Risk Factors and Prevalence of Salmonella spp. in Poultry Carcasses in Slaughterhouses Under Official Veterinary Inspection Service in Brazil
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Collection
2.3. Microbiological Analysis
2.4. Statistical Analysis
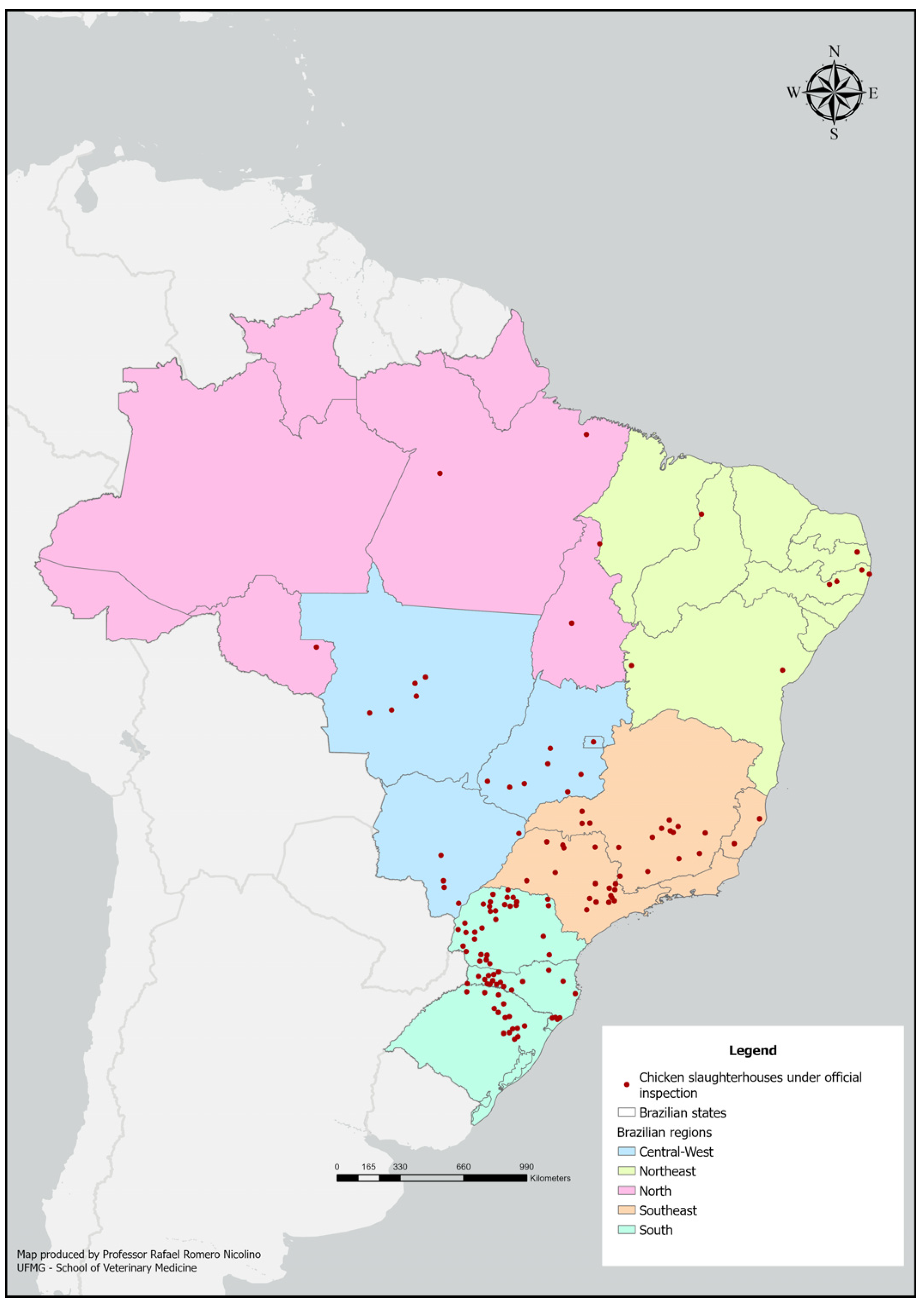
2.5. Geoprocessing
3. Results
| Region | No. of Slaughter Establishments Sampled | % Salmonella | CI (95%) |
|---|---|---|---|
| South | 63 | 18.42 | 1.38–24.14 |
| Central-west | 11 | 17.48 | 10.35–27.98 |
| Southeast | 33 | 16.58 | 10.5–25.19 |
| North/Northeast | 8 | 16.05 | 0.51–40.31 |
| Commercial Qualification | % Salmonella | CI (95%) |
|---|---|---|
| IM | 19.59 | 8.5–39.9 |
| EM (European Union) | 18.82 | 12.7–26.9 |
| EM (Other) | 16.98 | 13.1–21.8 |
4. Discussion
4.1. Brazilian Prevalence Comparison with Other Countries
4.2. Slaughterhouse Size
4.3. Regional Influence
4.4. Operational Influences
4.5. Prevalence and Serotyping According to Establishments Qualification
4.6. Public Health Impact
4.7. Efforts Improvements on Surveillance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| DIPOA | Department of Inspection of Animal Products |
| EM | External Market |
| EU | European Union |
| IM | Internal Market |
| ISO | International Organization for Standardization |
| LFDAs | Federal Laboratories of Agriculture Defense |
| MAPA | Ministry of Agriculture, Livestock and Food Supply |
| MS | Member State |
| NM | National Market |
| No. | Number |
| SDA | Secretariat of Animal Defense |
| SUS | Unified Health System |
| UFMG | Federal University of Minas Gerais |
| USA | United States of America |
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States-Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef]
- European Food Safety Authority. The 2013 Joint ECDC/EFSA Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks Published. Sci. Rep. EFSA E ECDC 2015, 20, 6. [Google Scholar] [CrossRef]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef]
- Ashton, P.M.; Nair, S.; Peters, T.M.; Bale, J.A.; Powell, D.G.; Painset, A.; Tewolde, R.; Schaefer, U.; Jenkins, C.; Dallman, T.J.; et al. Identification of Salmonella for Public Health Surveillance Using Whole Genome Sequencing. PeerJ 2016, 4, e1752. [Google Scholar] [CrossRef]
- Whiley, H.; Ross, K. Salmonella and Eggs: From Production to Plate. Int. J. Environ. Res. Public Health 2015, 12, 2543–2556. [Google Scholar] [CrossRef]
- Fernandes, S.A.; Tavechio, A.T.; Ghilardi, Â.C.R.; Dias, Â.M.G.; Almeida, I.A.Z.C.D.; Melo, L.C.V.D. Salmonella Serovars Isolated from Humans in São Paulo State, Brazil, 1996–2003. Rev. Inst. Med. Trop. São Paulo 2006, 48, 179–184. [Google Scholar] [CrossRef]
- Popa, G.L.; Popa, M.I. Salmonella Spp. Infection—A Continuous Threat Worldwide. GERMS 2021, 11, 88–96. [Google Scholar] [CrossRef]
