Enhanced Muscle Flavor in Male Chinese Mitten Crab (Eriocheir sinensis) Driven by Feed-Induced Reconfiguration of Intestinal Volatile Compounds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Experimental Crabs and Culture Conditions
2.3. Consumer Sensory Evaluation
2.4. Electronic Nose Analysis
2.5. Volatile Compound Analysis by GC–IMS
2.6. Statistical Analysis
3. Results
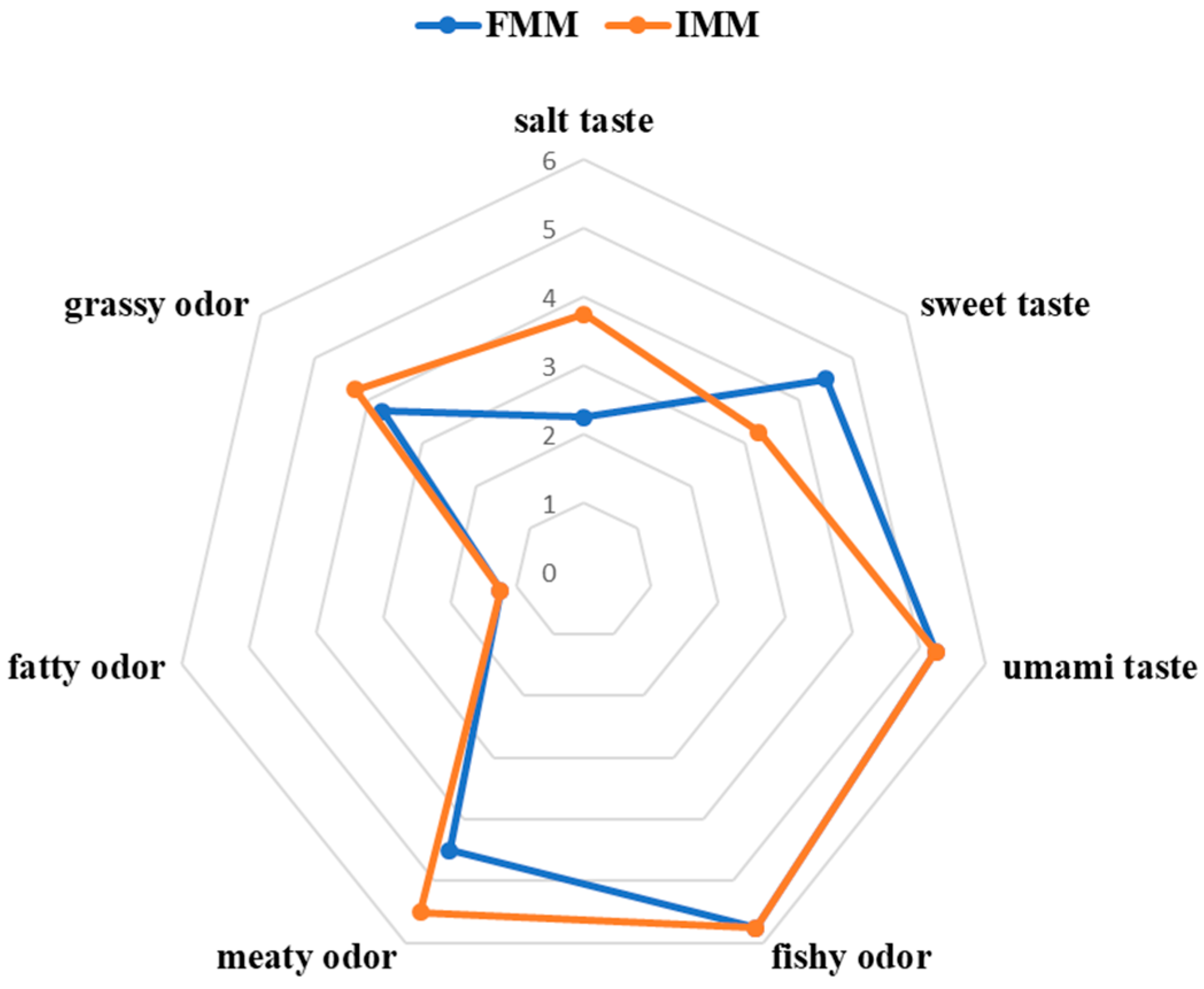
3.1. Consumer Sensory Evaluation
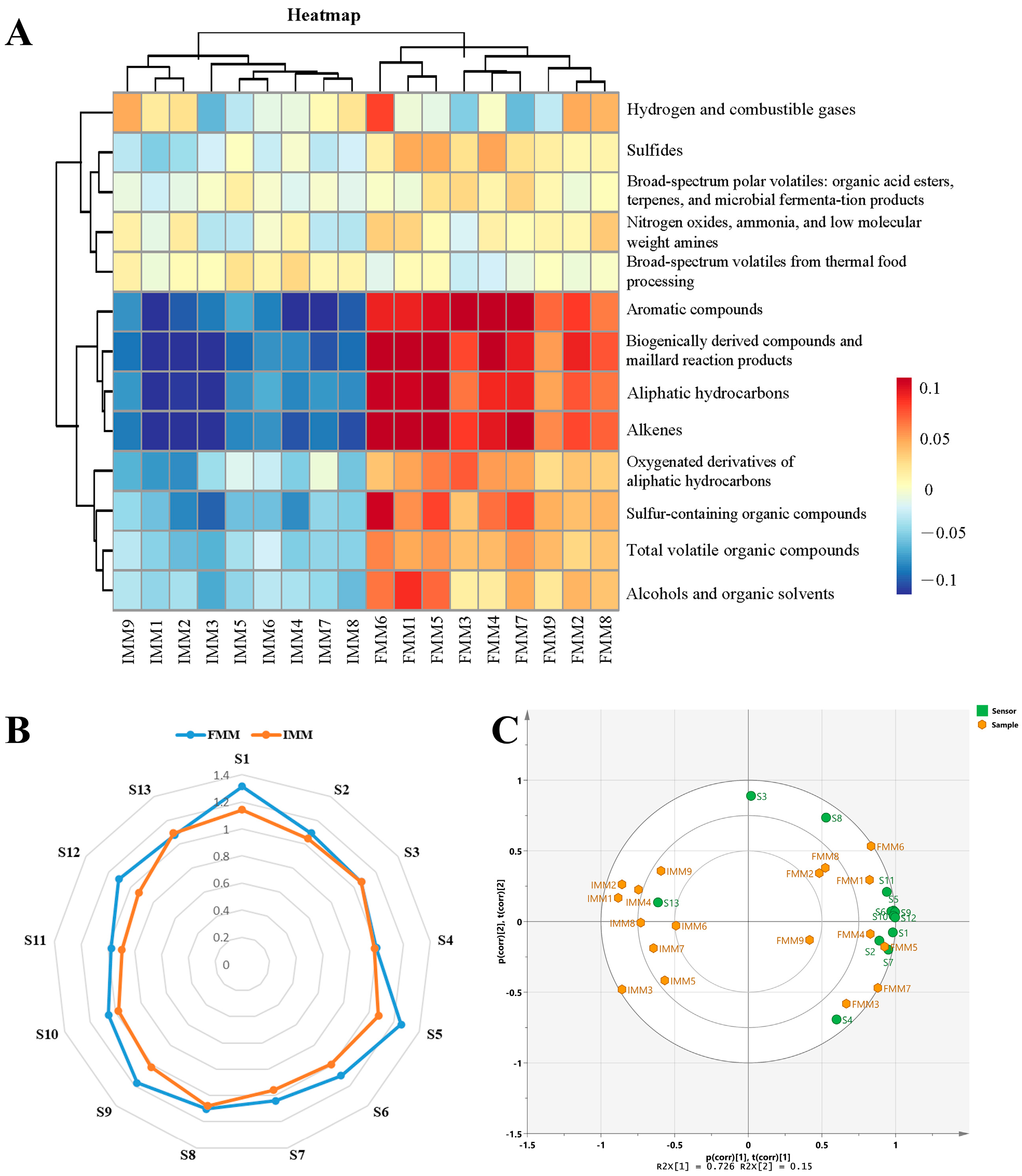
3.2. E-Nose Analysis of the Crab Fed with Different Diets
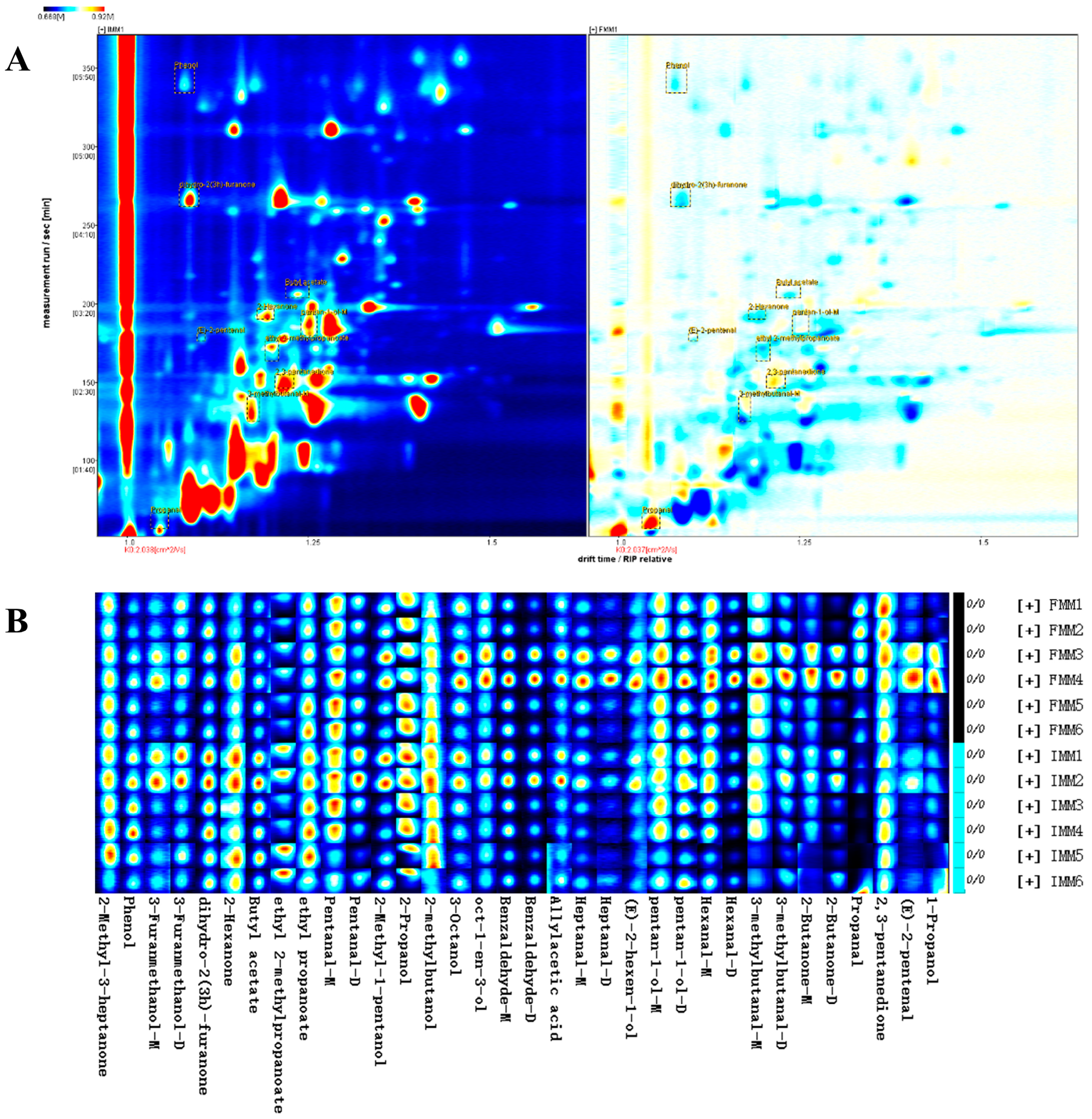
3.3. Differences in Volatile Compounds in the Muscle of the Crab
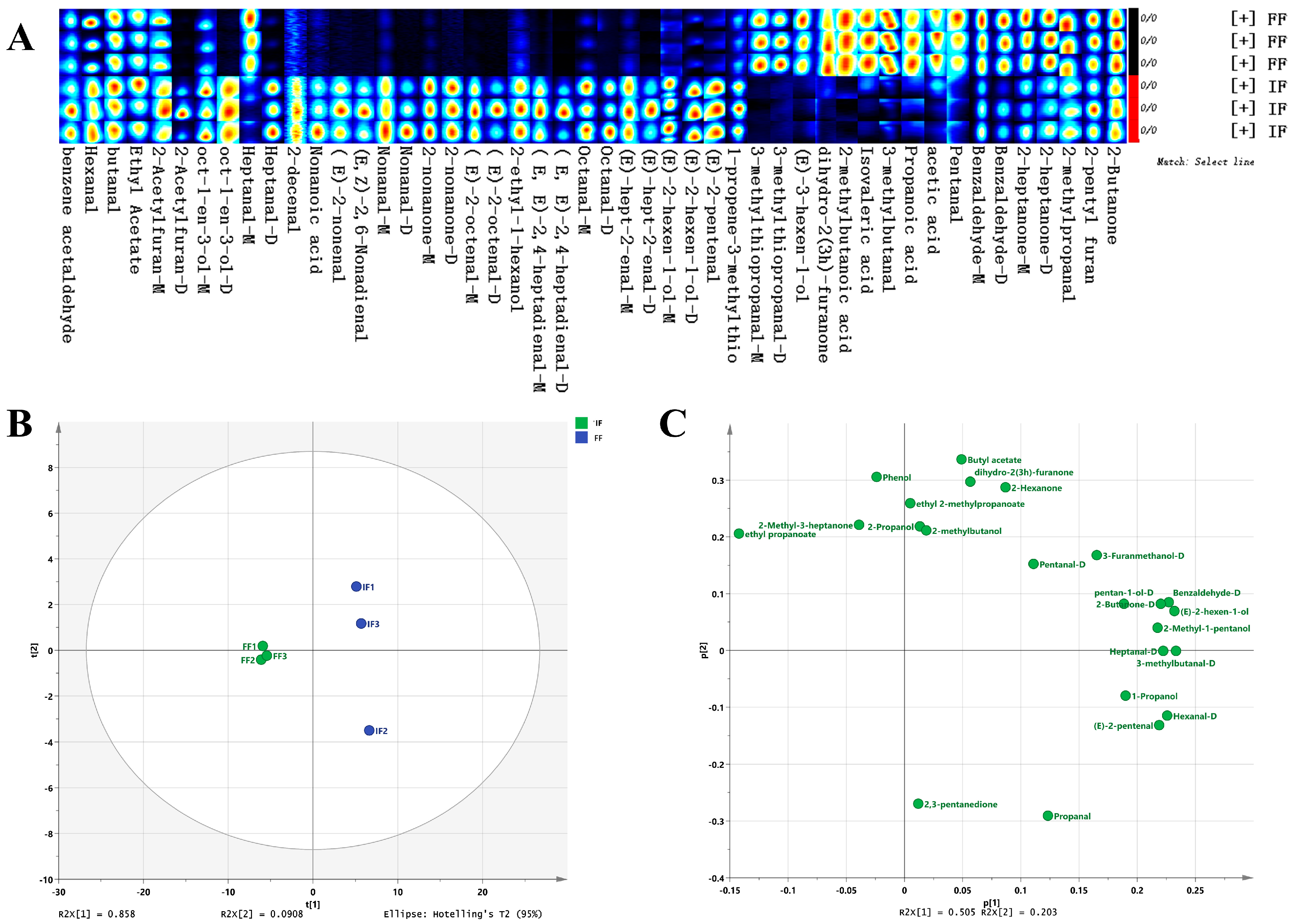
3.4. Differences in Volatile Compounds in the Intestine of the Crab Fed with Formulated Feed and Iced Trash Fish
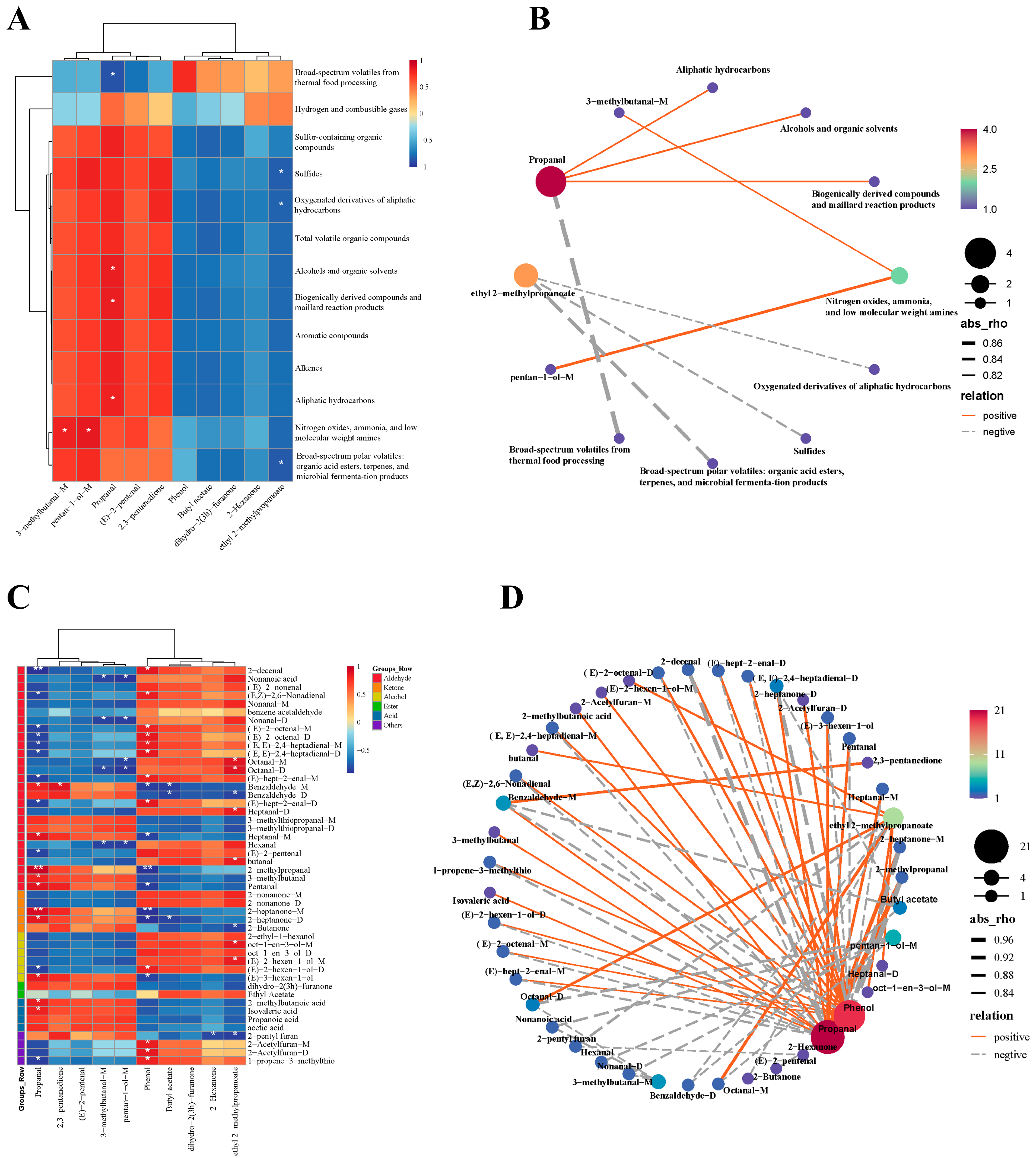
3.5. Correlation Analysis of the Odor Profiles and Volatiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fishery Bureau of Ministry of Agriculture of the People’s Republic of China. China Fishery Statistics Yearbook 2021; China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Zou, J.; Song, C.; Meng, S.; Hu, G.; Qiu, L.; Fan, L.; Chen, J. Effects of Feed on Fatty Acid Composition in Muscles and Gonads of the Chinese Mitten Crab (Eriocheir sinensis). Oceanol. Hydrobiol. Stud. 2021, 50, 338–351. [Google Scholar] [CrossRef]
- Yi, X.; Gao, J.; Li, L.; Du, J.; Nie, Z.; Zhang, X.; Xu, G. Effects of Fattening Diets on the Nutritional Quality and Flavor of the Adult Female Chinese Mitten Crab (Eriocheir sinensis). Aquac. Rep. 2022, 25, 101223. [Google Scholar] [CrossRef]
- Que, Y.; Yang, Z.; Ji, L.; He, J.; Shao, L.; Wang, C.; Yang, X.; Cheng, Y.; Yang, J. Effects of Formulated Dietary Replacement of Trash Fish on Growth Performance, Body Composition and Fatty Acid Composition of Eriocheir sinensis. J. Fish. China 2012, 36, 1612. [Google Scholar] [CrossRef]
- Shao, L.; Wang, C.; He, J.; Wu, X.; Cheng, Y. Meat Quality of Chinese Mitten Crabs Fattened with Natural and Formulated Diets. J. Aquat. Food Prod. Technol. 2014, 23, 59–72. [Google Scholar] [CrossRef]
- Shao, L.; Wang, C.; He, J.; Wu, X.; Cheng, Y. Hepatopancreas and Gonad Quality of Chinese Mitten Crabs Fattened with Natural and Formulated Diets. J. Food Qual. 2013, 36, 217–227. [Google Scholar] [CrossRef]
