Simple Summary
This study explores how low-protein amino acid-balanced (LPAB) diets with Astragalus polysaccharides (APSs) influence laying hens under heat stress. The results show that APSs and LPAB diets can mitigate the adverse effects of heat stress on hens. APSs mainly regulate the secretion of gonadotropin by reducing the concentration of corticosterone in serum, thereby increasing the concentration of follicle-stimulating hormone (FSH) and FSH receptor (FSHR) mRNA expression to regulate primary follicle development and increase the egg production rate. While low-protein diets improve yolk color and boost FSH, they also decrease immunoglobulin and triglyceride levels. Adding APSs enhances immunoglobulin concentrations and helps recover cholesterol levels. LPAB diets and APSs can both enhance cholesterol uptake and suppress its synthesis. Overall, LPAB diets and APSs are deemed valuable feeding strategies for improving production performance and alleviating heat stress in laying hens.
Abstract
The objective of the study was to investigate the effects of low-protein amino acid-balanced (LPAB) diets supplemented with Astragalus polysaccharides (APSs) on the production performance, antioxidants, immunity, and biochemical index of laying hens in an elevated-temperature environment. Fifty-two-week-old Hy-Line Brown chickens (n = 768) were randomly divided into four groups, with eight replicates of 24 hens each. The control group was kept at 24 °C with a basal diet (CON), while the treatment groups were exposed to 32 °C and given the following diets: basal (HB), LPAB (HL), and LPAB with 0.5% APSs (HLA). Under heat stress, APSs increased the egg production rate and number of small white follicles, improved the yolk color, and lowered the feed conversion ratio. LPAB diets increased follicle-stimulating hormone, antioxidant enzyme activities, and anti-inflammatory cytokine activity and up-regulated related genes, whereas they reduced stress-related hormones, malondialdehyde concentrations, and triglyceride concentrations and down-regulated related genes. The addition of APSs enhanced immunoglobulin concentrations and cholesterol recovery and altered the expression of related genes. The study found that the adverse effects of high temperatures are directly related to oxidative stress. LAPB diets and APSs relatively alleviate these adverse effects. Therefore, the importance of feeding strategies such as LPAB diets and APSs for laying hens under heat stress conditions has been identified.
1. Introduction
Rising ambient temperatures beyond the thermoneutral zone are a primary cause of heat stress in poultry, impairing performance [1], while in southern China, summer heat readily triggers acute or chronic stress in laying hens because they lack sweat glands and have dense plumage [2,3]. Heat stress directly damages proteins and indirectly generates ROS [4]. High temperatures also bring about adverse effects such as reduced feed conversion ratio (FCR), feed intake, body weight, egg production, egg weight, and egg quality, which can lead to severe egg loss [5,6]. In addition, high-temperature environments can lead to an increase in plasma corticosterone (CORT) concentrations and affect humoral and cellular immune responses, potentially leading to immune dysfunction [7,8]. Moreover, a study has shown that heat stress impairs leukocyte protein synthesis, lowers plasma immunoglobulin G (IgG), immunoglobulin M (IgM), and anti-inflammatory cytokine concentrations, and increases the levels of pro-inflammatory cytokines [9].
To counter high temperatures, farmers often use wet curtain cooling and mechanical ventilation. However, these temperature control systems are expensive and hard to maintain consistently in large or open-sided poultry houses. Crude protein has a higher thermic effect because it generates a greater caloric increment [10]. Reducing protein levels in laying hen diets and supplementing with synthetic amino acids to meet essential amino acid (EAA) needs may be a better strategy. Studies have shown that reducing dietary protein levels from 16.5% to 12% under heat stress conditions and supplementing EAAs can improve the resistance of laying hens to heat stress while maintaining laying performance [11]. Dietary supplementation of methionine and L-lysine can improve protein utilization and production performance [12,13].
In addition, Astragalus polysaccharides (APSs) play a role due to their anti-inflammatory, immune stimulation, and antioxidant effects [14,15]. In the APS group, the serum levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) were significantly decreased, and the expression of related genes was also down-regulated [16].
This study investigates the effects of APSs on the production performance, antioxidants, immunity, and lipid metabolism in heat-stressed laying hens based on a low-protein amino acid-balanced diet to provide a reference. This helps to develop a heat stress mitigation strategy that is more suitable for laying hen production systems.
2. Material and Methods
2.1. Experimental Design and Diets
In the same hen house, 768 Hy-Line Brown laying hens (52 weeks old), with nearly the same body weight, were randomly divided into 4 experimental groups, with 8 replicates in each group and 24 hens in each replicate. Laying hens were observed for 10 days to record individual egg production, feed intake, and health status. The experiment was conducted when the hens were between 52 and 58 weeks of age. According to the egg production, individuals with low egg production or seemingly weak egg production were excluded, and then, the remaining hens were randomly divided into groups. The control group (CON) was kept at a constant 24 ± 1 °C, and the treatment groups (TRT) were exposed to 32 ± 1 °C for 7 h per day (heat stress). Experimental laying hens were from Jurong Haoyuan Ecological Agriculture Technology Co., Ltd. (Zhenjiang, China). The control diet based on maize–soybean meal was formulated to supply the nutrient requirements of the birds [17] (Table 1); the crude protein (CP) level in the basal diet was 15.85%, and in the low-protein diet, it was 13.85%. The CON was fed with basal diet, while the TRT groups were fed with basal diet (HB), low-protein amino acid-balanced (LPAB) diet (HL), and LPAB diets supplemented with 0.5% APSs (HLA). During the experimental period, the hens had unrestricted access to feed and water under a lighting programme of 16 h light: 8 h dark. The chicken cage was a three-layer cage with a length of 40 cm × width of 37 cm × height of 37 cm. Each cage was equipped with a trough and two automatic water dispensers. The diets were prepared, respectively, and all the diets used the same batch of corn, soybean meal and other ingredients. The test additive was APSs powder, purity 98% (w/w), supplied by Xi’an Shennong Technology Co., Ltd. (Xi’an, China). The polysaccharide was extracted from dried Astragalus membranaceus roots by combined ethanol–water extraction, followed by deproteinization, decoloration and lyophilization, yielding a water-soluble, off-white powder. Immediately before diet preparation, APSs were first pre-blended with ten times its weight of corn meal to ensure homogeneity, then incorporated into the basal mash at 0.5% (w/w) using a horizontal ribbon mixer (5 min mixing time). Heat stress was applied in climate-controlled rooms. From 09:00 to 16:00 each day, temperature was maintained at 32 ± 1 °C and relative humidity at 65 ± 5% using a PID-regulated combination of electric heaters and a variable-speed wet-curtain system; outside this period the rooms were held at 24 ± 1 °C.

Table 1.
Composition and nutrient levels of basal diets (g/kg air-dry basis).
