injury in High-Soya-Meal-Fed Epinephelus coioides. Animals. 2025; 15(16):2392. https://doi.org/10.3390/ani15162392
Chicago/Turabian StyleWang, Aozhuo, Ruyi Xiao, Cong Huo, Kun Wang, and Jidan Ye. 2025. "Glutamate Supplementation Ameliorated Growth Impairment and Intestinal Injury in High-Soya-Meal-Fed Epinephelus coioides" Animals 15, no. 16: 2392. https://doi.org/10.3390/ani15162392
APA StyleWang, A., Xiao, R., Huo, C., Wang, K., & Ye, J. (2025). Glutamate Supplementation Ameliorated Growth Impairment and Intestinal Injury in High-Soya-Meal-Fed Epinephelus coioides. Animals, 15(16), 2392. https://doi.org/10.3390/ani15162392







