Simple Summary
This study demonstrates the use of organic plant ingredients with low levels of fishmeal inclusion in the diet of organic juvenile sea bass. The diets were formulated to contain 25%, 30%, and 35% fishmeal, with a fourth treatment comprising 30% fishmeal and conventional ingredients, serving as the control. The research demonstrated that the control diet resulted in optimal growth rates and biometric indices. However, specific variations were identified in the nutritional and qualitative characteristics of the fillet. However, these differences remained within the established reference ranges. Additionally, a higher level of preference and intention to purchase sea bass fed with organic feed was observed. It is evident that when offering an organic product, the types of ingredients utilized are of crucial importance. This is because they can facilitate consumer choice, thus achieving an encouraging outlook for organic aquaculture and promoting research.
Abstract
The objective of the present study was to ascertain the effect of diverse organic feeds (25ECO, 30ECO, and 35ECO) containing varying levels of fishmeal (25%, 30%, and 35%) on the growth and fillet quality of juvenile sea bass. The ECO diets were composed of a blend of organic vegetable proteins. The control diet contained 30% fishmeal without any organic ingredients. The experimental period spanned 196 days, during which the fish were fed twice daily, with an initial mean weight of 40 g. The results indicated that reducing fishmeal to 25% in the 25ECO diet negatively affected growth and increased feed consumption. The 30ECO diet, which contains 30% fishmeal, exhibited no adverse effects; however, its biometric outcomes diverged from those of the control diet. The 25ECO diet demonstrated superior Met retention levels, and certain free amino acids that enhance flavour (SER, ALA, ASP, and GLU) exhibited higher concentrations in fillets from fish fed ECO diets (35ECO and 30ECO). No substantial disparities were observed in the fatty acid profile or fillet nutritional indexes, which were deemed to be satisfactory and conducive to good health. From an economic perspective, the 30 ECO diet was deemed optimal and exhibited the greatest inclination towards purchase.
1. Introduction
Sea bass (Dicentrachus labrax L.) is the second most important marine fish produced in the European Union aquaculture, with 90.883 tons, but the first in value, with 563 million euros. In Spain, sea bass is the most important fish, with 24.580 tons produced annually [1].
According to the report presented by APROMAR (2004) [2], in 2023, sea bass had a consumption share of 22.5 thousand tons in the last 12 months, ranking third after salmon and sea bream. The Scientific, Technical and Economic Committee for Fisheries (STECF) [3], in projecting a vision towards the year 2030, estimates a 20% increase in this sector, as did the Food and Agriculture Organization (FAO) [4] in 2022. Conversely, within the context of the Mediterranean region, the species that are most widely consumed are sea bass and sea bream. Collectively, these two species account for approximately 97% of the global production of both species, a factor that must be taken into consideration within the context of the global market [4,5,6].
When discussing the nutritional properties of sea bass (Dicentrarchus labrax), it is imperative to emphasize the significance of consumer preferences in Europe. This demographic highly values the species for its distinctive flavour, unique aroma, and low fat content, as it is classified as a semi-lean fish. These attributes contribute to the prevention of coronary heart disease and the enhancement of cognitive functions [7,8,9,10,11]. Conversely, the ongoing pursuit of sustainability in aquaculture has encouraged the investigation of innovative trends that consider alternative raw materials with a reduced ecological footprint, such as fishmeal and fish oil. These alternatives facilitate the optimization of circular economy strategies [12,13,14,15,16], thereby contributing to enhancements in zootechnical performance, nutritional efficiency, health, and technological progress associated with sea bass production [9,17,18].
Within aquaculture, the highest cost of production comes from the cost of feed, which comprises between 50 and 60% of total production; this percentage has fluctuated as aquaculture has developed in recent years. This underscores the vital importance of effective feed management, reliable access to ingredients for formulation, and the sustainability of feed production [17,19,20,21,22]. The composition of a sustainable feed with a reduced inclusion of fishmeal for carnivorous fish varies between 20% and 40%, depending on the proteins of vegetable origin. These can be readily substituted with soybean meal [23,24]. However, for sea bass (Dicentrarchus labrax), the level of inclusion without compromising their growth can be as low as 25% fishmeal [25], and for Japanese sea bass (Lateolabrax japonicus), 30% is considered [26]. In addition, other studies have demonstrated that increased utilization of soybean meal has been associated with diminished intake and reduced weight gain, in addition to deleterious effects on fish health, including alterations in intestinal integrity [24,26,27,28].
The European Sea bass (Dicentrarchus labrax) is among the most ecologically significant species in the Mediterranean region. The production of this species has increased substantially, with a rise from 2000 tons in 2015 to 2750 tons in 2020 [29,30]. Greece is positioned as the primary producer of this species within the European Union. Nevertheless, economic factors, including rising production costs and increasing retail prices, have given rise to concerns regarding investment in this sector, a sentiment that is particularly pronounced among both farmers and consumers. This circumstance impedes progress toward sustainable growth in organic sea bass production [31]. However, research is being conducted on species that are more in demand among European consumers, including sea bass, sea bream, and trout. Furthermore, efforts are being made to promote the production of these species, with the objective of achieving certification [32]. This approach entails the exploration of new markets and the establishment of a commitment to an ecological alternative, with a focus on fish welfare [16,33,34,35,36,37].
The utilization of organic inputs and by-products derived from organic agriculture and livestock has been demonstrated to hold considerable promise, given their status as a distinct market segment distinguished by an ecological and sustainable approach [31,38]. An increase in demand for these products has been observed, as well as a growing adaptation to innovative technologies in infrastructure [39,40,41,42,43]. However, restrictions imposed by the European Union, along with certain standards that this production system must meet [34,44,45,46], pose challenges for organic aquaculture in its search for sustainable protein sources for feeding carnivorous fish [16,35,36,37,40,47,48,49,50]. This context is essential for the development of an eco-friendly feed that does not compromise fish health. However, given the existence of reports indicating that there are no substantial disparities between organic and conventional aquaculture productions with regard to health parameters, such as blood tests and immunological responses, further research is necessary to ascertain the validity of these findings [50,51].
Given this problem, the feed industry has sought alternative protein sources, taking as an example those of vegetable origin that are more widely available on the market. However, such alternatives present implications due to the presence of anti-nutritional factors present, lower protein quality, and reduced palatability [52]. In recent years, the digestibility of plant-based proteins has improved through effective thermal processing—particularly extrusion during feed manufacture—and it is now possible to eliminate antinutrients, making the feed nutritionally suitable for fish in accordance with species-specific requirements [53,54,55,56,57].
Today, an increasing amount of information is available regarding diets containing organic inputs, as well as the relationship between conventional diets and organic diets, which is significantly influenced by Regulation (EU) No. 2018/848 [46]. According to Article 6, aquatic organisms may be fed with feed derived from sustainably exploited fisheries, in accordance with Regulation (EU) No. 1380/2013, or with organic feed composed of agricultural ingredients from organic production, including organic aquaculture, and natural, non-agricultural substances.
The approach of replacing fishmeal with other protein ingredients has been widely studied [15,24,58,59,60,61,62,63,64,65,66,67]. However, organic diets have not been extensively examined; only Tefal et al. [16] studied animal and plant ingredients derived from organic production in the absence of fishmeal. Their results, however, were inferior to those obtained with the control diet containing fishmeal. The issue of sustainability in organic aquaculture is of particular importance when formulating feed with reduced levels of fishmeal inclusion. The objective of the present study was to examine the impact of organic feeds varying in fishmeal content (25, 30, and 35%), combined with organic vegetable ingredients, as well as a control diet containing 30% fishmeal and conventional ingredients, on growth performance, biometric parameters, body composition, nutritional efficiency, fillet nutritional indexes, and economic viability.
2. Materials and Methods
2.1. Production System
The experimental study was conducted within a saltwater recirculation system, with a total volume of 65 m3. This system was equipped with a rotary mechanical filter and a gravity biofilter, each with an estimated capacity of 6 m3. The system also comprised twelve cylindrical fibreglass tanks, each with a capacity of 1750 L, divided into three tanks for each treatment. A system for the purpose of aeration was installed in all tanks located at the Aquaculture Laboratory of the Department of Animal Science of the Universitat Politècnica de València, Valencia, Spain.
2.2. Fish and Experimental Conditions
Organic sea bass (Dicentrachus labrax) fingerlings were obtained from the commercial hatchery Sonrionansa, located in the Autonomous Community of Cantabria in Spain.
At the beginning of the experiment, the fish had an average initial weight of 40 g, and 50 fish were distributed per tank. Fish were manually fed to satiety twice a day from Monday to Friday (at 9.00 a.m. and 2.00 p.m.) and once (10.00 a.m.) on Saturdays, over a 196-day period. The amount of ingested food was recorded daily. The temperature and oxygen dissolved in water (portable oximeter or OxyGuard Handy Polaris probe) were also checked daily, and salinity (refractometer, Hanna Instruments), pH (litmus paper strips), and concentration of ammonium, nitrites, and nitrates were monitored three times a week (MERCK test colourimeter). The average values obtained were as follows: 21.53 ± 2.2 °C (temperature), 7.62 ± 0.41 mg L−1 (dissolved oxygen), 7.02 ± 0.32 (pH), 16.15 ± 2.20 g L−1 salt (salinity 0/00), 0.18 ± 0.24 mg L−1 (ammonium), 0.25 mg L−1 (nitrites), and 92.32 mg L−1 (nitrates). The photoperiod used was natural, and all tanks had similar lighting conditions.
2.3. Experimental Feed
The experimental diets were manufactured at the Universitat Politècnica of Valencia’s facilities through a process of cooking-extrusion, using a semi-industrial extruder (CLEXTRAL BC 45, St. Etienne, France) belonging to the Department of Animal Science.
For the manufacture and formulation of experimental feed, the requirements established by organic production regulations were followed, using ecological raw materials which had their corresponding labelling and excluding formulation of non-permitted chemical substances such as synthetic amino acids. Four isolipid (17%) and isoproteic (46%) diets were formulated to evaluate the partial replacement of fishmeal with vegetable sources. Three organic experimental diets, FM 35 ECO, FM 30 ECO, and FM 25 ECO, containing 35, 30, and 25% fishmeal in their composition and complied with organic regulations. A fourth diet (FM 30 Control), also contained 30% fishmeal but included non-organic ingredients and served as the control.

Table 1.
Formulation and nutritional composition of experimental feed.

Table 2.
Amino acid composition of experimental diets.

Table 3.
Fatty acid composition (g 100g−1 of diets) of experimental diets.
2.4. Proximate Composition and Amino Acid Analysis
The dietary ingredients, feed (Table 1), and whole fish were analyzed according to the procedures established by the AOAC [68]. Dry matter (dried at 105 °C at constant weight), ashes (incinerated at 550 °C at constant weight), and crude protein were measured using a LECO CN628 machine, Geleen, The Netherlands, based on the Dumas method. Crude lipids were extracted using diethyl ether according to the ANKOM XT10 Technology Method 2 (2000).