2.2. Animals and Sample Collection
During the experiment, the total egg production, total egg weight, feed intake and mortality number were recorded every day, and the average egg weight, egg production rate, breakage rate (broken egg number/total egg number), mortality rate, average daily feed intake and FCR were counted with repetition as the unit. Three eggs were randomly selected from each replicate on day 40, day 41 and day 42 for egg quality analysis. Eggshell strength was measured by Egg Force Reader (Orka Technology Ltd., West Bountiful, UT, USA). Eggshell thickness was measured by Egg Force Shell Thickness (Orka Technology Ltd., West Bountiful, UT, USA). The egg shape index was calculated as the vertical diameter divided by the horizontal diameter, following the NY/T 823-2020 method [18]. Egg yolk weight and egg weight were measured by electronic balance (precision, 0.01 g) to calculate the relative egg weight. The egg yolk color, albumen height and Haugh unit were measured by Egg NnalyzerTM (Orka Technology Ltd., West Bountiful, UT, USA). On day 42 of the experimental period, 24 healthy Hy-Line laying hens were randomly selected from each treatment, and blood samples were taken from their brachial veins. The blood samples were centrifuged at 826× g for 10 min to prepare serum, which was then divided into enzyme-free tubes and stored in a −20 °C freezer. Experimental laying hens were euthanized after cervical dislocation, the ovary and liver were removed after death, the ovary was rinsed with cold PBS, and the number of follicles in the ovaries was determined by diameter, small white follicle (SWF, 2–4 mm), big yellow follicle (BYF, 4–10 mm), graded follicle (GF, diameter > 10 mm) and the number of follicles in the ovaries was counted [19], and all visible hierarchical and pre-hierarchical follicles were carefully removed. The remaining ovarian tissue (excluding the follicles) was then rapidly frozen in liquid nitrogen and stored at −80 °C until RNA extraction for determining the mRNA expression of follicle-stimulating hormone receptor (FSHR), luteinizing hormone receptor (LHR), estrogen receptor 1 (ESR1), estrogen receptor 2 (ESR2), β-actin, superoxide dismutase (SOD1), nuclear factor erythroid 2-related factor 2 (Nrf2), glutathione peroxidase (GPx), tumor necrosis factor-α (TNF-α), IL-1β, interleukin-10 (IL-10), interferon-γ (IFN-γ), sterol regulatory element-binding protein (SREBP2), 3-hydroxy-3-methylglutaryl-CoA (HMGCR), low-density lipoprotein receptor (LDLR).
2.3. Measurement of Serum Hormone, Antioxidant, Immunoglobulin, Inflammatory and Biochemical Indicators
Serum concentration of adrenocorticotropic hormone (ACTH), CORT, estradiol (E2), FSH, LH and progesterone (P4) were measured using a one-step sandwich ELISA with a double antibody kit from Elabscience (Houston, TX, USA). Serum concentration of immunoglobulin A (IgA), IgG, and IgM, inflammatory factors IL-1β, interleukin-6 (IL-6), IL-10, monocyte chemokine-1 (MCP-1), IFN-γ, and tumor necrosis factor-α (TNF-α) were measured using ELISA kits purchased from Shanghai Yubo Biotechnology Co., Ltd. (Shanghai, China). The total protein concentration in the serum was measured using a kit, and the activities of total SOD (T-SOD), GPx, total antioxidant capacity (T-AOC), and malondialdehyde (MDA) were measured using kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Total protein (TP), albumin (ALB), globulin (GLB), serum total cholesterol (T-CHO), high-density lipoprotein cholesterol (HDL-C), LDL cholesterol (LDL-C), triglyceride (TG) and glucose (GLU) content, as well as the activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in the serum using a UniCel DXC 800 Synchron fully automated biochemical analyzer (Beckman Coulter Inc., Brea, CA, USA).
2.4. Expression of Gonadotropin-Releasing Hormone mRNA in the Liver and Ovaries
Total RNA isolation, cDNA synthesis, and quantitative PCR were performed according to the methods described in a previously published paper [20]. The specificity of the primers was confirmed by melting curve analysis. The reference gene was β-actin, and the primer sequences for each gene are listed in Table 2. The 2−ΔΔCt method was used to calculate the relative expression levels of the target genes.

Table 2.
Sequences of the primers for the target genes.
2.5. Statistical Analysis
Microsoft Excel 2019 (v. 1808, Microsoft, Redmond, WA, USA) was used for preliminary data processing. Statistical analysis was conducted using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). Prior to all parametric tests, the homogeneity of variances was assessed using Levene’s F-test. One-way analysis of variance (ANOVA) was performed to compare differences among the four experimental groups (CON, HB, HL, and HLA), followed by post hoc multiple comparisons using Tukey’s test. The experimental design was not a fully factorial arrangement. Specifically, the LPAB and APS treatments were only applied under heat stress conditions (32 °C), while the thermoneutral group (24 °C) received only the basal diet. Therefore, temperature, protein level, and APS supplementation were not treated as independent factors in a multifactorial model. Data were presented as mean and pooled standard error of the mean (SEM), and statistical significance was set at p < 0.05.
3. Results
3.1. Production Performance and Follicle Development
As presented in Table 3 and Table 4, compared with the CON, the breakage rate, mortality, average daily feed intake and average egg weight of HB, HL and HLA were not significantly different. In terms of egg production rate (EPR), the CON was the highest, and HB and HL were significantly decreased (p < 0.05). Compared with CON, HL and HLA, the FCR of HB worsened significantly (p < 0.05). The number of SWF in the CON, HL and HLA was more than HB (p < 0.05).

Table 3.
Effects of Astragalus polysaccharides in low-protein diet on production performance of laying hens.

Table 4.
Effects of Astragalus polysaccharides in low-protein diet on follicular development of laying hens.
3.2. Egg Quality and Egg Components
According to Table 5, compared to the CON, the LPAB diets deepened yolk color (p < 0.05). Temperature, protein level in the diets and APS addition had no significant effect on albumen height, Haugh unit, yolk ratio, eggshell strength, eggshell thickness and egg shape index (p > 0.05).

Table 5.
Effects of Astragalus polysaccharides in low-protein diet on egg quality.
3.3. Serum Hormone Indicators and Relevant Gene Expression in the Ovary
According to Table 6, the HB had higher concentration of ACTH, CORT, whereas FSH was lower compared to the other groups (p < 0.05). Additionally, the HL had higher concentration of CORT compared to CON and HLA (p < 0.05). Figure 1 demonstrated that the FSHR in the ovary was up-regulated in CON, HL and HLA (p < 0.05).

Table 6.
Effects of Astragalus polysaccharides on serum hormone indexes in low-protein diet under high-temperature environment.
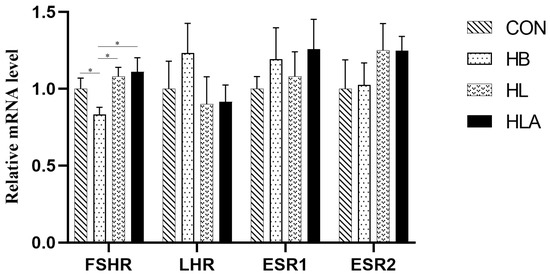
Figure 1.