- Zhuang, K.; Wu, N.; Wang, X.; Wu, X.; Wang, S.; Long, X.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir sinensis). J. Food Sci. 2016, 81, S968–S981. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparison of Volatiles of Cultured and Wild Sea Bream (Sparus aurata) during Storage in Ice by Dynamic Headspace Analysis/Gas Chromatography-Mass Spectrometry. J. Agric. Food. Chem. 2005, 53, 2616–2622. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat Flavor Precursors and Factors Influencing Flavor Precursors-A Systematic Review. Meat Sci. 2015, 110, 278–284. [Google Scholar]
- Wu, N.; Gu, S.; Tao, N.; Wang, X.; Ji, S. Characterization of Important Odorants in Steamed Male Chinese Mitten Crab (Eriocheir sinensis) Using Gas Chromatography--Mass Spectrometry--Olfactometry. J. Food Sci. 2014, 79, C1250–C1259. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Priolo, A. Ruminant Fat Volatiles as Affected by Diet. A Review. Meat Sci. 2006, 73, 218–228. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Trabalza-Marinucci, M.; Miraglia, D.; Roila, R.; Acuti, G.; Giusepponi, D.; Dal Bosco, A.; Ranucci, D. Effects of Olive Mill Vegetation Water Phenol Metabolites Transferred to Muscle through Animal Diet on Rabbit Meat Microbial Quality. Sustainability 2021, 13, 4522. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. A 21st Century Technique for Food Control: Electronic Noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Wang, Y.; Tao, N.; Wu, X.; Wang, X.; Qiu, W.; Ma, M. Comparison of Flavour Qualities of Three Sourced Eriocheir sinensis. Food Chem. 2016, 200, 24–31. [Google Scholar] [CrossRef]
- Gu, S.Q.; Wang, X.C.; Tao, N.P.; Wu, N. Characterization of Volatile Compounds in Different Edible Parts of Steamed Chinese Mitten Crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent Progress in Food Flavor Analysis Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Xu, X.; Sun, C.; Liu, B.; Zhou, Q.; Xu, P.; Liu, M.; Wang, A.; Tian, H.; Luo, W.; Jiang, Q. Flesh Flavor of Red Swamp Crayfish (Procambarus clarkii Girard, 1852) Processing by GS-IMS and Electronic Tongue Is Changed by Dietary Animal and Plant Protein. Food Chem. 2022, 373, 131453. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, C.; Rao, W.; Chen, P.; Lei, K.; Mai, K.; Zhang, W. Dietary phospholipids improve growth performance and change the lipid composition and volatile flavor compound profiles in the muscle of abalone Haliotis discus hannai by affecting the glycerophospholipid metabolism. Aquac. Rep. 2023, 30, 101567. [Google Scholar] [CrossRef]