The dietary composition and amino acid profile of the fish were examined utilizing a Waters high-performance liquid chromatography (HPLC) system, which includes two pumps (Model 515; Waters), an automatic sampler (Model 717; Waters), a fluorescence detector (Model 474; Waters), and a temperature control module, as outlined by Bosch et al. [69]: Subsequently, following the hydrolysis procedure, an internal standard of aminobutyric acid was incorporated into the analysis. The process of derivatization to facilitate the quantification of the amino acids employed 6-aminoquinolyl N-hydroxysuccinimidyl carbamate (AQC). The final results of the aforementioned analysis are displayed in Table 2.
2.5. Determination of Fatty Acid
Total fatty acid methyl esters (FAMEs) of lipids were produced directly, as indicated by O’Fallon et al. [70]. The samples were prepared according to the percentage of crude fat present in each sample; 12 samples were prepared with a weight corresponding to 20 milligrams of fat. 0.5 μL of the final sample was injected into a focusing A gas chromatograph (Thermo, Milan, Italy) equipped with a split/splitless injector and a flame ionization detector upon completing the procedures described in the aforementioned protocol. The separation of methyl esters was achieved by utilizing an SPTM 2560 fused silica capillary column (Supelco, PA, USA), which has 100 m × 0.25 mm × 0.2 μM film thickness. Helium was used as the transport gas, moving at 20 cm per second. The ratio for splitting was established at 1/100 while the sample was being injected. The split ratio was set at 1/100 during the injection of the sample. The initial oven temperature setting was 140 °C for five minutes, followed by an increase to 240 °C at a rate of 4 °C min−1 for 30 min. The cycle ended with a return to the original state. By comparing the retention times of specific fatty acids with Supelco’s fatty acid methyl ester criteria, one may identify them. Table 3 presents the results of the fatty acid composition of the feeds assessed in this study.
2.6. Growth and Nutrient Efficiency Indices
The following variables of growth and nutrient efficiency were determined as follows: specific growth rate (SGR), feed intake (FI), feed conversion rate (FCR), mortality (%). In order to obtain results that are more reliable, the biomass of dead fish reported in monthly reports was considered [35].
2.7. Biometric Indices
In order to obtain the biometric parameters, five randomly selected fish were euthanized from each tank, yielding a total of 15 fish per treatment in the final sampling. The sacrifice procedure was carried out by administering an overdose of anesthetic, whose formulation was based on clove oil (150 mg L−1). Subsequently, the total length (cm), total weight (g), carcass weight (g), liver weight (g), and visceral fat weight (g) were measured to calculate the biometric indexes: Condition Factor (CF), Viscerosomatic Index (VSI), Visceral Fat Index (VFI), and Hepatosomatic Index (HHI). Finally, following the appropriate identification and labelling of the fish samples, they were stored in a mixed manner at −30 °C for subsequent analysis.
2.8. Retention Efficiency
Protein, fat and energy retention were calculated as productive protein value (PPV), productive fat value (PFV), and productive energy value (PEV):
PPV (%) = Retained protein (final fish protein × Final biomass (g)) × 100 − Initial fish protein × initial biomass (g)/Protein ingested (Kg of food ingested × % crude protein of feed)
PFV (%) = Retained fat (final fish fat × Final biomass (g)) × 100 − initial fish fat × initial biomass (g)/Fat ingested (Kg food ingested × % crude fat of feed)
PEV (%) = Retained Energy (Final fish energy × Final biomass (g)) × 100 − Initial fish energy × initial biomass (g)/energy ingested (Kg feed ingested × % feed energy)
Amino acid retention efficiency (AARE) were estimated as:
AARE (%) = 100 × fish amino acid gain (g)/ingested amino acid (g).
2.9. Free Fillet Amino Acids
The free amino acids profile was carried out according to the protocol adapted from Bidlingmeyer et al. [71]. The free amino acid content of the fillet was evaluated by liquid chromatography (HPLC). A 2 g sample of fish meat was weighed, 10 mL of 0.1 N HCl was added, and the mixture was homogenized with Ultraturrax. Following homogenization, the sample underwent a series of centrifugation and filtration steps. Specifically, it was subjected to a 20 min centrifugation at 10,000× g, resulting in the interphase collection. This interphase was then filtered through a 0.45-micron PVDF filter, further purifying the sample. Subsequently, 300 μL of the filtrate was mixed with 50 μL of internal standard and 875 μL of acetonitrile. The mixture was left to stand until the following day. Subsequently, the sample underwent cooling and centrifugation at 10,000× g for five minutes. Subsequently, 300 μL of the resulting filtrate was subjected to centrifugal evaporation at 1750 rpm, 42 °C, and 35 min under vacuum conditions. Subsequently, 20 μL of drying reagent—comprising 3.5 mL of methanol, 0.5 mL of water, 0.5 mL of trimethylamine, and 0.5 mL of phenyl isothiocyanate was added, the mixture was shaken, and the solution was evaporated again until dry for 20 min. Subsequently, 15 μL of derivatization reagent (PITC) should be added to the residue. The mixture was then subjected to a drying process, followed by a 20 min rest period. The result was diluted with 250 μL of dilution reagent (i.e., disodium phosphate buffer, PO4HNa2, 5 nM, pH 7.4 with 5% CAN) and subjected to centrifugation. Subsequently, 200 μL of the centrifugate was collected, and 20 μL was injected into the HPLC.
The following conditions were employed for HPLC: phase A: 70 mM sodium acetate buffer, pH 6.55, with 2.5% acetonitrile; phase B: 450 mL acetonitrile, 400 mL water, and 150 mL methanol; column: The Movapack C-18 (Waters, S.A.) was a 300 mM × 3.9 i.d. (particle size five μM) apparatus. The precolumn was Novapack 20 mM. The temperature was as follows: 52 °C with a flow of 1 mL min−1. The detection was monitored in an UV detector at 254 nM.
2.10. Nutritional Quality Indexes of the Fillet
2.10.1. Determination of Fatty Acid in Fillet
To the fatty acid profile, fat was extracted using the adapted protocol of O’Fallon et al. [69], and each fatty acid amount was obtained by gas chromatography. An aliquot of 20 mg from the ground sample was weighed according to the % raw fat and placed in a 15 mL Pyrex tube with a Teflon stopper. Then, 0.7 mL of KOH (potassium hydroxide) 10 N, 5.3 mL of MeOH (methanol), and 1 mL of a solution composed of 0.5 mg mL−1 of C13:0 in MeOH were vortexed. The sample was brought to 55 °C for 1.5 h and stirred vigorously for 5 s every 20 min. The sample was then cooled with water at room temperature, and 0.58 mL of H2SO4 24 N was added. The sample was brought back at 55 °C for 1.5 h, stirring each vigorously for 20 min for 5 s. Then, it was cooled with water to room temperature, and 1.5 mL of hexane was added, followed by vortexing for 5 min. For 5 min, it was centrifuged through a machine at 3000 revolutions per minute, after which the liquid above the solid material was collected. The measurement of fatty acids was performed using GC software, following the specifications for a Supelco column SP-2560. (Merck KGaA, Darmstadt, Germany).
2.10.2. The Lipid Quality Indexes
The Lipidic Quality Index were calculated using the following equations [72]:
Atherogenicity Index (AI)
AI = (12:0 + (4 × 14:0) + 16:0)/(∑MUFA + ∑PUFA n − 6 + ∑PUFA n − 3)
The AI considers the sum of the major saturated and unsaturated fatty acids. In general, saturated fatty acids have some proatherogenic effects, which could favour the adhesion of lipids to the cellular units of the circulatory and immune systems. While unsaturated fatty acids are considered antiatherogenic, reducing the levels of esterified fats, cholesterol and phospholipids, as well as inhibiting plaque aggregation at the arterial level and preventing macro- and microcoronary disease [11,72,73,74,75,76,77].
Thrombogenicity Index (TI)
TI = (14:0 + 16:0 + 18:0)/(0.5 × ∑MUFA + 0.5 × (PUFA n − 6 × PUFA n − 3) + (PUFA n − 3/PUFA n − 6))
The TI has been identified as a key factor in the process of clot formation within blood vessels. A relationship has been established between prothrombogenic fatty acids, more commonly referred to as saturated fats, and antithrombogenic acids, which include monounsaturated fats (MUFA, n-6 PUFA y n-3 PUFA) [11,72,73,74,75,76,77].
Cholesterolenic Index (h/H)
h/H = (18:1n − 9 + 18:2n − 6 + 20:4n − 6 + 18:3n − 3 + 20:5n − 3 + 22:5n − 3 +22:6 n − 3)/(14:0 + 16:0)
The h/H Index is indicative of the ratio of hypocholesterolemic and hypercholesterolemic fatty acids. The range of indexes in fish is from 1.54 to 4.83. These indexes may be affected by the provision of aquaculture fish diets with higher concentrations of SFA, or by higher consumption of algae in wild species, resulting in values below the range [78,79,80,81,82].
Food Lipid Quality (FLQ)
FLQ = 100 × (EPA + DHA)/Total lipids
This index has been employed to determine the degree of correlation between omega-3 polyunsaturated fatty acids (EPA and DHA) and total lipids. The FLQ Index was originally developed as a means to assess the quality of lipids in fish [76,82,83], and meat products [74,84]. Elevated values of the index are indicative of a higher quality of lipids in the diet. However, these values can vary depending on factors such as age, season of harvest, presence of dietary substances, and habitat [74,75,82,84].
Linoleic acid/α-Linolenic Acid Index (LA/ALA)
LA/ALA = (C18:2n − 6)/(C18:3n − 3)
The LA/ALA ratio has been demonstrated to indicate a moderate improvement in omega-3 (n − 3) fatty acid levels (EPA, DHA, DPA), with a greater impact on the diet and health of children than adults [82,85].
2.11. Fish Purchase Intention
For the purposes of this analysis, which precedes the sensometric study by Calanche et al., [86], a supplementary questionnaire (Table 4) was administered to the judges. This questionnaire collected information regarding which samples the judges would be willing to purchase, their awareness of organic food, their opinion on it, and their willingness to pay a higher price for any of the selected sea bass (organic and conventional).

Table 4.
Intent to purchase.
2.12. Economic Analysis
A cost–benefit analysis of the present study was conducted by determining the Economic Conversion Ratio (ECR) and the Economic Profit Index (EPI) adapted from Martínez-Llorens et al. [87] and Jauralde et al. [88]. The total price of the diet per kilogram was calculated, taking into account the value of the referential cost of each ingredient per metric ton (MT).
E.C.R = (EUR kg−1) = FCR × Diet Price
E. P. I. = Sea bass price − ECR
2.13. Statistical Analysis
Growth data, biometric indices, fatty acids of diets were subjected to multivariable analysis of variance (ANOVA), taking the initial weight as a covariate in the study of the final average weight and TCI. The Newman-Keuls test was used to observe significant differences between feeding with a level of significance p < 0.05, corresponding to a range of 95% confidence. The statistical programme was used was Statgraphics Plus 5.1 the results were expressed as the mean ± the standard error of the mean.