Relative gene expression of productive hormone-related genes. CON: a group was maintained at 24 °C with a basal diet; HB: a group was exposed to 32 °C, fed basal diet; HL: a group was exposed to 32 °C, low-protein amino acid-balanced diet; HLA: a group was exposed to 32 °C low- protein amino acid-balanced diet with 0.5% Astragalus polysaccharides. n = 6; * indicates p < 0.05 relative to the control group. All data are expressed as mean ± SEM in the figure.
3.4. Serum Antioxidant Index and Relevant Gene Expression in the Liver
According to Table 7, T-SOD activity in CON was significantly higher than that in HB and HL groups (p < 0.05), and HLA was significantly higher than that in HB group (p < 0.05); MDA concentrations in the CON and HLA were lower than those in the HB and HL (p < 0.05), and the HLA was higher than the CON (p < 0.05). GPx concentration in the CON was higher than in the HB and HL (p < 0.05), the HB was lower than all other groups (p < 0.05), and the HLA showed no significant difference compared with CON and HL (p > 0.05). From Figure 2, the SOD1 and GPx in the liver were up-regulated in CON, HL and HLA compared to HB (p < 0.05).

Table 7.
Effects of Astragalus polysaccharides in low-protein diet on antioxidation performance of serum.
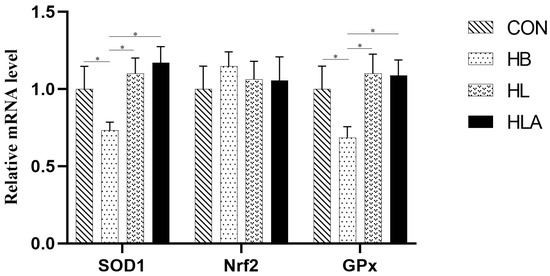
Figure 2.
The mRNA expression of antioxidant-related genes. CON: a group was maintained at 24 °C with a basal diet; HB: a group was exposed to 32 °C, fed basal diet; HL: a group was exposed to 32 °C, low-protein amino acid-balanced diet; HLA: a group was exposed to 32 °C low-protein amino acid-balanced diet with 0.5% Astragalus polysaccharides. n = 6; * indicates p < 0.05 relative to the control group. All data are expressed as mean ± SEM in the figure.
3.5. Serum Immunoglobulin, Inflammatory Cytokine Indicators and Relevant Gene Expression in the Liver
As presented in Table 8 and Table 9, the HLA had the highest concentrations of IgA, IgG, and IgM compared to the other groups (p < 0.05). Specifically, the CON had higher IgA and IgM concentration compared to the HB and HL (p < 0.05), while the HB had lower IgG and IgM concentration compared to the HL (p < 0.05). IL-1β concentration was higher in the HB compared to other groups (p < 0.05), while the HL and HLA were not significantly different from each other (p > 0.05), but were both higher than CON (p < 0.05). For IL-10, the HLA showed a higher concentration than the other groups (p < 0.05), which did not differ significantly from each other (p > 0.05). As shown in Figure 3, in the liver, the IL-1β mRNA level was highest in HB (p < 0.01), and was also higher in HL and HLA than in CON (p < 0.05). The relative expression level of IL-10 mRNA in HLA was significantly higher than that in other groups (p < 0.05).

Table 8.
Effects of Astragalus polysaccharides on serum immunoglobulin content of laying hens under high-temperature environment in low-protein diet.

Table 9.
Effects of Astragalus polysaccharides on serum inflammatory cytokines in laying hens under high-temperature environment in low-protein diet.
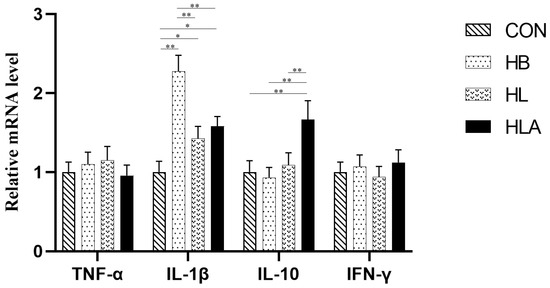
Figure 3.
The mRNA expression of inflammatory-related genes. CON: a group was maintained at 24 °C with a basal diet; HB: a group was exposed to 32 °C, fed basal diet; HL: a group was exposed to 32 °C, low-protein amino acid-balanced diet; HLA: a group was exposed to 32 °C low-protein amino acid-balanced diet with 0.5% Astragalus polysaccharides. n = 6; * indicates p < 0.05 and ** indicates p < 0.01 relative to the control group. All data are expressed as mean ± SEM in the figure.
3.6. Serum Biochemical Indicators and Relevant Gene Expression in the Liver
According to Table 10, T-CHO concentration was lowest in the CON compared to the other groups (p < 0.05). In addition, the HL and HLA had lower T-CHO concentrations and AST activity than the HB (p < 0.05). Moreover, the HL exhibited higher concentration of HDL-C than the CON (p < 0.05), while the HB showed higher concentration of LDL-C compared to the CON and HLA (p < 0.05). Regarding TG and GLU content, the CON had lower concentration than the TRT (p < 0.05), while the HL and HLA had lower concentration than the HB (p < 0.05). From Figure 4, in the liver, the HMGCR mRNA level was highest in HB (p < 0.01), and was also higher in HL and HLA than in CON (p < 0.05). LDLR was up-regulated in CON, HL and HLA compared to HB (p < 0.05), and also higher in HL and HLA than in CON (p < 0.05).

Table 10.
Effects of Astragalus polysaccharides on serum biochemical indexes under high-temperature environment in low-protein diet.
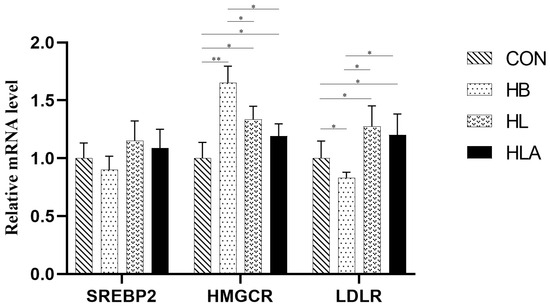
Figure 4.
The mRNA expression of reproductive lipid metabolism-related genes. CON: a group was maintained at 24 °C with a basal diet; HB: a group was exposed to 32 °C, fed basal diet; HL: a group was exposed to 32 °C, low-protein amino acid-balanced diet; HLA: a group was exposed to 32 °C low-protein amino acid-balanced diet with 0.5% Astragalus polysaccharides. n = 6; * indicates p < 0.05 and ** indicates p < 0.01 relative to the control group. All data are expressed as mean ± SEM in the figure.