- Chi, C.; Lin, Y.; Miao, L.; Liu, B.; Ge, X. Effects of Dietary Supplementation of a Mixture of Ferulic Acid and Probiotics on the Fillet Quality of Megalobrama amblycephala Fed with Oxidized Oil. Aquaculture 2022, 549, 737786. [Google Scholar] [CrossRef]
- Ding, W.; Lu, Q.; Fan, L.; Yin, M.; Xiao, T.; Guo, X.; Zhang, L.; Wang, X. Correlation of Taste Components with Consumer Preferences and Emotions in Chinese Mitten Crabs (Eriocheir sinensis): The Use of Artificial Neural Network Model. Foods 2022, 11, 4106. [Google Scholar] [CrossRef]
- Yang, F.; Guo, H.; Gao, P.; Yu, D.; Xu, Y.; Jiang, Q.; Xia, W. Comparison of Methodological Proposal in Sensory Evaluation for Chinese Mitten Crab (Eriocheir sinensis) by Data Mining and Sensory Panel. Food Chem. 2021, 356, 129698. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Yi, D.; Luo, Y.; Zheng, C.; Wu, Y. Insights into the volatile flavor profiles of two types of beef tallow via electronic nose and gas chromatography–ion mobility spectrometry analysis. Foods 2024, 13, 1489. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, D.; Chen, R.; Yang, X.; Liu, H.; Wang, Z.; Hui, T. Comprehensive evaluation of flavor in charcoal and electric-roasted tamarix lamb by HS-SPME/GC-MS combined with electronic tongue and electronic nose. Foods 2021, 10, 2676. [Google Scholar] [CrossRef]
- Qi, K.; Ge, K.; Zhang, R.; Wang, B.; Tao, X.; Qin, K.; Xu, Z. Unveiling the impact of muscle fiber composition on taste and aroma compounds in Jinhua pig skeletal muscles. Food Chem. 2025, 493, 145764. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Qu, Z.; Shi, W.; Wang, X.; Wu, X. A Comparative Study on the Flavour of Wild Chinese Mitten Crab (Eriocheir sinensis) along the Eastern Coast of China. J. Food Compos. Anal. 2024, 127, 105949. [Google Scholar] [CrossRef]