2.14. Ethical Aspects
The experimental protocol was carried out following the European Union Council Directive 2010/63/EU, and Spanish state law (Spanish Royal Decree 53/2013) on the protection of animals used for research scientific after evaluation and approval by the Animal Ethics and Welfare Committee of the Polytechnic University of Valencia (UPV).
Fish in the tanks were checked daily. Also, fish were weighed individually every four weeks, and their health status was assessed through observation, after sedation with clove oil dissolved in water (0.01 mg L−1 of water) to minimize animal suffering. Animals were euthanized by an excess of clove oil (150 mg L−1) and then dissected.
3. Results
3.1. Growth and Nutritional Parameters
During the 196-day study period, a significant increase in weight was observed in all diets, although diet 25 ECO originated a lower weight since three first months (Figure 1).
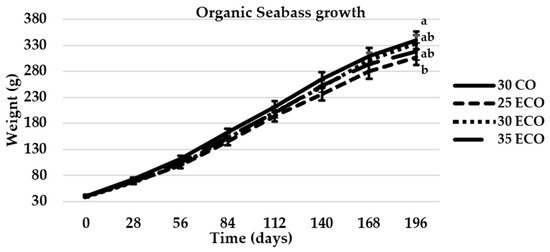
Figure 1.
Mean live weight of sea bass evolution during the 196-day experiment. The values that were represented as means (n = 3) exhibited different letters in each sampling, indicating significant differences (p < 0.05).
As demonstrated in Table 5, some statistical differences were observed among the ecological diet treatments and the control diet with respect to the FW, SGR, FI, and FCR. Fish fed the control diet (30 CO) exhibited higher final weight and higher specific growth rate (SGR), reaching values of 340 g and 1.09% day−1 respectively, but differences only appeared with diet 25 ECO, 304 g and 1.04% day−1. Conversely, the lowest FI and FCR indices were observed in fish fed the 30 CO diet, which exhibited a FI of 1.19 g 100 g fish−1 day−1 and a FCR of 1.47, only different from 25 ECO, 1.35 g 100g fish−1 day−1 and 1.73, respectively.

Table 5.
Final results of growth and nutritional use of sea bass fed with the different experimental feeds for 196 days.
3.2. Biometric Parameters of Sea Bass Fed Experimental Diets
As illustrated in Table 6, the biometric indices for the final period are presented.

Table 6.
Final biometric parameters of sea bass fed with the different experimental feeds for 196 days.
A statistically significant difference was observed for the condition factor (CF), hepatosomatic index (HIS), Viscerosomatic index (VSI), and mesenteric fat (MF) (p < 0.05). The indexes with lower values corresponded to the 30 CO diet for CF, VSI, and MF (1.33 g cm−3; 11.82%; 5.26%, respectively). However, this was not the case for the HIS, whose value was higher (2.16%).
3.3. Body Composition and Nutrient Retention Efficiency
The final results of the body composition of sea bass fed the experimental diets, as well as nutrient retention, are presented in Table 7. Dry matter and fat protein increased in final sea bass from initial sea bass, and ash reduced. No statistically significant differences (p > 0.05) were observed for dry matter, crude protein, crude fat, and ash between the ECO and CO treatments. The protein, fat and energy retention efficiencies (PPV; PFV; PEV), showed significant differences (p< 0.05) between fish fed the ECO and CO diets, demonstrating that the high values for PPV and PFV indexes were found for the 30 CO diet (25.34% and 76.34%, respectively), and the lowest value was for the 25 ECO diet (21.89% and 62.60%, respectively). However, as for PEV, the highest value was obtained by those fish fed with the 35 ECO diet (30.17%), while the lowest value was represented by the 25 ECO diet (26.27%)

Table 7.
Whole-body composition and retention efficiency of sea bass at the end of the experiment.
3.4. Amino Acid Retention Efficiency
The results of amino acid retention are shown in Table 8. The study revealed no statistically significant differences in most the essential amino acid retention between fish subjected to the CO and ECO treatments at a significance level of p > 0.05. However, a statistically significant difference was observed in methionine retention, with the highest value recorded in fish fed the 25 ECO diet (33.18 mg kg body−1 weight), and the lowest value observed in fish fed the 30 CO diet (18.20 mg kg body−1 weight). Conversely, for the retention efficiency of non-essential amino acids, only three amino acids exhibited significant differences (p < 0.05), glutamine, proline, and serine. The highest values of glutamine and proline were observed in fish fed the 35 ECO diet (26.34, 31.75 g kg−1 wet weight, respectively), while the lowest values were observed in fish fed the 30 CO treatment (18.76, 21.15 g kg−1 wet weight, respectively). In regard to serine retention efficiency, the highest value was observed in fish fed the 25 ECO diet (26.69 g kg−1 wet weight), while the lowest value was observed in fish fed the 30 ECO diet (18.82 g kg−1 wet weight). No significant differences (p > 0.05) were observed for the remaining non-essential amino acids.

Table 8.
Sea bass amino acid retention efficiency (AARE) at the end of the experiment.
3.5. Proximal Composition of Fillets, Fatty Acid, and Free Amino Acid Profile
The nutritional composition of the fillets from sea bass fed with ECO and CO feeds is shown in Table 9. No significant differences were observed for total dry matter, protein, and fat, with the exception of ash, which exhibited a higher value in fish fed diets 25 ECO and 30 ECO, while the lowest value was observed in fish fed diet 35 ECO.

Table 9.
Proximal composition of fillets from sea bass fed the experimental diets.
A comparative analysis of the fatty acid composition of fillets of sea bass fed different diets (Table 10) revealed that no differences appeared in total saturated, monounsaturated, polysaturated and PUFAs. For monounsaturated fatty acids, samples fed diet 25 ECO demonstrated significantly higher levels (p < 0.05) of C17:1, C18:1n-9t, and C20:1. Conversely, diet 30 CO fillets exhibited elevated levels of fatty acids C14:1 and C16:1. In long-chain fatty acids (PUFAs), diet 25 ECO exhibited higher values for LA, ALA, C20:2, and C 20:4n-6, while diet 30 CO demonstrated a higher value for C 22:4n-6. No differences were obtained in EPA, DHA, n-3 HUFAs, n-3/n-6 ratio, and EPA/DHA.

Table 10.
Fatty acid profile (g 100g−1) of fillets from sea bass fed organic diets.
Table 11 presents the free amino acids detected in sea bass fillets from fish fed on the ECO and CO diets. The analysis revealed that the most of free amino acids in fillets were higher in sea bass fed with the 35 ECO, TAU, LYS, GLN, SER, ARG, LEU, PHE, and TYR On the other hand, CYS, MET, TRP, BAL, and ANS levels were higher in the fillets from the 25 ECO treatment group, while HIS, THR and PRO in the sea bass fillets fed the Control 30 CO diet.

Table 11.
Free amino acid profile (mg 100g−1) in fillets from sea bass fed with the experimental diets ECO and CO.
3.6. Nutritional Quality Indexes of the Fillet
No significant differences were observed for several indexes between the ECO treatments (Table 12).

Table 12.
Nutritional and qualitative indexes of European sea bass fillets.
3.7. Purchase Intention
As demonstrated in Table 4, the data was collated from 100 panellists, and a Kruskal–Wallis test with a 95% confidence level was employed to estimate the preference for sea bass fillets fed with the ECO and CO diets. The results of this test are presented in Table 13. A greater preference was expressed for the purchase of sea bass fillets from treatment 30 ECO, while for fillets from treatment 25 ECO, the opposite was true. Conversely, Figure 2 demonstrates that the B-plot design strengthens this inclination in purchase intention, as determined by the Kruskal–Wallis test. Conversely, Figure 3 illustrates the extent of awareness regarding organic food, with a notable inclination towards the following statements: The present study posits that the adoption of environmentally friendly practices, a greater focus on animal welfare, and enhanced availability are imperative for the advancement of the field. In the subsequent question of the survey, 52% of respondents expressed a willingness to incur a higher cost for organic food products. As demonstrated in Figure 4, the willingness to pay is shown to be relatively consistent across the three categories, with no significant differences observed (p > 0.05) when classified according to the total number of panellists. The distribution of the categories is as follows: Category 1 accounts for 25%; Category 2 accounts for 41%; and Category 3 accounts for 34%.

Table 13.
Willingness to pay for sea bass obtained in the experimental trial, assessed using the Kruskal–Wallis test.
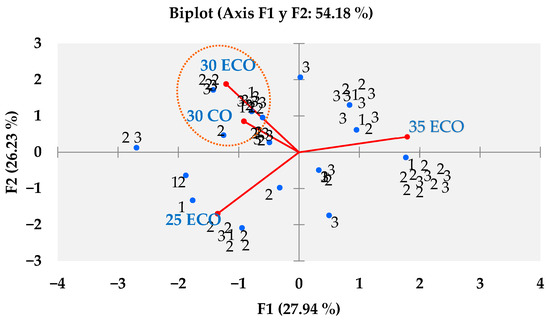
Figure 2.
B-plot design of preference in purchase intention for sea bass fillets fed diets 25 ECO, 30 ECO, 35 ECO, and 30 CO.
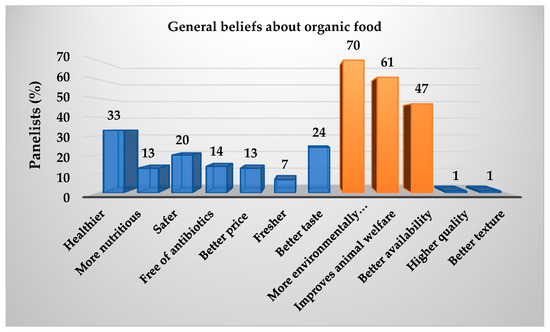
Figure 3.
Panellists’ overall knowledge of organic food.
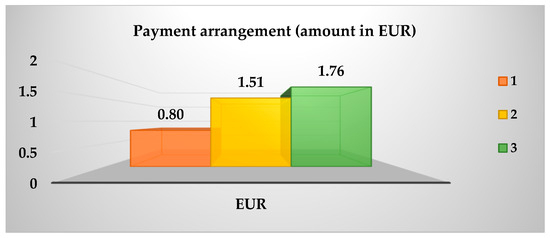
Figure 4.
Additional amount (EUR) willing to pay in the price.
3.8. Economic Analysis
A cost–benefit analysis of the present study was conducted, and the results are presented in Table 14. With regard to the economic implications of feed, no substantial differences were identified in economic conversion ratio among the several ECO diets (EUR 1.62–1.69 kg−1); however, the diet 30 CO, with a cost of EUR 1.29 kg−1, was determined to be the most economical option. With regard to the Economic Conversion Ratio (ECR), some differences appeared, being the lowest value for 30 CO (EUR 1.91 kg−1) and the highest for 25 ECO (EUR 2.94 kg−1). The economic profit index (EPI) exhibited a high value for 30 ECO and 35 ECO diet, reaching EUR 8.41 and 8.42 kg−1, while the lowest value was registered for the 30 CO diet, at EUR 3.60 kg−1, as consequence of sea bass sell price (EUR 5.50 kg−1 for conventional sea bass and EUR 11.00 kg−1 for organic sea bass).