4. Discussion
4.1. Production Performance, Follicle Development, Serum Hormone and Related Gene Expression in the Ovary
The results showed that heat stress led to a severe decline in EPR, and that low-protein amino acid-balanced diets and APSs offset some of the effects of heat stress, but still not to the same extent as the CON. Furthermore, the trend in FCR was consistent with EPR. Previous studies have shown that high environmental temperatures disadvantageously influence the production performance of birds, probably due to long-term behavioral, metabolic and physiological changes in response to heat stress [21,22]. A lot of research has shown that high dietary CP can result in reduced protein use and increased heat output, while restricting consumption of protein and adding synthetic DL-methionine and L-lysine could increase the efficiency of hens’ protein use and productivity [13,23]. CP has a higher thermal gain and is conducive to more metabolism heat production compared to fat and carbohydrate [24]. Therefore, a low-protein diet effectively mitigates heat stress and boosts protein efficiency in laying hens. This process is achieved by supplementing amino acids (AAs) in order to reduce feeding cost [25,26] and environmental pollution [27]. The mechanism of increased FCR in heat-stressed laying hens fed a low-CP diet is not clear. Reduction in dietary CP (1–3%) did not significantly affect the egg weight and egg production performance of laying hens with a balanced amino acid diet [28,29]. Moreover, it has been reported that if synthetic AA (methionine, lysine, threonine, tryptophan and ILE) is added, the CP in the egg diets can be reduced by 2% [30].
APS has many beneficial properties, such as anti-oxidation [31], immune regulation [32,33], anti-inflammatory [34], hypoglycemic [35] and other biological activities. For increased reactive oxygen species in laying hens due to heat stress, APS can be a kind of inhibiting agent to decrease the side effects of heat stress. Normal ovulation relies on the development of hierarchical follicles [36]. This study showed that the number of SWF in the HL and HLA groups was higher than in the HB group, which may be due to the APSs, which can promote blood circulation of laying hens and further promote follicular development. The LPAB diets can reduce the adverse effects of heat stress, and APSs also have antioxidant and anti-inflammatory effects, which have positive effects on protecting follicular health.
ACTH is the main regulator of adrenal nutrition and fascicular steroidogenesis [37]. Under the conditions of this experiment, the high-temperature environment significantly reduced the ACTH concentration in the serum of laying hens. In birds, gonadotropin-releasing hormone released by the hypothalamus stimulates pituitary secretion of FSH and LH, supporting follicular development and ovulation [38]. FSH and LH can promote the formation of oocytes by stimulating the production of ovarian gonadal hormones (such as E2, P4, etc.) [39]. Studies have shown that the release of ACTH and CORT in serum is affected under heat stress [40]. Studies have found that elevated serum ACTH and CORT concentration under heat stress conditions inhibit the secretion of FSH and LH [41]. In this experiment, the addition of APSs in the LPAB diet could reduce the concentration of ACTH and CORT in serum, which was basically consistent with the above results. It can be inferred that the possible mechanism is that the protein is metabolized in the body to provide precursor products for hormone synthesis, or it may be directly involved in the normal regulation of the hypothalamus–pituitary–ovarian axis that affects the secretion of gonadotropin, thereby reducing the synthesis, storage and secretion of FSH and LH, while APSs mainly regulate the secretion of gonadotropin by reducing the concentration of CORT in serum, thereby increasing the concentration of FSH and FSHR mRNA expression to regulate primary follicle development and increase egg production rate.
4.2. Effects of Astragalus Polysaccharides in Low-Protein Diet on Egg Quality
Research has shown that most egg quality traits remain unaffected by protein level [42]. Additionally, observations have indicated that eggshell characteristics do not change when the methionine content in the feed is increased [43]. Haugh unit scores were significantly lower in hens fed 13% CP diet than 16% CP diet, but yolk color was significantly higher than in hens fed 16% CP diet [23]. Low-protein diets contain a high proportion of maize, which is rich in lutein; adding more maize to the diet can improve yolk pigmentation [44]. Research indicates that appropriate levels of EAAs can prevent the harmful effects of low-CP diets on shell quality [45]. Eggshell strength was also not influenced by dietary CP levels, suggesting that the addition of L-threonine and lysine to low-crude-protein diets can maintain eggshell quality when digestible sulfur-containing amino acid requirements are considered.
4.3. Serum Antioxidant and Relevant Gene Expression in the Liver
The SOD activity and MDA content are important indicators of antioxidant performance. GPx is an important peroxidase enzyme widely present in the body, and is one of the indicators of the body’s resistance to peroxidation. It has been shown that high-temperature conditions induce the production of free radicals and oxidative stress, which induce lipid peroxidation and cell membrane damage [46]. A decrease in SOD activity and GPx concentration, consistent with an increase in free radical production in the high-temperature group, has been confirmed [47]. Meanwhile, findings indicate that heat stress increases the levels of SOD and MDA in broiler livers [48]. Nrf-2, a key transcription factor in the antioxidant system, protects cells by upregulating antioxidants like SOD and GPx to counteract stressors [49], which is consistent with the findings of this study. Low-protein diets, supplemented with essential amino acids to meet animals’ nutritional needs and maintain amino acid balance, can boost antioxidant enzyme synthesis and enhance antioxidant capacity [50]. Beneficial effects of feeding low-protein diets (with recommended ME) under heat stress have been observed, and this finding is consistent with several other studies conducted under similar conditions [51,52]. Furthermore, it has been shown that oxidative stress dominates the damage caused by heat stress; heat stress causes enhanced metabolism in livestock organisms, causing excessive accumulation of ROS and causing an oxidative–antioxidant imbalance in the organism and oxidative stress; this in turn causes oxidative damage to tissue cells, proteins and nucleic acids [53,54].
4.4. Serum Immunoglobulin, Inflammatory Cytokines and Relevant Gene Expression in the Liver
IgA, IgG and IgM play an important role in the immune process of poultry [55]. IgM is an early antibody of humoral immunity, and IgG has the function of neutralizing viruses and bacteria [56]. Supplementation of 0.498% tryptophan in low-protein diet (14.05% CP) significantly increased serum IgG and IgM contents in broilers [57]. Studies have reported that heat stress can inhibit the production of chicken antibodies, and the production of IgA is the lowest [58], which is consistent with the results of [59]. In this experiment, high temperature reduced the content of IgA, IgG and IgM in serum, and low-protein diet significantly increased the content of IgG and IgM, and the low-protein diet supplemented with APSs reversed the adverse effects of high temperature and increased the content of immunoglobulin.
IL-1β is mainly released by monocytes, macrophages and non-immune cells during cell injury and infection, which can promote the production of other cytokines and play an important role in immune response [60]. IL-10 exerts strong anti-inflammatory effects by suppressing pro-inflammatory cytokine production and modulating cytokine receptor expression [61]. Under the conditions of this experiment, the high-temperature environment increased the content of IL-1β in the serum of laying hens. The concentration of IL-1β in the serum of LPAB diets decreased by 14.4% and 17.00%, respectively, and IL-1β relative mRNA expression in the liver was decreased, but it still failed to completely offset the damage caused by heat stress. After adding APSs to the LPAB diet, the concentration of IL-10 increased by 38.67%, and IL-10 relative mRNA expression in the liver was upregulated, indicating that although the high temperature destroyed the balance of its cytokines and adversely affected the immune system of laying hens, the LPAB diets and APSs started the anti-inflammatory reaction. At the same time, it also inhibits inflammation, and reduces body damage.