- Feng, W.; Feng, W.; Ge, J.; Li, J.; Su, S.; Jia, R.; Yu, J.; Xu, P.; Tang, Y. Alterations of Amino Acid Metabolism and Intestinal Microbiota in Chinese Mitten Crab (Eriocheir sinensis) Fed on Formulated Diet and Iced Trash Fish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, H.; Liu, M.; Zhao, X.; Luo, H. Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat. Foods 2020, 9, 1178. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Li, C.; Xu, B. Insight into the Effect of Frozen Storage on the Changes in Volatile Aldehydes and Alcohols of Marinated Roasted Beef Meat: Potential Mechanisms of Their Formation. Food Chem. 2022, 385, 132629. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Lin, Z.; Li, Y.; Chen, F.; Liu, S.; Li, C. Effects of Different Cooking Methods on Volatile Flavor Compounds of Chicken Breast. J. Food Biochem. 2021, 45, e13770. [Google Scholar] [CrossRef]
- Brewer, M.S. Irradiation Effects on Meat Flavor: A Review. Meat Sci. 2009, 81, 1–14. [Google Scholar] [CrossRef]
- Grabež, V.; Bjelanović, M.; Rohloff, J.; Martinović, A.; Berg, P.; Tomović, V.; Rogić, B.; Egelandsdal, B. The Relationship between Volatile Compounds, Metabolites and Sensory Attributes: A Case Study Using Lamb and Sheep Meat. Small Rumin. Res. 2019, 181, 12–20. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Xue, J.; Yu, X.; Long, X.; Wu, X.; Wang, H. Phospholipidomics quality evaluation of swimming crabs (Portunus trituberculatus) cultured with formulated feed, frozen trash fish, and mixed feed, a non-target approach by HILIC-MS. J. Chromatogr. B 2021, 1179, 122845. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, H.; Gao, P.; Yang, F.; Yu, D.; Xia, W.; Yu, D. Effects of different thermal methods and degrees on the flavor of channel catfish (Ictalurus punctatus) fillets: Fatty acids, volatile flavor and taste compounds. Food Chem. 2024, 461, 140887. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Huang, A. A Metabolomics-Based Approach Investigates Volatile Flavor Formation and Characteristic Compounds of the Dahe Black Pig Dry-Cured Ham. Meat Sci. 2019, 158, 107904. [Google Scholar] [CrossRef]
- Corral, S.; Salvador, A.; Flores, M. Elucidation of Key Aroma Compounds in Traditional Dry Fermented Sausages Using Different Extraction Techniques. J. Sci. Food Agric. 2015, 95, 1350–1361. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Tamogami, S.; Chen, J.; Zhang, H. Volatile Profile and Flavor Characteristics of Ten Edible Oils. Anal. Lett. 2021, 54, 1423–1438. [Google Scholar] [CrossRef]
- Bintsis, T.; Robinson, R.K. A Study of the Effects of Adjunct Cultures on the Aroma Compounds of Feta-Type Cheese. Food Chem. 2004, 88, 435–441. [Google Scholar] [CrossRef]