Table 14.
Economic analysis of sea bass production fed with the different experimental feeds for 196 days.
4. Discussion
4.1. Fish Performance
In general, fishmeal and fish oil are considered as complete ingredients for aquaculture feed because they present a good profile of amino acids, polyunsaturated fatty acids and minerals. The challenges when replacing these ingredients is to find that they contain the same properties, and that meet the nutritional needs of the species. It should be noted that fishmeal is very palatable, which is not the case with vegetable sources, because they have low palatability, they are deficient in amino acids, and they have an endless number of anti-nutritional factors depending on the ingredient [54]. In current trial, any reduction on feed ingestion was observed in ECO diets, in the case of 30 ECO growth was similar to the control diets (30 CO) and in 25 ECO the feed intake (FI) was highest. Compared to other research in sea bream, sea bass and trout, it was confirmed that there is a higher feed intake in organic diets compared to conventional diets and that it could be deduced from the absence of synthetic amino acids by trying to compensate with a higher intake [16,37,50]. Nevertheless, the inclusion of high levels of plant ingredients (25 ECO) resulted in a poorer growth and food conversion, and this observation is consistent with the results of other studies that have incorporated organic subproducts of animal origin into the diet, replacing conventional fishmeal. However, these studies have not yielded superior results compared to the control diet with 30% FM [16,35,36].
In sea bass, good results have been obtained when using feed with high degrees of substitution of fishmeal by vegetable protein mixtures in a conventional feed [96,97]. There are few studies where growth is currently evaluated in sea bass fed with organic feed with high substitutions of fishmeal and fish oil. Some previous studies with sea bass compare conventional and organic feed. However. they cannot be compared with these results since the organic feed presented high amounts of fishmeal (56%) compared to conventional (20%), resulting in better growths and feed conversion rates in fish fed with organic feed [50]. Similar studies carried out in other species. the organic feed also presented better growth than conventional feed, without showing significant differences. On the other hand, as did the work of Di Marco et al. [50] in the case of sea bream, the organic feed was also formulated with a higher percentage of fishmeal (63%) than the conventional one (50%) [41]. Another study with organic peameal and organic rapeseed vegetable protein concentrate (PPC) as substitutes for fishmeal in rainbow trout diets, at substitution levels of 0, 16, 31, and 47%, reported no significant differences in the specific growth rate (SGR) and feed conversion rate (FCR). This outcome is attributed to the fact that up to 47% of rapeseed protein concentrate (PPC) while still maintaining a balanced profile of essential amino acids in the feed [98].
As stated in previous works, the utilization of organic animal by-products as substitutes for conventional fishmeal has been demonstrated to have a substantial impact on the circular economy and to promote the incorporation of organic ingredients. However, these by-products have been observed to exert an adverse effect on growth and final weight [16], which in this study demonstrated minimal disparities in weight gain between ECO and CO diets at an inclusion level of 30% of FM. This ensured that parameters such as feed intake and Feed Conversion Ratio (FI, FCR) would be similar.
4.2. Final Biometric Parameters
Sea bass fed control diet 30 CO presented a lower Viscerosomatic index (11.8%) than ECO diets (13.33–13.83%) which agrees with others studies with a high substitution of fishmeal by ecological vegetable sources resulted in a significant increase in the VSI that ranged between 8.39 and 14.0%, and the same happened with the HSI high values 1.12 and 2.64% [50,99].
A study by Gaylord et al. [100] identified a correlation between dietary methionine deficiency and intraperitoneal fat increment, which present study has demonstrated, because all organic diets exhibit a deficiency of this essential amino acid, and an increment of visceral or mesenteric fat (MF) and Viscerosomatic index (VSI) (Table 5). However, the incorporation of methionine of vegetable origin in studies involving sea bream and sea bass, with diets comprising trout and sea bass by-products as hydrobiological ingredients, without the presence of conventional FM, has emerged as a promising alternative. This approach holds significant potential for the revaluation of fishery by-products and the promotion of sustainability. Consequently, it necessitates a re-examination of the optimal proportions of alternative protein ingredients to be incorporated into the formulation, with the objective of meeting the nutritional requirements of the European sea bass [36,101].
4.3. Body Composition and Retention Nutrient Efficiency
In regard to body composition, no statistically significant differences (p > 0.05) between the ECO and CO group diets were observed with respect to dry matter, crude protein, crude fat, and ash, corroborating the findings reported in European sea bass by Tefal et al. [35], who utilized vegetable ingredients and organic animal by-products (soybean meal, Iberian pork, insect meal, trout by-product) without fishmeal. Conversely, other studies on organic diets for sea bream exhibited considerably lower outcomes concerning body composition levels, this can be attributed to the incorporation of poultry meal, which is known to reduce body fat content [36,102]. However, Sabbagh et al. [103] achieved favourable outcomes without compromising growth and protein quality. In the present study, the protein and fat retention efficiency (PPV, PFV) exhibited superiority over the findings reported by Tefal et al. [35].
Nutrient retention efficiency showed variability among treatments, with fish fed organic diets having lower protein productive value (PPV) rates. This phenomenon can be attributed to a deficiency of essential amino acids (EAA), which limited the synthesis of proteins in the fish. In addition, Fat Productive Value (FPV) and Energy Productive Value (EPV) were significantly increased in fish fed ECO diets, especially in the 35 ECO treatment. This increase reflects a significant crude fat and energy content in the final body composition of the fish due to alterations in lipid metabolism. Such alterations are associated with inadequate lipid distribution at the tissue level, thereby compromising the activity of lipogenic enzymes [16,104,105].
The former utilized organic subproducts of animal origin in sea bass feed, yielding ranges of 17 to 12% for PPV and 65 to 56% for PFV. In contrast, the present study employed a greater quantity of organic ingredients of vegetable origin (spelt bran, corn meal, and soybean meal), yielding ranges of 25 to 22% for PPV and 76 to 62% for PFV. This discrepancy can be attributed to the distinct composition of the organic ingredients utilized in each study. On the other hand, the use of these alternative plant ingredients is promising at a 30% fishmeal inclusion level, as reflected in the present study. However, high substitutions by other organic animal sources in diets for carnivorous fish could negatively affect growth, protein productivity, and retention values of essential amino acids [36].
4.4. Retention Amino Acid Efficiency
Only a significant difference was observed in the efficiency of retention of methionine, the 25 ECO diet, which incorporated 25% fishmeal (FM), demonstrated the highest methionine retention rate of 33.18%, indicating a notable increment in retention efficiency in relation the others ECO diets (25.9%) and control diet 30 CO (18.2%). These differences can be attributed to the need, for compensating the dietary methionine deficiency, (0.7–0.9% in ECO diets, and 1.25% in control diet). This compensation was effective in the case of sea bass fed 20 ECO and 35 ECO, which presented a similar growth than control, but sea bass fed 25 ECO was no able to reach the same weight. Likewise, as indicated Tefal et al. [16], European bass fed organic diets containing by-products from trout, Iberian pork, and insect meal exhibited significant levels of methionine; despite diets were supplemented, Iberian pork and mix diets presented lowest level of methionine (0.5% compared 0.7–1.0%), and although its retention was higher (24% compared 12–15%) the growth could not be compensated, which agree with diet 25 ECO in current trial. However, the efficiency of the retention of other essential amino acids remained largely unaltered, a property that can be ascribed to the level of incorporation of conventional fishmeal at levels of 25%, 30%, and 35%.
4.5. Proximal Composition of Fillets, Fatty Acid, and Free Amino Acid Profile
In the proximal composition (Table 9) of the sea bass fillets fed with ECO and CO feed, no significant differences were found for crude protein, dry matter, and ether extract. However, a lower ash content was observed in sea bass fillets fed with the 35 ECO diet. Conversely, to interpret the proximate composition of the fillets, it is imperative to consider samples of the lipid profile and free amino acids, which can contribute to sensory and quality studies [106].
The fatty acid composition of sea bass muscle is associated with the fatty acid profile of the diets provided. The composition of the fish’s diet can influence its growth and health. The predominant saturated fatty acids are typically C16:0 and C18:0, while monounsaturated fatty acids are principally represented by C18:1n9 [107,108]. A higher presence of these fatty acids has been shown to promote better membrane fluidity when the ambient temperature changes [109]. Conversely, marine fish species lack the capacity to synthesize unsaturated fatty acids (HUFAs) from PUFAs, a deficiency that accounts for the substantial accumulation of linoleic acid (LA) in the fillets of sea bass fed conventional and organic diets. This phenomenon can be attributed to the absence of one or more physiological synthesis processes [110]. Furthermore, the deposition of fatty acids in the musculature of sea bass, for ∑ SFA, ∑ MUFA, and ∑ PUFA, can be influenced by the diet and the nature of the protein and lipid sources, whether conventional or organic (including by-products from agriculture, terrestrial livestock, or aquatic sources). It is imperative to take into account the prior treatment of the specimens, as well as whether they are wild or farmed, given that this can lead to ineffective or efficient distribution of fatty acids in the dorsal or ventral regions of carnivorous fish species such as sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata) [16,36,76,111,112,113].
In contrast, the variation in the concentration of fatty acids in carnivorous fish, even between species of the same phylum, is influenced by a variety of factors. The primary factor contributing to this imbalance is the absence of the enzyme’s delta-12 desaturase and delta-15 desaturase, which are essential for the synthesis of ALA and LA from C 18:1n-9. The degree of their composition is determined by their ability to elongate and desaturate, thereby becoming long-chain PUFAs [114,115,116]. However, as illustrated in Table 10, a higher concentration of n-6 is observed in fish fed organic diets. This finding suggests that an increased n-6 intake may influence the ratio of PUFA to n-3 fatty acids. As indicated in the works of Monroig et al. [115], Saini et al. [117], Tefal et al. [36], and Glencross et al. [116].
Tefal et al. [36] indicate that when sea bream were fed organic diets that include trout by-products (TRO), sea bass (SBS), and poultry (POU), as well as a mixture of these (MIX) and an ECO diet containing 30% fishmeal, better growth performance is observed in the CO and ECO diets. However, the CO diet exhibited enhanced integration of fatty acids in the fillet, a finding that contrasts with the results at present study of elevated levels of fatty acids in the 35 ECO diets comprising 35% fishmeal. The fish that demonstrated optimal weight gain were those fed the 30 CO and 30 ECO diets, both containing 30% fishmeal. The nature of the ingredients has been demonstrated to influence palatability, resulting in increased intake to meet nutritional requirements. This, in turn, affects the lipid metabolism of fatty acids in fillets on the ECO and CO diets, as indicated by several authors in their studies on fatty acid composition in marine fish [116,118,119,120].