- Voss-Rech, D.; Vaz, C.S.L.; Alves, L.; Coldebella, A.; Leao, J.A.; Rodrigues, D.P.; Back, A. A Temporal Study of Salmonella enterica Serotypes from Broiler Farms in Brazil. Poult. Sci. 2015, 94, 433–441. [Google Scholar] [CrossRef]
- Finstad, S.; O’Bryan, C.A.; Marcy, J.A.; Crandall, P.G.; Ricke, S.C. Salmonella and Broiler Processing in the United States: Relationship to Foodborne Salmonellosis. Food Res. Int. 2012, 45, 789–794. [Google Scholar] [CrossRef]
- Gieraltowski, L.; Higa, J.; Peralta, V.; Green, A.; Schwensohn, C.; Rosen, H.; Libby, T.; Kissler, B.; Marsden-Haug, N.; Booth, H.; et al. National Outbreak of Multidrug Resistant Salmonella Heidelberg Infections Linked to a Single Poultry Company. PLoS ONE 2016, 11, e0162369. [Google Scholar] [CrossRef] [PubMed]
- Ramtahal, M.A.; Amoako, D.G.; Akebe, A.L.K.; Somboro, A.M.; Bester, L.A.; Essack, S.Y. A Public Health Insight into Salmonella in Poultry in Africa: A Review of the Past Decade: 2010–2020. Microb. Drug Resist. 2022, 28, 710–733. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Shimp, W.R.; Lau, P.W.; Badie, G.; Enioutina, E.Y.; Daynes, R.A.; Byrne, B.A.; House, J.K.; Mahan, M.J. Human Salmonella Clinical Isolates Distinct from Those of Animal Origin. Appl. Environ. Microbiol. 2008, 74, 1757–1766. [Google Scholar] [CrossRef]
- Siddique, A.; Wang, Z.; Zhou, H.; Huang, L.; Jia, C.; Wang, B.; Ed-Dra, A.; Teng, L.; Li, Y.; Yue, M. The Evolution of Vaccines Development across Salmonella Serovars among Animal Hosts: A Systematic Review. Vaccines 2024, 12, 1067. [Google Scholar] [CrossRef]
- Rabsch, W.; Fruth, A.; Simon, S.; Szabo, I.; Malorny, B. The Zoonotic Agent Salmonella. In Zoonoses—Infections Affecting Humans and Animals; Sing, A., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 179–211. ISBN 978-94-017-9456-5. [Google Scholar]
- Steele, J.H. Salmonellosis: A Major Zoonosis. Arch. Environ. Health Int. J. 1969, 19, 871–875. [Google Scholar] [CrossRef]
- Stevens, M.P.; Humphrey, T.J.; Maskell, D.J. Molecular Insights into Farm Animal and Zoonotic Salmonella Infections. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2709–2723. [Google Scholar] [CrossRef]
- Michael, G.B.; Schwarz, S. Antimicrobial Resistance in Zoonotic Nontyphoidal Salmonella: An Alarming Trend? Clin. Microbiol. Infect. 2016, 22, 968–974. [Google Scholar] [CrossRef]
- Rabsch, W.; Hargis, B.M.; Tsolis, R.M.; Kingsley, R.A.; Hinz, K.-H.; Tschäpe, H.; Bäumler, A.J. Competitive Exclusion of Salmonella Enteritidis by Salmonella Gallinarum in Poultry. Emerg. Infect. Dis. 2000, 6, 443–448. [Google Scholar] [CrossRef]
- Zhou, X.; Kang, X.; Zhou, K.; Yue, M. A Global Dataset for Prevalence of Salmonella Gallinarum between 1945 and 2021. Sci. Data 2022, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, F.; Smulders, F.J.M.; Chopra-Dewasthaly, R.; Paulsen, P. Salmonella in the Wildlife-Human Interface. Food Res. Int. 2012, 45, 603–608. [Google Scholar] [CrossRef]