- Tikk, K.; Haugen, J.E.; Andersen, H.J.; Aaslyng, M.D. Monitoring of Warmed-over Flavour in Pork Using the Electronic Nose—Correlation to Sensory Attributes and Secondary Lipid Oxidation Products. Meat Sci. 2008, 80, 1254–1263. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, W.; Peng, Y.; Ji, H.; Gao, J. Evaluation of Flavor Improvement in Antarctic Krill Defluoridated Hydrolysate by Maillard Reaction Using Sensory Analysis, E-Nose, and GC-MS. J. Aquat. Food Prod. Technol. 2020, 29, 279–292. [Google Scholar] [CrossRef]
- Takagi, S.; Sato, Y.; Murata, Y.; Kokubun, A.; Touhata, K.; Ishida, N.; Agatsuma, Y. Quantification of the Flavor and Taste of Gonads from the Sea Urchin Mesocentrotus nudus Using Gc–Ms and a Taste-Sensing System. Sensors 2020, 20, 7008. [Google Scholar] [CrossRef]
- Kourkoutas, D.; Elmore, J.S.; Mottram, D.S. Comparison of the Volatile Compositions and Flavour Properties of Cantaloupe, Galia and Honeydew Muskmelons. Food Chem. 2006, 97, 95–102. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Relationship of Sensory and Instrumental Aroma Measurements of Dark Chocolate as Influenced by Fermentation Method, Roasting and Conching Conditions. J. Food Sci. Technol. 2013, 50, 909–917. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of Several Volatile Phenols and Their Glycoconjugates to Smoke-Related Sensory Properties of Red Wine. J. Agric. Food. Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Park, M.K.; Choi, Y.S. Effective strategies for understanding meat flavor: A review. Food Sci. Anim. Resour. 2025, 45, 165. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef]
- Yang, J.; Qin, K.; Wang, Q.; Li, S.; Zhu, Y.; Yang, X. Comparative analysis of amino acids and volatile compounds as markers in Lueyang black-boned chicken and Arbor Acres broilers. Poult. Sci. 2025, 104, 104897. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Hansen, L.L.; Andersen, H.J. Transfer of the Meat Aroma Precursors (Dimethyl Sulfide, Dimethyl Disulfide and Dimethyl Trisulfide) from Feed to Cooked Pork. LWT-Food Sci. Technol. 2002, 35, 485–489. [Google Scholar] [CrossRef]