As demonstrated in Table 11, the analysis of marine and continental fish species reveals the presence of essential amino acids, which are essential for a nutritionally balanced diet. However, external factors such as diet, time, processing, and storage at varying temperatures have been demonstrated to exert a substantial influence on the composition of muscle and free amino acids [106,121,122,123,124]. In the case of SER, values were found in sea bass fillets fed with the 35 ECO diet, which determine the sweetness indicator [106,124,125]. Concurrently, in diverse aquatic organisms, certain free amino acids—including ORN, GLU, ASP, ALA, SER, and GLY—play a pivotal a key role in the organoleptic characteristics of food, affecting umami flavour, sweetness, aroma, and bitter-sweet taste [106,124,126]. In addition, some of these compounds are precursors of secondary metabolites that produce volatile compounds [124]. These free amino acids were studied in sea bream [106], herbivorous carp [126] and yellowfin sea bream [127], Barramundi [128], and shrimp [124,129]. In contrast, the present study demonstrated that the ECO and CO treatments exhibited elevated levels of six out of the twenty-six free amino acids, with TAU registering at 370.6 mg 100 g−1 (35 ECO), GLY at 56.5 mg 100 g−1 (30 ECO), LYS at 67.3 mg 100 g−1 (35 ECO), HIS at 51.6 mg 100 g−1 (30 CO), ALA at 29.01 mg 100 g−1 (35 ECO), and GLU at 28.50 mg 100 g−1 (30 ECO).
However, other studies carried out on fillets of gilthead sea bream (Sparus aurata) (whole fish, gutted, and filleted) that were frozen reported levels of TAU ≥ 150 mg 100 g−1 [106]. A paucity of research was identified on free amino acids in fish fed organic diets, underscoring the significance of this study in treatments applied to sea bass. Consequently, a preceding sensory study [86], published prior to the present study, demonstrated that sea bass fillets fed the 30 ECO diet exhibited optimal sensory attributes, whereas the fillets from the 25 ECO treatment exhibited contrasting outcomes. This divergence may be attributed to the fact that the concentration of each amino acid can affect the sensory quality of sea bass.
4.6. Nutritional Quality Indexes of the Fillet
In relation to the nutritional and qualitative indices of the fillets of sea bass that were fed the ECO and CO diets, it was observed that both the nutritional parameters (IA, TI, HH, FLQ) and the qualitative parameters (ω6/ω3, PUFA/SFA, MUFA/SFA, PUFA/MUFA, (PUFA + MUFA)/SFA, and LA/ALA) were found to be within the ranges defined by the references of various authors. Concurrently, research on sea bass indicates that the atherogenic index (AI) values exceed 0.50, while the thrombogenic index (TI) values reach 0.33, across both farmed and wild specimens [130]. Conversely, Monteiro et al. [90] have indicated that the AI ranges in sea bass vary between 0.40 and 0.42, suggesting that this fish exhibits a higher degree of adaptability to the artificial diets provided. Consequently, the quality of the final product may be contingent upon the quantity of vegetable oils incorporated during the manufacturing process, as well as the fatty acid profile present in the raw materials, particularly in the case of organic ingredients. This may result in a decrease in IA, TI, and FLQ levels, evidencing the lack of other parameters that could provide a better understanding of the nutritional value of the fillets and a higher proportion of beneficial fatty acids [82,111,130,131,132]. In contrast, Monteiro et al. [90] reported TI values of 0.191–0.63, which are considerably higher than those observed in the present study (0.00024–0.00038), without exceeding the defined range [11,72,76,89]. It is evident that these values may fluctuate as sea bass specimens attain larger sizes [133].
In the case of h/H, it was observed that the lowest value was recorded for sea bass fillets that were fed the control diet 30 CO (3.40). Conversely, in the ECO diets, the values were elevated for both the fillets from group 25 ECO and group 30 ECO (4.55 and 4.50, respectively). However, Santos-Silva et al., [91] have indicated that index levels may fluctuate without exerting an effect on specific fatty acids in the cholesterol metabolism. This phenomenon can be attributed, in large part, to the type of diet, the feeding time, and the nature of the oils used. Consequently, such disparities are discernible in other carnivorous species inhabiting analogous environments. In the case of Labeo rohita, when attempting to replace fish oil with peanut oil, up to 60% could be substituted without significantly impairing the growth and nutritional quality of the fillet (FLQ) [134], Merluccius gayi a value of 2.23 is reported, for Seriola lalandi a value of 2.14 [81], while for Salmon trutta a range of 1.88 to 2.16 is established [78].
In the present study, no significant differences were observed in FLQ between the ECO and CO diets (8.97–8.63), indicating minimal values, attributable to the organic sources and the incorporation of 50% fish oil and soybean oil. The primary objective of this index is to assess the nutritional quality of the fillet, a particularly salient aspect in the context of marine species. It has been demonstrated that an elevated proportion of fish oil in the diet is associated with enhanced nutritional quality and elevated FLQ values, attributable to an increase in the incorporation of EPA and DHA [82,84]. For Labeo rohita, a superior nutritional quality index (32.70) was observed in diets containing 100% fish oil. A decrease in fish oil and the introduction of an alternative energy source have been demonstrated to result in a reduction in FLQ values [134]. In the case of trout, a value of 17.97 was reported [74], and for sea bream, ranges of 23.7 to 13.5, these values being lower than those of feeds that did not contain fish oil [84,135].
However, the other indices remain within the established range [92,93]. A higher ratio of omega-6 to omega-3 fatty acids indicates a higher incorporation of vegetable oils or oilseed meal, which may cause an imbalance in the sources of polyunsaturated fatty acids (PUFAs), as well as in the concentration of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the fillets. A notable increase in the omega-6/omega-3 ratio has been observed in sea bream and sea bass, with values rising from 0.22 to 0.26 and from 0.44 to 0.48, respectively [130]. In a separate study that utilized organic diets, it was observed that the productive value of fatty acids in fillets of sea bream fed with certified organic animal by-products (trout, sea bass, poultry, MIX) [36] was also affected, both in terms of growth and the deposition of lipids in the fillets. In contrast, it has been posited that the ratio of PUFA/SFA in the human diet should exceed 0.45 [93], a figure that exceeds the values found in this study on sea bass fillets that received ECO and CO diets (1.60 to 2.06) [82,92].
4.7. Purchase Intention
The sea bass fed a diet containing 30% fishmeal (30ECO) was the most preferred, while the sea bass fed a diet containing 25% fishmeal (25ECO) was the least preferred. This was due to changes in their organoleptic characteristics, small but substantial variations in the free amino acids GLY and GLU, which were responsible for the seafood and umami flavours that influenced this choice. Meanwhile, according to the panellists, the concept of organic food refers more to food that is “environmentally friendly,” “animal welfare-friendly,” and “more readily available,” thereby maintaining a somewhat clear vision of sustainable production. The majority of panellist (Figure 4) indicated their willingness to pay between EUR 0.80 and 1.76 more for organic sea bass than for conventional sea bass (EUR 5.50 per kg). The amount with the highest percentage of panellists willing to pay the reference price was EUR 1.51 (41%), resulting in a tentative sale price for organic sea bass fillets of EUR 7.0 per kg.
4.8. Economic Analysis
The price of organic diets and the economic conversion ratio were higher in ECO diets than conventional diet, due the cost of organic ingredients, which could be an inconvenient for organic production. Nevertheless, the economic profit index also was higher with organic diets, due the higher sell price of organic sea bass, EUR 11 per kg respect EUR 5.5 per kg, but the broken price would be EUR 7 per kg, price more affordable for consumers on the other hand, the organic label would open new fish market, interested in this ecological production.
5. Conclusions
The present study demonstrates the feasibility of feeding European sea bass with organic diets containing vegetable ingredients with varying levels of fishmeal. Optimal results in terms of growth performance, FCR, PPV, PFV, PEV, fillet quality, and economic profit were achieved with the diet containing 30% fishmeal and organic plant ingredients (30 ECO), without compromising the nutritional quality indices of the fillet. However, it should be noted that these outcomes may vary depending on the origin and composition of the organic ingredients used. Nevertheless, this study offers an optimistic perspective for future research on organic aquaculture, allowing for the optimization of feed and enhanced utilization of natural resources and the production of high-quality, sustainable aquatic food products.
Author Contributions
Conceptualization, A.T.-V., S.M.-L. and M.J.-C.; data curation, E.R.C.-P. and A.T.-V.; formal analysis, E.R.C.-P., M.S.-G. and A.T.-V.; funding acquisition, M.J.-C.; investigation, E.R.C.-P., M.S.-G., J.B.C.-M., A.T.-V., S.M.-L. and M.J.-C.; methodology, E.R.C.-P., M.S.-G., J.B.C.-M., A.T.-V., S.M.-L. and M.J.-C.; project administration, M.J.-C.; resources, M.J.-C.; software, E.R.C.-P., M.S.-G., J.B.C.-M., A.T.-V., S.M.-L. and M.J.-C.; supervision, A.T.-V. and M.J.-C.; validation, A.T.-V. and M.J.-C.; visualization, S.M.-L.; writing—original draft, E.R.C.-P.; writing—review and editing, E.R.C.-P. and M.J.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This project was developed with the collaboration of the Biodiversity Foundation (Spanish Ministry for Ecological Transition and the Demographic Challenge) through the Pleamar Programme, co-financed by the European Maritime and Fisheries Fund (EMFF).
Institutional Review Board Statement
Following the Royal Decree 53/2013 and the European Directive 2010/63/EU on the protection of animals used for scientific research, the experimental protocol was reviewed and approved by the Ethics and Animal Welfare Committee of the Universitat Politècnica de València (Official Bulletin No. 80 of 26 June 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- APROMAR. Guía Sobre el Bienestar de los Peces en la Acuicultura Española: Bienestar de las Lubinas; Asociación Empresarial de Acuicultura de España: Cádiz, Spain, 2024; Volume 2, p. 49. [Google Scholar]
- APROMAR. La Acuicultura en España. 2024. Available online: https://apromar.es/wp-content/uploads/2025/03/Informe2024_v1.4.pdf (accessed on 1 May 2025).
- European Commission: Scientific, Technical and Economic Committee for Fisheries; Nielsen, R.; Llorente, I.; Guillen, J.; Virtanen, J. The 2024 Aquaculture Economic Report (STECF 24–14); Nielsen, R., Llorente, I., Guillen, J., Virtanen, J., Eds.; Publications Office of the European Union: Luxembourg, 2025; Available online: https://op.europa.eu/publication-detail/-/publication/0c13327d-fd6a-11ef-b7db-01aa75ed71a1 (accessed on 1 May 2025).