4.5. Serum Biochemical and Relevant Gene Expression in the Liver
Cholesterol and triglyceride are mainly related to lipid metabolism in laying hens. The contents of CHOL and LDL-C in 21-day-old male broilers were significantly increased under heat stress [62]. APSs could reverse the increase in serum T-CHO, TG and LDL-C levels and the decrease in serum HDL-C levels induced by alcohol in mice [63]. Under the condition of high-temperature stress, the addition of APSs to the diet can significantly reduce the content of total cholesterol and LDL-C in serum, which is basically consistent with the above studies. It may be due to the fact that APSs are good lipid regulators and can improve plasma lipoprotein levels [59,64]. Under the condition of this experiment, heat stress and low-protein diet led to the increase in total cholesterol and triglyceride content in serum of laying hens, which may be due to the decrease in basal metabolism of laying hens in high-temperature environment or low-protein state, affecting the normal metabolism of lipids in vivo, and the decrease in lipid concentration. The energy required for cell metabolism is only provided by related enzymes to decompose nutrients, so the contents of TG and T-CHO involved in lipid metabolism are significantly increased. ALT and AST are liver function markers associated with amino acid metabolism, and their elevated levels under stress reflect tissue damage [65,66]. In this experiment, the addition of APSs in the diet can reduce the activity of AST in serum, protect the liver and reduce the damage caused by heat stress. In different treatments of low-crude-protein diets, no significant changes were observed in TP, ALB, GLB, and AST (standard CP 2, 4, and 6% control diets) [67]. It is speculated that the addition of APSs to the low-protein diet has no obvious effect on improving the protein metabolism of laying hens under high-temperature environment. In addition, heat stress can destroy the integrity of the intestinal barrier, promote the absorption of glucose, and increase serum glucose content [68]. Giving 4% low-protein diet to pregnant mice will lead to dysfunction of islet β cells in their offspring and reduce the secretion of insulin and amylin [69]. Studies have shown that APSs can reduce serum GLU levels, increase insulin sensitivity, and reduce insulin resistance [70]. The results of this experiment are basically consistent with the above results. Some studies found that when broilers were fed low-protein diets (2–4% CP), there was no change in serum total protein [71,72]. Further observations indicated that total protein is only affected when diets lack amino acids [73]. Thus, meeting AA needs seems more critical than CP itself. As shown in this study, low-CP diets can boost hepatic lipogenesis, raising triglyceride levels. Cholesterol biosynthesis starts with acetyl-CoA, with HMGCR acting as the rate-limiting enzyme [74]. Cells also take up cholesterol via LDLR-mediated endocytosis [75]. Our data showed significantly upregulated HMGCR expression in HB livers. Compared to HB, hepatic LDLR expression increased by 53% in HL and 45% in HLA, suggesting heat stress boosts blood cholesterol by reducing extrahepatic cholesterol absorption. The LPAB diets and APSs can both enhance cholesterol uptake and suppress its synthesis.
5. Conclusions
In conclusion, heat stress caused by a high-temperature environment reduces production performance. LPAB diets and APSs can reduce the adverse effects of heat stress, improving FCR and follicular development, increasing egg yolk color, and adding 0.5% APSs can also enhance EPR. In addition, LPAB diets and APSs significantly improve antioxidant and immune performance in heat stress. Based on LPAB diets, this study added APSs to explore its effect on laying performance, antioxidant performance, immune performance and lipid metabolism of laying hens. This provides a reference for the use of LPAB diets and plant-derived feed additives in laying hen production. At the same time, it provides a feasible method to mitigate the negative impact of high-temperature environments on laying hen production. APSs can improve the health status of laying hens, and it is expected to be a green, healthy, production-friendly, and antioxidant feed additive.
Author Contributions
Conceptualization, H.Y.; Methodology, H.Y.; Investigation, W.L., Z.W., H.Y.; Data curation, W.L.; Writing—original draft preparation, W.L.; Writing—review and editing, W.L., X.W., Z.W., H.Y.; Supervision, H.Y.; Project administration, H.Y.; Funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Application Demonstration Project of Agricultural Science and Technology Achievements in Jiangsu Province (Rural industry revitalization) (grant number BN2022054); Zhenjiang Key R & D Program-Modern Agriculture (grant number NY2021025). Yangzhou University-Hai’an City Agricultural Science and Technology Modernization Pilot County Project (grant number HA-XXY-2023-01).
Institutional Review Board Statement
All animal experiments were conducted in accordance with the Institutional Animal Care and Use Committee of Yang Zhou University, Yang Zhou, China (Approval No. SYXK(Su)2022-0020, approved on 15 March 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Jiasen Jiang for technical support and the barn staff for managing the animals.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tučková, K.; Filipčík, R. Effect of Heat Stress on Age in Conception of Holstein Heifers. Acta. Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 173–177. [Google Scholar] [CrossRef]