- Jiang, W.; Jia, X.; Xie, N.; Wen, C.; Ma, S.; Jiang, G.; Li, X.; Chi, C.; Zhang, D.; Liu, W. Aquafeed Fermentation Improves Dietary Nutritional Quality and Benefits Feeding Behavior, Meat Flavor, and Intestinal Microbiota of Chinese Mitten Crab (Eriocheir sinensis). Anim. Nutr. 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Qian, X.; Ji, J.; Ning, X.; Zhu, F.; Yin, S.; Zhang, K. Effects of Hypoxia and Reoxygenation on Apoptosis, Oxidative Stress, Immune Response and Gut Microbiota of Chinese Mitten Crab, Eriocheir sinensis. Aquat. Toxicol. 2023, 260, 106556. [Google Scholar] [CrossRef]
- Gao, J.; Tai, X.; Shao, N.; Sun, Y.; Nie, Z.; Wang, Y.; Li, Q.; Xu, P.; Xu, G. Effects of Effective Microorganisms on the Growth Performance, Nutritional Composition and Flavour Quality of the Pond-Cultured Eriocheir sinensis. Aquac. Res. 2021, 52, 871–880. [Google Scholar] [CrossRef]
- Ghosh, A.; Rathore, A.; Gaba, S.; Kamli, M.R.; Maigoro, A.Y.; Kwon, H.W.; Mahajan, N.; Kim, C.B.; Malik, A. The Chinese Mitten Crab (Eriocheir sinensis) and Its Microbiome: A Review. Aquaculture 2025, 595, 741518. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Li, N.; Zhong, H.; Xu, H.; Zhu, Q.; Liu, Y. Comparative Analysis of the Gut Microbial Composition and Meat Flavor of Two Chicken Breeds in Different Rearing Patterns. BioMed Res. Int. 2018, 2018, 4343196. [Google Scholar] [CrossRef] [PubMed]
- Pignoli, G.; Bou, R.; Rodriguez-Estrada, M.T.; Decker, E.A. Suitability of Saturated Aldehydes as Lipid Oxidation Markers in Washed Turkey Meat. Meat Sci. 2009, 83, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Victoria Moreno-Arribas, M.; Bartolomé, B. Gut Microbial Catabolism of Grape Seed Flavan-3-Ols by Human Faecal Microbiota. Targetted Analysis of Precursor Compounds, Intermediate Metabolites and End-Products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef]

| Ingredients | Proximate Composition | ||
|---|---|---|---|
| Fishmeal 1 | 32.00 | Crude protein | 39.72 |
| Soybean meal 1 | 10.00 | Crude lipid | 7.69 |
| Rapeseed meal 1 | 10.00 | Ash | 10.02 |
| Peanut meal 1 | 10.00 | Gross energy (MJ/kg) | 18.49 |
| Blood meal 1 | 6.00 | ||
| α-starch 2 | 22.50 | ||
| Fish oil 1 | 2.00 | ||
| Soybean oil 1 | 2.00 | ||
| Monocalcium phosphate 1 | 2.00 | ||
| Choline chloride (50%) 3 | 1.00 | ||
| Vitamin and mineral premix 3 | 1.00 | ||
| Bentonite 3 | 1.00 | ||
| Salt | 0.50 |
| Volatiles | NO. | Compounds | CAS# | Formula | RI | Retention Time (s) | Drift Time (ms) | Intensity (Volume) | |