- Bangtrakulnonth, A.; Pornreongwong, S.; Pulsrikarn, C.; Sawanpanyalert, P.; Hendriksen, R.S.; Wong, D.M.A.L.F.; Aarestrup, F.M. Salmonella Serovars from Humans and Other Sources in Thailand, 1993–2002. Emerg. Infect. Dis. 2004, 10, 131–136. [Google Scholar] [CrossRef]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- Mellor, K.C.; Petrovska, L.; Thomson, N.R.; Harris, K.; Reid, S.W.J.; Mather, A.E. Antimicrobial Resistance Diversity Suggestive of Distinct Salmonella Typhimurium Sources or Selective Pressures in Food-Production Animals. Front. Microbiol. 2019, 10, 708. [Google Scholar] [CrossRef]
- Mesquita, F.B.; Nicolino, R.R.; Brasileiro, A.C.M.; Brenner, S.; Haddad, J.P.A. Costs Estimation of Human Salmonellosis Outbreaks Associated to Animal Products Consumption in Brazil, 2008/2016. In Proceedings of the 15th International Symposium of Veterinary Epidemiology and Economics, Chiang Mai, Thailand, 12–16 November 2018; Abstract Book. p. 661. [Google Scholar]
- World Health Organization (WHO). WHO Estimates of the Global Burden of Foodborne Diseases. Who 2015, 255, 51–57. [Google Scholar] [CrossRef]
- Snary, E.L.; Swart, A.N.; Simons, R.R.L.; Domingues, A.R.C.; Vigre, H.; Evers, E.G.; Hald, T.; Hill, A.A. A Quantitative Microbiological Risk Assessment for Salmonella in Pigs for the European Union. Risk Anal. 2016, 36, 437–449. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-Analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; Hendriksen, R.S.; Carrique-Mas, J.J. Practical Considerations of Surveillance of Salmonella Serovars Other than Enteridis and Typhimurium. Rev. Sci. Tech. OIE 2013, 32, 509–519. [Google Scholar] [CrossRef]
- Baggesen, D.L.; Sandvang, D.; Aarestrup, F.M. Characterization of Salmonella enterica Serovar Typhimurium DT104 Isolated from Denmark and Comparison with Isolates from Europe and the United States. J. Clin. Microbiol. 2000, 38, 1581–1586. [Google Scholar] [CrossRef]
- Ward, L.R.; Threlfall, J.; Smith, H.R.; O’Brien, S.J. Salmonella enteritidis Epidemic. Science 2000, 287, 1753. [Google Scholar] [CrossRef]
- Sun, H.; Wan, Y.; Du, P.; Bai, L. The Epidemiology of Monophasic Salmonella Typhimurium. Foodborne Pathog. Dis. 2020, 17, 87–97. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, Colonization, and Virulence Differences among Serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef]
- Carrasco, E.; Morales-Rueda, A.; García-Gimeno, R.M. Cross-Contamination and Recontamination by Salmonella in Foods: A Review. Food Res. Int. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- Nauta, M.; Corbellini, L.G.; Aabo, S. The Use of Risk Assessment to Support Control of Salmonella in Pork. In Proceedings of the Proceedings Book; Embrapa Suínos e Aves: Foz do Iguaçu, Brasil, 2017; pp. 36–39. [Google Scholar]