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Zoli, M.; Rossi, L.; Fronte, B.; Aubin, J.; Jaeger, C.; Wilfart, A.; Bacenetti, J. Environmental impact of different Mediterranean technological systems for European sea bass (Dicentrarchus labrax) and Gilthead Sea bream (Sparus aurata) farming. Aquac. Eng. 2024, 107, 102457. [Google Scholar] [CrossRef]
- Rosati, S.; Maiuro, L.; Lombardi, S.J.; Iaffaldano, N.; Di Iorio, M.; Cariglia, M.; Sorrentino, E. Integrated Biotechnological Strategies for the Sustainability and Quality of Mediterranean Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus aurata). Foods 2025, 14, 1020. [Google Scholar] [CrossRef]
- Kyrana, V.R.; Lougovois, V.P. Sensory, chemical and microbiological assessment of farm-raised European sea bass (Dicentrarchus labrax) stored in melting ice. Int. J. Food Sci. Technol. 2002, 37, 319–328. [Google Scholar] [CrossRef]
- Zoli, M.; Rossi, L.; Costantini, M.; Bibbiani, C.; Fronte, B.; Brambilla, F.; Bacenetti, J. Quantification and characterization of the environmental impact of sea bream and sea bass production in Italy. Clean. Environ. Syst. 2023, 9, 100118. [Google Scholar] [CrossRef]
- Chen, J.M.; Chen, J.C. Study on the free amino acid levels in the hemolymph, gill, hepatopancreas and muscle of Penaeus monodon exposed to elevated ambient ammonia. Aquat. Toxicol. 2020, 50, 27–37. [Google Scholar] [CrossRef]
- Cortés-Sánchez, A.D.J.; Diaz-Ramírez, M.; Torres-Ochoa, E.; Espinosa-Chaurand, L.D.; Rayas-Amor, A.A.; Cruz-Monterrosa, R.G.; Salgado-Cruz, M.D.L.P. Processing, quality and elemental safety of fish. Appl. Sci. 2024, 14, 2903. [Google Scholar] [CrossRef]
- Tarricone, S.; Ragni, M.; Carbonara, C.; Giannico, F.; Bozzo, F.; Petrontino, A.; Colonna, M.A. Growth Performance and Flesh Quality of Sea Bass (Dicentrarchus labrax) Fed with Diets Containing Olive Oil in Partial Replacement of Fish Oil—With or Without Supplementation with Rosmarinus officinalis L. Essential Oil. Animals 2024, 14, 3237. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Pereira, J.; Lemoine, A.; Neubauer, P.; Junne, S. Perspectives for improving circular economy in brackish shrimp aquaculture. Aquac. Res. 2022, 53, 1169–1180. [Google Scholar] [CrossRef]
- Le Féon, S.; Dubois, T.; Jaeger, C.; Wilfart, A.; Akkal-Corfini, N.; Bacenetti, J.; Aubin, J. DEXiAqua, a model to assess the sustainability of aquaculture systems: Methodological development and application to a French Salmon Farm. Sustainability 2021, 13, 7779. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Chary, K.; van Riel, A.J.; Muscat, A.; Wilfart, A.; Harchaoui, S.; Verdegem, M.; Wiegertjes, G.F. Transforming sustainable aquaculture by applying circularity principles. Rev. Aquac. 2024, 16, 656–673. [Google Scholar] [CrossRef]
- Tefal, E.; Jauralde, I.; Martínez-Llorens, S.; Tomás-Vidal, A.; Milián-Sorribes, M.C.; Moyano, F.J.; Jover-Cerdá, M. Organic ingredients as alternative protein sources in the diet of juvenile organic seabass (Dicentrarchus labrax). Animals 2023, 13, 3816. [Google Scholar] [CrossRef]
- Hodar, A.R.; Vasava, R.J.; Mahavadiya, D.R.; Joshi, N.H. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zool. India 2020, 23, 13–21. [Google Scholar]
- Milián-Sorribes, M.C.; Martínez-Llorens, S.; Cruz-Castellón, C.; Jover-Cerdá, M.; Tomás-Vidal, A. Effect of fish oil replacement and probiotic addition on growth, body composition and histological parameters of yellowtail (Seriola dumerili). Aquac. Nutr. 2021, 27, 3–16. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Preliminary nutritional evaluation of Mucuna seed meal (Mucuna pruriens var. utilis) in common carp (Cyprinus carpio L.): An assessment by growth performance and feed utilisation. Aquaculture 2001, 196, 105–123. [Google Scholar] [CrossRef]
- Reigh, R.C. Underutilized and Unconventional Plant Protein Supplements. In Alternatives Protein Sources in Aquaculture Diets; Lim, C., Webster, C.D., Lee, C.-S., Eds.; The Howarth Press: New York, NY, USA, 2008; pp. 433–447. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Sæther, B.S.; Albrektsen, S.; Noble, C. Review on European sea bass (Dicentrarchus labrax, Linnaeus, 1758) nutrition and feed management: A practical guide for optimizing feed formulation and farming protocols. Aquac. Nutr. 2015, 21, 129–151. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal alternative protein sources for aquaculture feeds. Feed. Aquac. Sect. Curr. Situat. Altern. Sources 2018, 1–28. [Google Scholar] [CrossRef]
- Storebakken, T.; Refstie, S.; Ruyter, B. Soy Products as Fat and Protein Sources in Fish Feeds for Intensive Aquaculture. In Soy in Animal Nutrition; Drackley, J.K., Ed.; Federation of Animal Science Societies: Savoy, IL, USA, 2000; pp. 127–170. [Google Scholar]
- Randazzo, B.; Di Marco, P.; Zarantoniello, M.; Daniso, E.; Cerri, R.; Finoia, M.G.; Cardinaletti, G. Effects of supplementing a plant protein-rich diet with insect, crayfish or microalgae meals on gilthead sea bream (Sparus aurata) and European seabass (Dicentrarchus labrax) growth, physiological status and gut health. Aquaculture 2023, 575, 739811. [Google Scholar] [CrossRef]
- Tibaldi, E.; Hakim, Y.; Uni, Z.; Tulli, F.; de Francesco, M.; Luzzana, U.; Harpaz, S. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax). Aquaculture 2006, 261, 182–193. [Google Scholar] [CrossRef]
- Li, Y.; Ai, Q.; Mai, K.; Xu, W.; Cheng, Z. Effects of the partial substitution of dietary fish meal by two types of soybean meals on the growth performance of juvenile Japanese seabass, Lateolabrax japonicus (Cuvier 1828). Aquac. Res. 2012, 43, 458–466. [Google Scholar] [CrossRef]
- Deng, J.; Mai, K.; Ai, Q.; Zhang, W.; Wang, X.; Xu, W.; Liufu, Z. Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2006, 258, 503–513. [Google Scholar] [CrossRef]
- Chen, W.; Ai, Q.; Mai, K.; Xu, W.; Liufu, Z.; Zhang, W.; Cai, Y. Effects of dietary soybean saponins on feed intake, growth performance, digestibility and intestinal structure in juvenile Japanese flounder (Paralichthys olivaceus). Aquaculture 2011, 318, 95–100. [Google Scholar] [CrossRef]
- Zubiaurre, C. The Current Status and Future Perspectives of European Organic Aquaculture. Aquac. Eur. Eur. Aquac. Soc. 2013, 38, 14–21. [Google Scholar]
- EUMOFA—European Market Observatory for Fisheries and Aquaculture. Organic Aquaculture in the Eu. In Current Situation, Drivers, Barriers, Potential for Growth; EUMOFA: Brussels, Belgium, 2022; ISBN 9789276476221. [Google Scholar]
- Willer, H.; Trávníček, J.; Claudia, M.; Schlatter, B. (Eds.) The World of Organic Agriculture-Statistics & Emerging Trends; IFOAM: Bonn, Germany, 2021; ISBN 9783037363935. [Google Scholar]
- Castellini, A.; Mauracher, C.; Procidano, I.; Sacchi, G. Italian market of organic wine: A survey on production system characteristics and marketing strategies. Wine Econ. Policy 2014, 3, 71–80. [Google Scholar] [CrossRef]
- Mauracher, C.; Tempesta, T.; Vecchiato, D. Consumer preferences regarding the introduction of new organic products. The case of the Mediterranean Sea bass (Dicentrarchus labrax) in Italy. Appetite 2013, 63, 84–91. [Google Scholar] [CrossRef]
- Gould, D.; Compagnoni, A.; Lembo, G. Organic aquaculture: Principles, standards and certification. In Organic Aquaculture: Impacts and Future Developments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–22. [Google Scholar]
- Tefal, E.; Jauralde, I.; Tomás-Vidal, A.; Martínez-Llorens, S.; Peñaranda, D.S.; Jover-Cerdá, M. New organic raw materials for gilthead seabream (Sparus aurata) feeding and the effects on growth, nutritive parameters, digestibility, and histology. Fishes 2023, 8, 330. [Google Scholar] [CrossRef]
- Tefal, E.; Tomás-Vidal, A.; Martínez-Llorens, S.; Jauralde, I.; Sánchez-Peñaranda, D.; Jover-Cerdá, M. Effects of Eco-Organic Feed on Growth Performance, Biometric Indices, and Nutrient Retention of Gilthead Seabream (Sparus aurata). Sustainability 2023, 15, 10750. [Google Scholar] [CrossRef]
- Tefal, E.; Peñaranda, D.S.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jauralde, I.; Lagos, L.; Jover-Cerdá, M. Feeding of rainbow trout (Oncorhynchus mykiss) with organic ingredients replacing fish meal. Aquaculture 2024, 592, 741257. [Google Scholar] [CrossRef]
- Cottee, S.Y.; Petersan, P. Animal welfare and organic aquaculture in open systems. J. Agric. Environ. Ethics 2009, 22, 437–461. [Google Scholar] [CrossRef]
- Lembo, G.; Mente, E. Organic Aquaculture; Springer International Publishing: Cham, Suiza, 2019; ISBN 978-3-030-05603-2. [Google Scholar]
- Mente, E.; Karalazos, V.; Karapanagiotidis, I.T.; Pita, C. Nutrition in organic aquaculture: An investigation and a discourse: Nutrition in organic aquaculture. Aquac. Nutr. 2011, 17, 798–817. [Google Scholar] [CrossRef]
- Mente, E.; Stratakos, A.; Boziaris, I.S.; Kormas, K.A.; Karalazos, V.; Karapanagiotidis, I.T.; Leondiadis, L. The effect of organic and conventional production methods on sea bream growth, health and body composition: A field experiment. Sci. Mar. 2012, 76, 549–560. [Google Scholar] [CrossRef]
- Angel, D.; Jokumsen, A.; Lembo, G. Aquaculture Production Systems and Environmental Interactions. In Organic Aquaculture: Impacts and Future Developments; Lembo, G., Mente, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 103–118. ISBN 978-3-030-05603-2. [Google Scholar] [CrossRef]
- Beg, M.M.; Roy, S.M.; Moulick, S.; Mandal, B.; Kim, T.; Mal, B.C. Economic feasibility study of organic and conventional fish farming systems of Indian major carps. Sci. Rep. 2024, 14, 7001. [Google Scholar] [CrossRef]
- Council Regulation (EC) No. 834/2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No. 2092/91. Off. J. Eur. Union 2007, 189, 1–23.
- Commission Regulation (EC) No 710/2009 Amending Regulation (EC) No 889/2008 Laying Down Detailed Rules for the Application of Regulation (EC) No 834/2007 as Regards the Laying Down of Detailed Rules for the Organic Production of Aquaculture Animals and Seaweed 2009. Available online: http://data.europa.eu/eli/reg/2009/710/oj (accessed on 2 May 2025).
- EU Regulation. Regulation (EU) 2018/848 of the European Parliament and of the Council. Official Journal of the European Union, 150/92. B Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32018R0848 (accessed on 2 May 2025).