|---|---|---|---|---|---|---|---|---|---|
| FMM | IMM | ||||||||
| Aldehydes | 1 | Benzaldehyde-M | C100527 | C7H6O | 957.7 | 311.3 | 1.1 | 588.8 ± 54.5 | 544.5 ± 44.7 |
| 2 | Benzaldehyde-D | C100527 | C7H6O | 957.0 | 310.8 | 1.5 | 213.0 ± 41.9 | 202.0 ± 30.9 | |
| 3 | Heptanal-D | C111717 | C7H14O | 899.3 | 260.8 | 1.7 | 74.2 ± 15.5 | 62.2 ± 7.8 | |
| 4 | Hexanal-D | C66251 | C6H12O | 791.5 | 198.1 | 1.6 | 497.3 ± 112.3 | 284.0 ± 51.5 | |
| 5 | Hexanal-M | C66251 | C6H12O | 791.8 | 198.3 | 1.3 | 565.4 ± 33.4 | 454.6 ± 48.3 | |
| 6 | Pentanal-M | C110623 | C5H10O | 698.6 | 152.8 | 1.2 | 435.2 ± 17.3 | 364 ± 46.1 | |
| 7 | Pentanal-D | C110623 | C5H10O | 700.2 | 153.5 | 1.4 | 492.9 ± 19.5 | 547 ± 90.5 | |
| 8 | 3-methylbutanal-M | C590863 | C5H10O | 654.1 | 132.5 | 1.2 | 723.0 ± 42.1 a | 501.8 ± 79.7 b | |
| 9 | 3-methylbutanal-D | C590863 | C5H10O | 656.9 | 133.8 | 1.4 | 1628.7 ± 292.8 | 1386.4 ± 188.2 | |
| 10 | Heptanal-M | C111717 | C7H14O | 900.4 | 261.7 | 1.3 | 245.2 ± 36 | 182.1 ± 19 | |
| 11 | Propanal | C123386 | C3H6O | 493.4 | 60.9 | 1.0 | 809 ± 108.3 a | 308.3 ± 112.8 b | |
| 12 | (E)-2-pentenal | C1576870 | C5H8O | 752.4 | 178.7 | 1.1 | 38.7 ± 7.1 a | 19.8 ± 3.7 b | |
| Ketones | 1 | 2-Methyl-3-heptanone | C13019200 | C8H16O | 1094.6 | 895.9 | 1.3 | 636.1 ± 17.0 | 688.0 ± 53.0 |
| 2 | 2-Hexanone | C591786 | C6H12O | 781.6 | 192.8 | 1.2 | 211 ± 17.7 b | 268.2 ± 17.6 a | |
| 3 | 2-Butanone-M | C78933 | C4H8O | 598.7 | 107.9 | 1.1 | 547.8 ± 76.7 | 406.1 ± 75.2 | |
| 4 | 2-Butanone-D | C78933 | C4H8O | 597.8 | 107.4 | 1.2 | 1163.2 ± 423.1 | 1234.3 ± 266.1 | |
| 5 | 2,3-pentanedione | C600146 | C5H8O2 | 693.4 | 150.3 | 1.2 | 726.3 ± 40.5 a | 615.6 ± 18.1 b | |
| Alcohols | 1 | 3-Octanol | C589980 | C8H18O | 998.6 | 356.6 | 1.4 | 253.7 ± 22.7 | 225.3 ± 21.4 |
| 2 | Oct-1-en-3-ol | C3391864 | C8H16O | 982.0 | 332.4 | 1.2 | 403.9 ± 35.1 | 331.0 ± 23.4 | |
| 3 | 3-Furanmethanol-M | C4412913 | C5H6O2 | 973.7 | 325.2 | 1.1 | 119.6 ± 15.8 | 130.0 ± 18.2 | |
| 4 | 3-Furanmethanol-D | C4412913 | C5H6O2 | 974.2 | 325.7 | 1.4 | 213.1 ± 18.8 | 226.7 ± 28.2 | |
| 5 | (E)-2-hexen-1-ol | C928950 | C6H12O | 849.3 | 230.6 | 1.2 | 172.7 ± 19.8 | 155.5 ± 18.0 | |
| 6 | Pentan-1-ol-D | C71410 | C5H12O | 764.0 | 184.3 | 1.5 | 474.1 ± 31.6 | 477 ± 50.5 | |
| 7 | Pentan-1-ol-M | C71410 | C5H12O | 767.1 | 185.8 | 1.3 | 732.7 ± 26.2 a | 573.7 ± 51.2 b | |