- Barrett, N.W.; Rowland, K.; Schmidt, C.J.; Lamont, S.J.; Rothschild, M.F.; Ashwell, C.M.; Persia, M.E. Effects of Acute and Chronic Heat Stress on the Performance, Egg Quality, Body Temperature, and Blood Gas Parameters of Laying Hens. Poult. Sci. 2019, 98, 6684–6692. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, M.; Usman, M.; Haider, U.; Raza Farhan, M.H.; Iftikhar, E.; Hassan, S.U.; Narayan, E. Role of Feed Additives in Mitigating the Impact of Heat Stress on Poultry Gut Health and Productivity. World’s Poult. Sci. J. 2024, 80, 1055–1075. [Google Scholar] [CrossRef]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat Stress and Poultry Production: Impact and Amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, W.; Mahrose, K.M.; Rizk, A.M.; Ouda, M.M.M.; Fathey, I.A.; Othman, S.I.; Allam, A.A.; Rudayni, H.A.; Almasmoum, H.A.; Taha, A.E.; et al. Impact of β-Glucan Dietary Supplementation on Productive, Reproductive Performance and Physiological Response of Laying Hens under Heat Stress Conditions. Poult. Sci. 2024, 103, 103183. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; Eltantawy, M.S.; Gawish, E.M.; Younis, H.H.; Amber, K.A.; Abd El-Moneim, A.E.-M.E.; Ebeid, T.A. Impact of Dietary Organic Mineral Supplementation on Reproductive Performance, Egg Quality Characteristics, Lipid Oxidation, Ovarian Follicular Development, and Immune Response in Laying Hens Under High Ambient Temperature. Biol. Trace Elem. Res. 2020, 195, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Maskal, J.M.; Duttlinger, A.W.; Kpodo, K.R.; McConn, B.R.; Byrd, C.J.; Richert, B.T.; Marchant-Forde, J.N.; Lay, D.C.; Perry, S.D.; et al. In Utero Heat Stress Alters the Postnatal Innate Immune Response of Pigs. J. Anim. Sci. 2020, 98, skaa356. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Kang, D.; Park, J.; Khan, M.; Belal, S.A.; Shin, D.; Shim, K. Altered Relationship between Gluconeogenesis and Immunity in Broilers Exposed to Heat Stress for Different Durations. Poult. Sci. 2021, 100, 101274. [Google Scholar] [CrossRef]
- Benyelloul, K.; Seddik, L.; Bouhadda, Y.; Bououdina, M.; Aourag, H.; Khodja, K. Effect of Pressure on Structural, Elastic and Mechanical Properties of Transition Metal Hydrides Mg7TMH16 (TM = Sc, Ti, V, Y, Zr and Nb): First-Principles Investigation. J. Phys. Chem. Solids 2017, 111, 229–237. [Google Scholar] [CrossRef]
- Teyssier, J.-R.; Brugaletta, G.; Sirri, F.; Dridi, S.; Rochell, S.J. A Review of Heat Stress in Chickens. Part II: Insights into Protein and Energy Utilization and Feeding. Front. Physiol. 2022, 13, 943612. [Google Scholar] [CrossRef]
- Torki, M.; Mohebbifar, A.; Ghasemi, H.A.; Zardast, A. Response of Laying Hens to Feeding Low-Protein Amino Acid-Supplemented Diets under High Ambient Temperature: Performance, Egg Quality, Leukocyte Profile, Blood Lipids, and Excreta pH. Int. J. Biometeorol. 2015, 59, 575–584. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Zhao, L.; Zhang, J.; Huang, S.; Ma, Q. Effect of Methionine Deficiency on the Growth Performance, Serum Amino Acids Concentrations, Gut Microbiota and Subsequent Laying Performance of Layer Chicks. Front. Vet. Sci. 2022, 9, 878107. [Google Scholar] [CrossRef] [PubMed]
- Poosuwan, K.; Bunchasak, C.; Kaewtapee, C. Long-Term Feeding Effects of Dietary Protein Levels on Egg Production, Immunocompetence and Plasma Amino Acids of Laying Hens in Subtropical Condition. J. Anim. Physiol. Anim. Nutr. 2010, 94, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Wang, R.-C.; Qu, Z.-Y.; Zhu, Y.-Z.; Li, Y.-L. Advances on Immunoregulation Effect of Astragalus Polysaccharides. Front. Nat. Prod. 2022, 1, 971679. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, Y.; Sun, L.; Yang, S.; Kuang, H.; Li, R.; Meng, Y.; Wu, Y. Identifying the Anti-Inflammatory Effects of Astragalus Polysaccharides in Anti-N-Methyl-D-Aspartate Receptor Encephalitis: Network Pharmacology and Experimental Validation. Comb. Chem. High Throughput Screen. 2024, 27, 1022–1032. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, C.; Guo, Y.; Zhang, W.; Guo, W.; Oleksandr, K.; Wang, Z. Polysaccharides Derived from Astragalus membranaceus and Glycyrrhiza uralensis Improve Growth Performance of Broilers by Enhancing Intestinal Health and Modulating Gut Microbiota. Poult. Sci. 2022, 101, 101905. [Google Scholar] [CrossRef]
- National Research Council, and Subcommittee on Poultry Nutrition. Nutrient Requirements of Poultry: 1994; National Academies Press: Washington, WA, USA, 1994; ISBN 0-309-04892-3. [Google Scholar]
- NY/T 823-2020; Terminology and Measurement Calculation Method for Poultry Performance. The National Standards of the People’s Republic of China: Beijing, China, 2020.
- Palmer, S.S.; Bahr, J.M. Follicle Stimulating Hormone Increases Serum Oestradial-17ß Concentrations, Number of Growing Follicles and Yolk Deposition in Aging Hens (Gallus Gallus Domesticus) with Decreased Egg Production. Br. Poult. Sci. 1992, 33, 403–414. [Google Scholar] [CrossRef]
- Osman, R.H.; Liu, L.; Xia, L.; Zhao, X.; Wang, Q.; Sun, X.; Zhang, Y.; Yang, B.; Zheng, Y.; Gong, D. Fads1 and 2 Are Promoted to Meet Instant Need for Long-Chain Polyunsaturated Fatty Acids in Goose Fatty Liver. Mol. Cell. Biochem. 2016, 418, 103–117. [Google Scholar] [CrossRef]
- Bohler, M.W.; Chowdhury, V.S.; Cline, M.A.; Gilbert, E.R. Heat Stress Responses in Birds: A Review of the Neural Components. Biology 2021, 10, 1095. [Google Scholar] [CrossRef]
- Kim, H.-R.; Ryu, C.; Lee, S.-D.; Cho, J.-H.; Kang, H. Effects of Heat Stress on the Laying Performance, Egg Quality, and Physiological Response of Laying Hens. Animals 2024, 14, 1076. [Google Scholar] [CrossRef]
- Torki, M.; Nasiroleslami, M.; Ghasemi, H.A. The Effects of Different Protein Levels in Laying Hens under Hot Summer Conditions. Anim. Prod. Sci. 2016, 57, 927–934. [Google Scholar] [CrossRef]
- Soares, K.R.; Lara, L.J.C.; da Silva Martins, N.R.; e Silva, R.R.; Pereira, L.F.P.; Cardeal, P.C.; Teixeira, M.d.P.F. Protein Diets for Growing Broilers Created under a Thermoneutral Environment or Heat Stress. Anim. Feed. Sci. Technol. 2020, 259, 114332. [Google Scholar] [CrossRef]
- Mosca, F.; Kuster, C.A.; Stella, S.; Farina, G.; Madeddu, M.; Zaniboni, L.; Cerolini, S. Growth Performance, Carcass Characteristics and Meat Composition of Milanino Chickens Fed on Diets with Different Protein Concentrations. Br. Poult. Sci. 2016, 57, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, S.; Zhai, W.; Zhao, Y. Effect of Reduction in Dietary Amino Acids and Energy on Growth Performance and Economic Return of Cobb 700 and Ross 708 Broilers. Animals 2025, 15, 890. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Qiao, Y.; Yang, X.; Chen, X.; Li, H.; Guo, Y.; Zhang, W.; Wang, Z. Protease and Bacillus Coagulans Supplementation in a Low-Protein Diet Improves Broiler Growth, Promotes Amino Acid Transport Gene Activity, Strengthens Intestinal Barriers, and Alters the Cecal Microbial Composition. Animals 2025, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, K.; Wang, H.; Lin, H.; Yang, X.; Wang, X.; Zhao, J.; Ma, B.; Shu, Q.; Lu, Y. Effects of Different Forms of Amino Acid Supplementation on the Performance and Intestinal Barrier Function of Laying Hens Fed a Low-Protein Diet. Poult. Sci. 2024, 103, 104375. [Google Scholar] [CrossRef]