- Lunger, A.N.; Craig, S.; McLean, E. Replacement of fish meal in cobia (Rachycentron canadum) diets using an organically certified protein. Aquaculture 2006, 257, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Trocino, A.; Xiccato, G.; Majolini, D.; Tazzoli, M.; Bertotto, D.; Pascoli, F.; Palazzi, R. Assessing the quality of organic and conventionally-farmed European sea bass (Dicentrarchus labrax). Food Chem. 2012, 131, 427–433. [Google Scholar] [CrossRef]
- Berge, G.M.; Jokumsen, A.; Lembo, G.; Spedicato, M.T. Challenges in Sourcing of Feed Ingredients for Organic Production of Carnivorous Fish. In Proceedings of the Aquaculture Europe, Rotterdam, The Netherlands, 20–23 October 2015. [Google Scholar]
- Di Marco, P.; Petochi, T.; Marino, G.; Priori, A.; Finoia, M.G.; Tomassetti, P.; Poli, B.M. Insights into organic farming of European sea bass Dicentrarchus labrax and gilthead sea bream Sparus aurata through the assessment of environmental impact, growth performance, fish welfare and product quality. Aquaculture 2017, 471, 92–105. [Google Scholar] [CrossRef]
- Pascoli, F.; Negrato, E.; De Lazzaro, P.; Poltronieri, C.; Radaelli, G.; Bertotto, D. Organic versus Conventional Sea Bass Aquaculture: Results from a Monitoring Study on Fish Welfare. In Proceedings of the EAS-WAS Conference-Aqua, Prague, Czech Republic, 1–5 September 2012; p. 855. [Google Scholar]
- Tacon, A.G.J.; Jackson, A.J.; Cowey, C.B.; Mackie, A.M.; Bell, J.G. Nutrition and Feeding in Fish; Academic Press: London, UK, 1985; pp. 119–145. [Google Scholar]
- Adelizi, P.D.; Rosati, R.R.; Warner, K.; Wu, Y.V.; Muench, T.R.; White, M.R.; Brown, P.B. Evaluation of fish-meal free diets for rainbow trout, Oncorhynchus mykiss. Aquac. Nutr. 1998, 4, 255–262. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Gatlin, D.M., III; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Wurtele, E. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Slawski, H.; Adem, H.; Tressel, R.P.; Wysujack, K.; Koops, U.; Kotzamanis, Y.; Schulz, C. Total fish meal replacement with rapeseed protein concentrate in diets fed to rainbow trout (Oncorhynchus mykiss Walbaum). Aquac. Int. 2012, 20, 443–453. [Google Scholar] [CrossRef]
- Tusche, K.; Wuertz, S.; Susenbeth, A.; Schulz, C. Feeding fish according to organic aquaculture guidelines EC 710/2009: Influence of potato protein concentrates containing various glycoalkaloid levels on health status and growth performance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2011, 319, 122–131. [Google Scholar] [CrossRef]
- Ballestrazzi, R.; Lanari, D.; D’Agaro, E.; Mion, A. The effect of dietary protein level and source on growth, body composition, total ammonia and reactive phosphate excretion of growing sea bass (Dicentrarchus labrax). Aquaculture 1994, 127, 197–206. [Google Scholar] [CrossRef]
- Tulli, F.; Tibaldi, E. Apparent nutrient digestibility of different protein sources for sea bass (Dicentrarchus labrax). In Proceedings of the 14th National People’s Congress, ASPA, Firenze, Italy, 12–15 June 2001; pp. 697–699. [Google Scholar]
- Altan, Ö.; Korkut, A.Y. Appearent digestibility of plant protein-based diets by European sea bass Dicentrarchus labrax L. 1758. Turk. J. Fish. Aquat. Sci. 2011, 11, 87–92. [Google Scholar] [CrossRef]
- Azeredo, R.; Machado, M.; Kreuz, E.; Wuertz, S.; Oliva-Teles, A.; Enes, P.; Costas, B. The European seabass (Dicentrarchus labrax) innate immunity and gut health are modulated by dietary plant-protein inclusion and prebiotic supplementation. Fish Shellfish. Immunol. 2017, 60, 78–87. [Google Scholar] [CrossRef]
- Bonvini, E.; Parma, L.; Badiani, A.; Fontanillas, R.; Gatta, P.P.; Sirri, F.; Nannoni, E.; Bonaldo, A. Integrated study on production performance and quality traits of European sea bass (Dicentrarchus labrax) fed high plant protein diets. Aquaculture 2018, 484, 126–132. [Google Scholar] [CrossRef]
- Estruch, G.; Collado, M.C.; Monge-Ortiz, R.; Tomás-Vidal, A.; Jover-Cerdá, M.; Peñaranda, D.S.; Martínez-Llorens, S. Long-term feeding with high plant protein-based diets in gilthead seabream (Sparus aurata, L.) leads to changes in the inflammatory and immune related gene expression at intestinal level. BMC Vet. Res. 2018, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Estruch, G.; Martínez-Llorens, S.; Tomás-Vidal, A.; Monge-Ortiz, R.; Jover-Cerdá, M.; Brown, P.B.; Peñaranda, D.S. Impact of high dietary plant protein with or without marine ingredients in gut mucosa proteome of gilthead seabream (Sparus aurata, L.). J. Proteom. 2020, 216, 103672. [Google Scholar] [CrossRef]
- Pérez-Pascual, D.; Estellé, J.; Dutto, G.; Rodde, C.; Bernardet, J.F.; Marchand, Y.; Ghigo, J.M. Growth performance and adaptability of European sea bass (Dicentrarchus labrax) gut microbiota to alternative diets free of fish products. Microorganisms 2020, 8, 1346. [Google Scholar] [CrossRef]
- Kotzamanis, Y.; Tsironi, T.; Brezas, A.; Grigorakis, K.; Ilia, V.; Vatsos, I.; Romano, N.; van Eys, J.; Kumar, V. High taurine supplementation in plant protein-based diets improves growth and organoleptic characteristics of European seabass (Dicentrarchus labrax). Sci. Rep. 2020, 10, 12294. [Google Scholar] [CrossRef]
- Mastoraki, M.; Panteli, N.; Kotzamanis, Y.P.; Gasco, L.; Antonopoulou, E.; Chatzifotis, S. Nutrient digestibility of diets containing five different insect meals in gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax). Anim. Feed. Sci. Technol. 2022, 292, 115425. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analiytical Chemistry. In Official method of Analysis, 16th ed.; Hoorwitz, N., Chialo, P., Reynold, H., Eds.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Bosch, L.; Alegría, A.; Farré, R. Application of the 6-aminoquinolyl-N-hydroxysccinimidyl carbamate (AQC) reagent to the RP-HPLC determination of amino acids in infant foods. J. Chromatogr. 2006, 831, 176–183. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmeyer, B.A.; Cohen, S.A.; Tarvin, T.L.; Frost, B. A new, rapid, high sensitivity analysis of amino acids in food type samples. J. AOAC 1987, 70, 241–247. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Telahigue, K.; Hajji, T.; Rabeh, I.; El Cafsi, M. The changes of fatty acid composition in sun dried, oven dried and frozen hake (Merluccius merluccius) and sardinella (Sardinella aurita). Afr. J. Biochem. Res 2013, 7, 158–164. Available online: https://academicjournals.org/journal/ajbr/article-full-text-pdf/4ef736a11679 (accessed on 2 May 2025).
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M.J. Mercury, fatty acids content and lipid quality indexes in muscles of freshwater and marine fish on the polish market. Risk assessment of fish consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B. Health risk assessment of heavy metals and lipid quality indexes in freshwater fish from lakes of Warmia and Mazury region, Poland. Int. J. Environ. Res. Public Health 2019, 16, 3780. [Google Scholar] [CrossRef]
- Tarricone, S.; Caputi Jambrenghi, A.; Cagnetta, P.; Ragni, M. Wild and farmed sea bass (Dicentrarchus labrax): Comparison of biometry traits, chemical and fatty acid composition of fillets. Fishes 2022, 7, 45. [Google Scholar] [CrossRef]
- Marques, A.; Canada, P.; Costa, C.; Basto, A.; Piloto, F.; Salgado, M.A.; Valente, L.M. Replacement of fish oil by alternative n-3 LC-PUFA rich lipid sources in diets for European sea bass (Dicentrarchus labrax). Front. Mar. Sci. 2023, 10, 1189319. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Roscini, V.; Castellini, C. Fillet fatty acid composition, estimated indexes of lipid metabolism and oxidative status of wild and farmed brown trout (Salmo trutta L.). Ital. J. Food Sci. 2013, 25, 83–89. [Google Scholar]
- Fernandes, C.E.; da Silva Vasconcelos, M.A.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; de Melo Filho, A.B. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef]
- Tonial, I.B.; Oliveira, D.D.; Coelho, A.R.; Matsushita, M.; Coró, F.A.G.; Souza, N.D.; Visentainer, J.V. Quantification of essential fatty acids and assessment of the nutritional quality indexes of lipids in tilapia alevins and juvenile tilapia fish (Oreochromis niloticus). J. Food Res. 2014, 3, 105–114. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and distribution of omega-3 fatty acids in South Pacific fish and shellfish species. Foods 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Krajnović-Ozretic, M.; Najdek, M.; Ozretić, B. Fatty acids in liver and muscle of farmed and wild sea bass (Dicentrarchus labrax L.). Comp. Biochem. Physiol. Part A Physiol. 1994, 109, 611–617. [Google Scholar] [CrossRef]
- Senso, L.; Suárez, M.D.; Ruiz-Cara, T.; García-Gallego, M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Calanche Morales, J.B.; Tomás-Vidal, A.; Cusiyunca Phoco, E.R.; Martínez-Llorens, S.; Marquina, P.L.; Jover-Cerdá, M.; Beltrán, J.A. An Approach to the Spanish Consumer’s Perception of the Sensory Quality of Environmentally Friendly Seabass. Foods 2021, 10, 2694. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Moñino, A.V.; Tomás Vidal, A.; Salvador, V.J.M.; Pla Torres, M.; Jover Cerdá, M. Soybean meal as a protein source in gilthead sea bream (Sparus aurata L.) diets: Effects on growth and nutrient utilization. Aquac. Res. 2007, 38, 82–90. [Google Scholar] [CrossRef]
- Jauralde, I.; Martínez-Llorens, S.; Tomás, A.; Ballestrazzi, R.; Jover, M. A proposal for modelling the thermal-unit growth coefficient and feed conversion ratio as functions of feeding rate for gilthead sea bream (Sparus aurata, L.) in summer conditions. Aquac. Res. 2013, 44, 242–253. [Google Scholar] [CrossRef]
- Tarricone, S.; Iaffaldano, N.; Colonna, M.A.; Giannico, F.; Selvaggi, M.; Caputi Jambrenghi, A.; Ragni, M. Effects of dietary red grape extract on the quality traits in juvenile European sea bass (Dicentrarchus labrax L.). Animals 2023, 13, 254. [Google Scholar] [CrossRef]
- Monteiro, M.; Matos, E.; Ramos, R.; Campos, I.; Valente, L.M. A blend of land animal fats can replace up to 75% fish oil without affecting growth and nutrient utilization of European seabass. Aquaculture 2018, 487, 22–31. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F.J.L.P.S. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Candela, C.G.; López, L.B.; Kohen, V.L. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health. Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. Available online: https://www.redalyc.org/pdf/3092/309226770013.pdf (accessed on 2 May 2025).