| 8 | 2-Methyl-1-pentanol | C105306 | C6H14O | 847.0 | 229.2 | 1.3 | 325.6 ± 33.7 | 290.6 ± 41.2 | |
| 9 | 2-Propanol | C67630 | C3H8O | 524.8 | 74.9 | 1.1 | 4227.3 ± 293.9 | 4647 ± 541 | |
| 10 | 2-methylbutanol | C137326 | C5H12O | 731.5 | 168.6 | 1.2 | 152.4 ± 6.3 | 155.4 ± 14.4 | |
| 11 | 1-Propanol | C71238 | C3H8O | 571.0 | 95.5 | 1.1 | 287.6 ± 70.1 | 245.2 ± 31.3 | |
| Esters | 1 | Dihydro-2(3h)-furanone | C96480 | C4H6O2 | 904.8 | 265.5 | 1.1 | 465.7 ± 24.2 b | 598.6 ± 38.0 a |
| 2 | Butyl acetate | C123864 | C6H12O2 | 805.8 | 206.2 | 1.2 | 190.6 ± 5.6 b | 241.8 ± 17.5 a | |
| 3 | Ethyl 2-methylpropanoate | C97621 | C6H12O2 | 733.5 | 169.6 | 1.2 | 200 ± 10.9 b | 308.6 ± 27.0 a | |
| 4 | Ethyl propanoate | C105373 | C5H10O2 | 713.7 | 160.1 | 1.2 | 790.0 ± 50.3 | 856.3 ± 46.3 | |
| Acids | 1 | Allylacetic acid | C591800 | C5H8O2 | 897.4 | 259.1 | 1.1 | 208.5 ± 12.0 | 202.4 ± 12.9 |
| 2 | Phenol | C108952 | C6H6O | 991.6 | 340.7 | 1.1 | 245.6 ± 11.3 b | 317.5 ± 19.3 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, J.; Liu, B.; Zhou, Q.; Zheng, X.; Xu, G.; Tian, H.; Miao, L.; Ding, H.; Zhao, Y.; Sun, C. Enhanced Muscle Flavor in Male Chinese Mitten Crab (Eriocheir sinensis) Driven by Feed-Induced Reconfiguration of Intestinal Volatile Compounds. Animals 2025, 15, 3101. https://doi.org/10.3390/ani15213101
Cen J, Liu B, Zhou Q, Zheng X, Xu G, Tian H, Miao L, Ding H, Zhao Y, Sun C. Enhanced Muscle Flavor in Male Chinese Mitten Crab (Eriocheir sinensis) Driven by Feed-Induced Reconfiguration of Intestinal Volatile Compounds. Animals. 2025; 15(21):3101. https://doi.org/10.3390/ani15213101
Chicago/Turabian StyleCen, Jin, Bo Liu, Qunlan Zhou, Xiaochuan Zheng, Gangchun Xu, Hongyan Tian, Linghong Miao, Huiming Ding, Yongfeng Zhao, and Cunxin Sun. 2025. "Enhanced Muscle Flavor in Male Chinese Mitten Crab (Eriocheir sinensis) Driven by Feed-Induced Reconfiguration of Intestinal Volatile Compounds" Animals 15, no. 21: 3101. https://doi.org/10.3390/ani15213101
APA StyleCen, J., Liu, B., Zhou, Q., Zheng, X., Xu, G., Tian, H., Miao, L., Ding, H., Zhao, Y., & Sun, C. (2025). Enhanced Muscle Flavor in Male Chinese Mitten Crab (Eriocheir sinensis) Driven by Feed-Induced Reconfiguration of Intestinal Volatile Compounds. Animals, 15(21), 3101. https://doi.org/10.3390/ani15213101