- Cabezas-Garcia, E.H.; Rodríguez-Aguilar, D.E.; Afanador-Téllez, G. Individual Egg Production of Hy-Line Brown Hens during the Early Laying Phase in Response to Dietary CP Levels. Anim.-Open Space 2022, 1, 100027. [Google Scholar] [CrossRef]
- Parenteau, I.A.; Stevenson, M.; Kiarie, E.G. Egg Production and Quality Responses to Increasing Isoleucine Supplementation in Shaver White Hens Fed a Low Crude Protein Corn-Soybean Meal Diet Fortified with Synthetic Amino Acids between 20 and 46 Weeks of Age. Poult. Sci. 2020, 99, 1444–1453. [Google Scholar] [CrossRef]
- Wang, J.-M.; Sun, X.-Y.; Ouyang, J.-M. Structural Characterization, Antioxidant Activity, and Biomedical Application of Astragalus Polysaccharide Degradation Products. Int. J. Polym. Sci. 2018, 2018, 5136185. [Google Scholar] [CrossRef]
- Liao, J.; Li, C.; Huang, J.; Liu, W.; Chen, H.; Liao, S.; Chen, H.; Rui, W. Structure Characterization of Honey-Processed Astragalus Polysaccharides and Its Anti-Inflammatory Activity in Vitro. Molecules 2018, 23, 168. [Google Scholar] [CrossRef]
- Li, K.; Cao, Y.; Jiao, S.; Du, G.; Du, Y.; Qin, X. Structural Characterization and Immune Activity Screening of Polysaccharides with Different Molecular Weights from Astragali Radix. Front. Pharmacol. 2020, 11, 582091. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, C.; Bai, L.; Wu, J.; Bo, R.; Ye, M.; Huang, L.; Chen, H.; Rui, W. Structural Differences of Polysaccharides from Astragalus before and after Honey Processing and Their Effects on Colitis Mice. Int. J. Biol. Macromol. 2021, 182, 815–824. [Google Scholar] [CrossRef]
- Zexi, Z.; Lei, Z.; Hansong, X. Effect of Astragalus Polysaccharide in Treatment of Diabetes Mellitus: A Narrative Review. J. Tradit. Chin. Med. 2019, 39, 133–138. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Tian, K.; Yan, S.; Xu, C.; Tian, Y.; Xiao, C.; Jia, X.; Shi, J.; Bai, Y.; et al. Dynamic Expression Profile of Follicles at Different Stages in High- and Low-Production Laying Hens. Genes 2023, 15, 40. [Google Scholar] [CrossRef]
- Tuğalay, Ç.Ş.; Bayraktar, Ö.H.; Genç, N. Effects of ACTH and Acute Heat Stress on Oxidative Stress in an Early Environmentally Enriched Broilers. J. Anim. Prod. 2021, 62, 93–98. [Google Scholar]
- Mishra, S.K.; Chen, B.; Zhu, Q.; Xu, Z.; Ning, C.; Yin, H.; Wang, Y.; Zhao, X.; Fan, X.; Yang, M.; et al. Transcriptome Analysis Reveals Differentially Expressed Genes Associated with High Rates of Egg Production in Chicken Hypothalamic-Pituitary-Ovarian Axis. Sci. Rep. 2020, 10, 5976. [Google Scholar] [CrossRef]
- Yin, Z.Z.; Dong, X.Y.; Cao, H.Y.; Mao, H.G.; Ma, Y.Z. Effects of Rearing Systems on Reproductive Hormones Secretion and Their Receptors Gene Expression in Xianju Chickens under Summer Conditions. Poult. Sci. 2018, 97, 3092–3096. [Google Scholar] [CrossRef]
- Titto, C.G.; Negrão, J.A.; Canaes, T.D.S.; Titto, R.M.; Leme-dos Santos, T.M.D.C.; Henrique, F.L.; Calviello, R.F.; Pereira, A.M.F.; Titto, E.A.L. Heat Stress and ACTH Administration on Cortisol and Insulin-like Growth Factor I (IGF-I) Levels in Lactating Holstein Cows. J. Appl. Anim. Res. 2017, 45, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, H.; Han, Y.; Yang, P. Mechanisms of Heat Stress on Neuroendocrine and Organ Damage and Nutritional Measures of Prevention and Treatment in Poultry. Biology 2024, 13, 926. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Hindawy, M.M.; El-Hack, M.E.A.; Arif, M.; El-Sayed, S.A. Influence of Low-Protein Diet with Different Levels of Amino Acids on Laying Hen Performance, Quality and Egg Composition. An. Acad. Bras. Ciências 2020, 92, e20180230. [Google Scholar] [CrossRef] [PubMed]
- van Harn, J.; Dijkslag, M.A.; van Krimpen, M.M. Effect of Low Protein Diets Supplemented with Free Amino Acids on Growth Performance, Slaughter Yield, Litter Quality, and Footpad Lesions of Male Broilers. Poult. Sci. 2019, 98, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Zurak, D.; Slovenec, P.; Janječić, Z.; Bedeković, X.; Pintar, J.; Kljak, K. Overview on Recent Findings of Nutritional and Non-Nutritional Factors Affecting Egg Yolk Pigmentation. World’s Poult. Sci. J. 2022, 78, 531–560. [Google Scholar] [CrossRef]
- Zhang, H.; Xuan, Y.; Guo, D.; Zeng, Q.; Bai, S.; Liu, Y.; Ding, X.; Zhang, K.; Wang, J. Effects of Dietary Low Protein Levels and Amino Acid Patterns on Production Performance, Egg Quality and Intestinal Function in Laying Hens. Poult. Sci. 2025, 104, 105578. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between Heat Stress and Oxidative Stress in Poultry; Mitochondrial Dysfunction and Dietary Interventions with Phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, M.; Xu, Q.; Chen, Y.; Wen, C.; Zhou, Y. Dietary Mannan Oligosaccharide Improves Growth Performance, Muscle Oxidative Status, and Meat Quality in Broilers under Cyclic Heat Stress. J. Therm. Biol 2018, 75, 106–111. [Google Scholar] [CrossRef]
- Tang, L.-P.; Liu, Y.-L.; Zhang, J.-X.; Ding, K.-N.; Lu, M.-H.; He, Y.-M. Heat Stress in Broilers of Liver Injury Effects of Heat Stress on Oxidative Stress and Autophagy in Liver of Broilers. Poult. Sci. 2022, 101, 102085. [Google Scholar] [CrossRef]
- Mahanty, A.; Mohanty, S.; Mohanty, B.P. Dietary Supplementation of Curcumin Augments Heat Stress Tolerance through Upregulation of Nrf-2-Mediated Antioxidative Enzymes and Hsps in Puntius Sophore. Fish Physiol. Biochem. 2017, 43, 1131–1141. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Q.; Zhou, H.; Li, G.; Wu, Y.; Yang, L. Effect of Dietary Tryptophan Supplementation on Serum Biochemical Indices, Antioxidant Capacities, Cytokine Levels and Mitochondrial Function of Broilers under Chronic Heat Stress. Trop. Anim. Health Prod. 2023. [Google Scholar] [CrossRef]