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Chang, N.W.; Huang, P.C. Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids 1998, 33, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Majdoub-Mathlouthi, L.; Saïd, B.; Kraiem, K. Carcass traits and meat fatty acid composition of Barbarine lambs reared on rangelands or indoors on hay and concentrate. Animal 2015, 9, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.J.; Coves, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Adamidou, S.; Nengas, I.; Alexis, M.; Foundoulaki, E.; Nikolopoulou, D.; Campbell, P.; Jauncey, K. Apparent nutrient digestibility and gastrointestinal evacuation time in European seabass (Dicentrarchus labrax) fed diets containing different levels of legumes. Aquaculture 2009, 289, 106–112. [Google Scholar] [CrossRef]
- Lund, I.; Dalsgaard, J.; Rasmussen, H.T.; Holm, J.; Jokumsen, A. Replacement of fish meal with a matrix of organic plant proteins in organic trout (Oncorhynchus mykiss) feed, and the effects on nutrient utilization and fish performance. Aquaculture 2011, 321, 259–266. [Google Scholar] [CrossRef]
- Estévez, A.; Vasilaki, P. Organic production of gilthead sea bream (Sparus aurata) using organic certified green pea protein and seaweed. Effects on growth, feed conversion and final product quality. Aquaculture 2023, 571, 739490. [Google Scholar] [CrossRef]
- Gaylord, T.G.; Barrows, F.T.; Teague, A.M.; Johansen, K.A.; Overturf, K.E.; Shepherd, B. Supplementation of taurine and methionine to all-plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 269, 514–524. [Google Scholar] [CrossRef]
- Cooney, R.; de Sousa, D.B.; Fernández-Ríos, A.; Mellett, S.; Rowan, N.; Morse, A.P.; Hayes, M.; Laso, J.; Regueiro, L.; Wan, A.H.; et al. A circular economy framework for seafood waste valorisation to meet challenges and opportunities for intensive production and sustainability. J. Clean. Prod. 2023, 392, 136283. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Psofakis, P.; Mente, E.; Malandrakis, E.; Golomazou, E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac. Nutr. 2019, 25, 3–14. [Google Scholar] [CrossRef]
- Sabbagh, M.; Schiavone, R.; Brizzi, G.; Sicuro, B.; Zilli, L.; Vilella, S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture 2019, 511, 734220. [Google Scholar] [CrossRef]
- Dias, J.; Alvarez, M.J.; Arzel, J.; Corraze, G.; Diez, A.; Bautista, J.M.; Kaushik, S.J. Dietary protein source affects lipid metabolism in the European seabass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Piccolo, G.; Tulli, F.; Messina, C.M.; Cardinaletti, G.; Tibaldi, E. Lipid composition and metabolism of European sea bass (Dicentrarchus labrax L.) fed diets containing wheat gluten and legume meals as substitutes for fish meal. Aquaculture 2013, 376, 6–14. [Google Scholar] [CrossRef]
- Calanche, J.; Tomas, A.; Martinez, S.; Jover, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Relation of quality and sensory perception with changes in free amino acids of thawed seabream (Sparus aurata). Food Res. Int. 2019, 119, 126–134. [Google Scholar] [CrossRef]
- Mourente, G.; Bell, J.G. Partial replacement of dietary fish oil with blends of vegetable oils (rapeseed, linseed and palm oils) in diets for European sea bass (Dicentrarchus labrax L.) over a long term growth study: Effects on muscle and liver fatty acid composition and effectiveness of a fish oil finishing diet. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 145, 389–399. [Google Scholar] [CrossRef]
- Rodríguez, C.; Acosta, C.; Badía, P.; Cejas, J.R.; Santamaría, F.J.; Lorenzo, A. Assessment of lipid and essential fatty acids requirements of black seabream (Spondyliosoma cantharus) by comparison of lipid composition in muscle and liver of wild and captive adult fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 619–629. [Google Scholar] [CrossRef]
- Sargent, J.; Bell, G.; McEvoy, L.; Tocher, D.; Estevez, A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture 1999, 177, 191–199. [Google Scholar] [CrossRef]
- Zheng, X.; Seiliez, I.; Hastings, N.; Tocher, D.R.; Panserat, S.; Dickson, C.A.; Bergot, P.; Teale, A.J. Characterization and comparison of fatty acyl Δ6 desaturase cDNAs from freshwater and marine teleost fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 269–279. [Google Scholar] [CrossRef]
- Testi, S.; Bonaldo, A.; Gatta, P.P.; Badiani, A. Nutritional traits of dorsal and ventral fillets from three farmed fish species. Food Chem. 2006, 98, 104–111. [Google Scholar] [CrossRef]
- Baki, B.; Gönener, S.; Kaya, D. Comparison of food, amino acid and fatty acid compositions of wild and cultivated sea bass (Dicentrarchus labrax L. 1758). Turk. J. Fish. Aquat. Sci. 2015, 15, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Carvalho, M.; Terova, G.; Fontanillas, R.; Serradell, A.; Ginés, R.; Torrecillas, S. Nutritional innovations in superior European sea bass (Dicentrarchus labrax) genotypes: Implications on fish performance and feed utilization. Aquaculture 2023, 572, 739486. [Google Scholar] [CrossRef]
- Kabeya, N.; Fonseca, M.M.; Ferrier, D.E.; Navarro, J.C.; Bay, L.K.; Francis, D.S.; Monroig, Ó. Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Sci. Adv. 2018, 4, eaar6849. [Google Scholar] [CrossRef] [PubMed]
- Monroig, Ó.; Lopes-Marques, M.; Navarro, J.C.; Hontoria, F.; Ruivo, R.; Santos, M.M.; Castro, L.F.C. Evolutionary functional elaboration of the Elovl2/5 gene family in chordates. Sci. Rep. 2016, 6, 20510. [Google Scholar] [CrossRef] [PubMed]
- Glencross, B.D.; Bachis, E.; Betancor, M.B.; Calder, P.; Liland, N.; Newton, R.; Ruyter, B. Omega-3 futures in aquaculture: Exploring the supply and demands for long-chain omega-3 essential fatty acids by aquaculture species. Rev. Fish. Sci. Aquac. 2025, 33, 167–216. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- Lee, S.M. Review of the lipid and essential fatty acid requirements of rockfish (Sebastes schlegeli). Aquac. Res. 2001, 32, 8–17. [Google Scholar] [CrossRef]
- Monge-Ortiz, R.; Tomás-Vidal, A.; Rodriguez-Barreto, D.; Martínez-Llorens, S.; Pérez, J.A.; Jover-Cerdá, M.; Lorenzo, A. Replacement of fish oil with vegetable oil blends in feeds for greater amberjack (Seriola dumerili) juveniles: Effect on growth performance, feed efficiency, tissue fatty acid composition and flesh nutritional value. Aquac. Nutr. 2018, 24, 605–615. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Changes in free amino acids during chilled storage of hake (Merluccius merluccius L.) in controlled atmospheres and their use as a quality control index. Eur. Food Res. Technol. 2001, 212, 302–307. [Google Scholar] [CrossRef]
- De Francesco, M.; Parisi, G.; Pérez-Sanchez, J.; Gomez-Réqueni, P.; Médale, F.; Kaushik, S.J.; Poli, B.M. Effect of high-level fish meal replacement by plant proteins in gilthead sea bream (Sparus aurata) on growth and body/fillet quality traits. Aquac. Nutr. 2007, 13, 361–372. [Google Scholar] [CrossRef]
- Rabie, M.; Simon-Sarkadi, L.; Siliha, H.; El-seedy, S.; El Badawy, A.A. Changes in free amino acids and biogenic amines of Egyptian salted-fermented fish (Feseekh) during ripening and storage. Food Chem. 2009, 115, 635–638. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, M.; Jin, Y.; Deng, S.; Tao, N.; Qiu, W. Simultaneous detection and analysis of free amino acids and glutathione in different shrimp. Foods 2022, 11, 2599. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wu, P.; Tang, R.J.; Liu, Y.; Kuang, S.Y.; Jiang, J.; Feng, L. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar] [CrossRef]
- Risso, S.J.; Carelli, A.A. Nutrient composition of raw and cooked meat of male southern king crab (Lithodes santolla Molina, 1782). J. Aquat. Food Prod. Technol. 2012, 21, 433–444. [Google Scholar] [CrossRef]
- Ahmed, J.; Habeebullah, S.F.K.; Alagarsamy, S.; Mulla, M.Z.; Thomas, L. Impact of high-pressure treatment on amino acid profile, fatty acid compositions, and texture of yellowfin seabream (Acanthopagrus arabicus) filets. Front. Sustain. Food Syst. 2022, 6, 857072. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Chung, W.H.; Howieson, J.; Fotedar, R. A combination of Hermetia illucens reared on fish waste and poultry by-product meal improves sensory and physicochemical quality of farmed Barramundi filets. Front. Nutr. 2022, 8, 788064. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, A.L.; Xian, J.A. Variation of free amino acid and carbohydrate concentrations in white shrimp, Litopenaeus vannamei: Effects of continuous cold stress. Aquaculture 2011, 317, 182–186. [Google Scholar] [CrossRef]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Montero, D.; Robaina, L.; Caballero, M.J.; Ginés, R.; Izquierdo, M.S. Growth, feed utilization and flesh quality of European sea bass (Dicentrarchus labrax) fed diets containing vegetable oils: A time-course study on the effect of a re-feeding period with a 100% fish oil diet. Aquaculture 2005, 248, 121–134. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Nomikos, T.; Demopoulos, C.A.; Zabetakis, I. Comparison of antiatherogenic properties of lipids obtained from wild and cultured sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem. 2007, 100, 560–567. [Google Scholar] [CrossRef]
- Poli, B.M.; Parisi, G.; Zampacavallo, G.; Mecatti, M.; Lupi, P.; Gualtieri, M.; Franci, O. Quality outline of European sea bass (Dicentrarchus labrax) reared in Italy: Shelf life, edible yield, nutritional and dietetic traits. Aquaculture 2001, 202, 303–315. [Google Scholar] [CrossRef]
- Siddiqua, K.S.; Khan, M.A. Replacement of fish oil with groundnut oil for developing sustainable feeds for Labeo rohita fingerling. Front. Sustain. Food Syst. 2022, 6, 862054. [Google Scholar] [CrossRef]
- Pérez, J.A.; Rodríguez, C.; Bolaños, A.; Cejas, J.R.; Lorenzo, A. Beef tallow as an alternative to fish oil in diets for gilthead sea bream (Sparus aurata) juveniles: Effects on fish performance, tissue fatty acid composition, health and flesh nutritional value. Eur. J. Lipid Sci. Technol. 2014, 116, 571–583. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).