- Zulkifli, I.; Akmal, A.F.; Soleimani, A.F.; Hossain, M.A.; Awad, E.A. Effects of Low-Protein Diets on Acute Phase Proteins and Heat Shock Protein 70 Responses, and Growth Performance in Broiler Chickens under Heat Stress Condition. Poult. Sci. 2018, 97, 1306–1314. [Google Scholar] [CrossRef]
- Salahi, A.; Shahir, M.H.; Attia, Y.A.; Fahmy, K.N.E.; Bovera, F.; Tufarelli, V. Impact of Low-Protein Diets on Broiler Nutrition, Production Sustainability, Gene Expression, Meat Quality and Greenhouse Gas Emissions. J. Appl. Anim. Res. 2025, 53, 2473419. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat Stress Effects on Livestock: Molecular, Cellular and Metabolic Aspects, a Review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Liao, X.D.; Ma, G.; Cai, J.; Fu, Y.; Yan, X.Y.; Wei, X.B.; Zhang, R.J. Effects ofClostridium Butyricum on Growth Performance, Antioxidation, and Immune Function of Broilers. Poult. Sci. 2015, 94, 662–667. [Google Scholar] [CrossRef]
- Song, X.; Qian, J.; Wang, C.; Wang, D.; Zhou, J.; Zhao, Y.; Wang, W.; Li, J.; Guo, R.; Li, Y. Correlation between the IgG/IgA Antibody Response against PEDV Structural Protein and Virus Neutralization. Microbiol. Spectr. 2023, 11, e05233-22. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, K.; Wang, J.; Zhang, H.; Qi, G.; Wu, S. Effect of Dietary Serine Supplementation on Performance, Egg Quality, Serum Indices, and Ileal Mucosal Immunity in Laying Hens Fed a Low Crude Protein Diet. Poult. Sci. 2021, 100, 101465. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, L.; Xu, Z.-J.; De Marco, M.; Briens, M.; Yan, X.-H.; Sun, L.-H. Hydroxy-Selenomethionine Improves the Selenium Status and Helps to Maintain Broiler Performances under a High Stocking Density and Heat Stress Conditions through a Better Redox and Immune Response. Antioxidants 2021, 10, 1542. [Google Scholar] [CrossRef]
- Alagawany, M.; Ashour, E.A.; El-Fakhrany, H.H.H.; Ismail, T.A.; Nasr, M. Early Nutrition Programming with Astragalus membranaceus Polysaccharide: Its Effect on Growth, Carcasses, Immunity, Antioxidants, Lipid Profile and Liver and Kidney Functions in Broiler Chickens. Anim. Biotechnol. 2022, 33, 362–368. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, W.-W. IL-1 Receptor Dynamics in Immune Cells: Orchestrating Immune Precision and Balance. Immune Netw. 2024, 24, e21. [Google Scholar] [CrossRef]
- Martinez-Espinosa, I.; Serrato, J.A.; Ortiz-Quintero, B. Role of IL-10-Producing Natural Killer Cells in the Regulatory Mechanisms of Inflammation during Systemic Infection. Biomolecules 2021, 12, 4. [Google Scholar] [CrossRef]
- Bueno, J.P.R.; de Mattos Nascimento, M.R.B.; da Silva Martins, J.M.; Marchini, C.F.P.; Gotardo, L.R.M.; de Sousa, G.M.R.; Mundim, A.V.; Guimarães, E.C.; Rinaldi, F.P. Effect of Age and Cyclical Heat Stress on the Serum Biochemical Profile of Broiler Chickens. Semin. Ciências Agrárias 2017, 38, 1383. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, N.; Zhao, L.; Wu, W.; Zhang, L.; Zhou, F.; Li, J. Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice. Foods 2021, 10, 2688. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; YaTao, X.; Rehman, Z.U.; Arain, M.A.; Soomro, R.N.; El-Hack, M.E.A.; Bhutto, Z.A.; Abbasi, B.; Dhama, K.; Sarwar, M.; et al. Nutritional and Healthical Aspects of Yacon (Smallanthus sonchifolius) for Human, Animals and Poultry. Int. J. Pharmacol. 2017, 13, 361–369. [Google Scholar] [CrossRef]
- Mokondjimobe, E.; Longo-Mbenza, B.; Akiana, J.; Ndalla, U.O.; Dossou-Yovo, R.; Mboussa, J.; Parra, H.-J. Biomarkers of Oxidative Stress and Personalized Treatment of Pulmonary Tuberculosis: Emerging Role of Gamma-Glutamyltransferase. Adv. Pharmacol. Sci. 2012, 2012, 465634. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Elkomy, M.H.; Fahim, H.I.; Ashour, M.B.; Naguib, I.A.; Alghamdi, B.S.; Mahmoud, H.U.R.; Ahmed, N.A. Rutin and Quercetin Counter Doxorubicin-induced Liver Toxicity in Wistar Rats via Their Modulatory Effects on Inflammation, Oxidative Stress, Apoptosis, and Nrf2. Oxidative Med. Cell. Longev. 2022, 2022, 2710607. [Google Scholar] [CrossRef]
- Badawi, M.E.-S.; Ali, A.H.; El-Razik, W.M.A.; Soliman, M.H. Influence of Low Crude Protein Diets on Broiler Chickens Performance. Adv. Anim. Vet. Sci. 2019, 7, 26–33. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Sheikhahmadi, A.; Wang, Y.; Jiao, H.; Lin, H.; Song, Z. Effects of Heat Stress on the Gene Expression of Nutrient Transporters in the Jejunum of Broiler Chickens (Gallus gallus domesticus). Int. J. Biometeorol. 2015, 59, 127–135. [Google Scholar] [CrossRef]
- Mathias, P.C.d.F.; Miranda, G.D.S.; Barella, L.F.; Miranda, R.A.; Pavanello, A.; Martins, I.P.; Facchi, J.C.; Costermani, H.d.O.; de Lima, T.A.L.; de Oliveira, J.C. Cholinergic-Pathway-Weakness-Associated Pancreatic Islet Dysfunction: A Low-Protein-Diet Imprint Effect on Weaned Rat Offspring. J. Dev. Origins Health Dis. 2020, 11, 484–491. [Google Scholar] [CrossRef]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; López, M.; Martínez, S.; Megías, M.D.; Catalá, P.; Madrid, J. Effect of Low-Protein Diets and Single Sex on Production Performance, Plasma Metabolites, Digestibility, and Nitrogen Excretion in 1-to 48-Day-Old Broilers. Poult. Sci. 2012, 91, 683–692. [Google Scholar] [CrossRef]
- Saleh, A.A.; Amber, K.A.; Soliman, M.M.; Soliman, M.Y.; Morsy, W.A.; Shukry, M.; Alzawqari, M.H. Effect of Low Protein Diets with Amino Acids Supplementation on Growth Performance, Carcass Traits, Blood Parameters and Muscle Amino Acids Profile in Broiler Chickens under High Ambient Temperature. Agriculture 2021, 11, 185. [Google Scholar] [CrossRef]
- Ahmadi, M.; Yaghobfar, A.; Tabatabaei, S.H. Study of Effects Difference Levels of Crude Protein and Amino Acid of Diet on Intestinal Morphological and Blood Biological Parameters of Poultry. Biological Forum 2015, 7, 666–670. [Google Scholar]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and Regulation of Cholesterol Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Yevstigneyev, N.; Madani, G.; McCormick, S. Approaches to Visualising Endocytosis of LDL-Related Lipoproteins. Biomolecules 2022, 12, 158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).