- Ravishankar, S.; Zhu, L.; Jaroni, D. Assessing the Cross Contamination and Transfer Rates of Salmonella enterica from Chicken to Lettuce under Different Food-Handling Scenarios. Food Microbiol. 2010, 27, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; Palmer, S.R.; Slader, J.; Humphrey, T. The South East Wales Infectious Disease Liaison Group Risk Factors for Salmonella Food Poisoning in the Domestic Kitchen—A Case Control Study. Epidemiol. Infect. 2002, 129, 277–285. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Street, D.A.; Fein, S.B.; Levy, A.S. Consumer Knowledge of Foodborne Microbial Hazards and Food-Handling Practices. J. Food Prot. 1996, 59, 287–294. [Google Scholar] [CrossRef]
- Fatica, M.K.; Schneider, K.R. Salmonella and Produce: Survival in the Plant Environment and Implications in Food Safety. Virulence 2011, 2, 573–579. [Google Scholar] [CrossRef]
- Ministério da Saúde Surtos de Doenças de Transmissão Hídrica e Alimentar Informe. Available online: https://higienealimentar.com.br/wp-content/uploads/2024/10/surtos-de-doencas-de-transmissao-hidrica-e-alimentar-no-brasil-informe-2024.pdf (accessed on 15 July 2025).
- Wang, T.; Li, W.; Zhang, R.; Wen, J.; Liu, S.; Jiang, Y.; Lin, L.; Chen, W.; Liang, J.; Ma, X.; et al. Epidemiological Features of Nontyphoidal Salmonella Infections Reported to Foodborne Disease Surveillance System in China, 2013–2022. BMC Public Health 2025, 25, 2258. [Google Scholar] [CrossRef]
- Ministry of Agriculture; Livestock and Food Supply; MAPA. Normative Instruction. In Establish the Microbiological Pathogen Reduction Program, Monitoring and Control of Salmonella sp. in Chicken and Turkey Carcasses; 2003. Available online: https://www.gov.br/agricultura/pt-br/assuntos/camaras-setoriais-tematicas/documentos/camaras-setoriais/aves-e-suinos/anos-anteriores/minuta-in-salmonella-aves.pdf (accessed on 15 January 2018).
- USDA; Food Safety and Inspection ServiceFSIS. The Nationwide Microbiological Baseline Data Collection Program: Raw Chicken Parts Survey; US Department of Agriculture: Washington, DC, USA, 2012; pp. 1–28. [Google Scholar]
- European Communities No 2073/2005. Commission Regulation on Microbiological Criteria for Foodstuffs. 2005. Available online: http://data.europa.eu/eli/reg/2005/2073/oj (accessed on 3 May 2018).
- Ministry of Agriculture; Livestock and Supply. MAPA Normative Instruction No. 20 of October 21, 2016. Establishes the Control and Monitoring of Salmonella spp. in Commercial Poultry Establishments of Broilers and Turkeys and in Establishments for the Slaughter of Chickens, Chickens, Broiler and Breeding Turkeys, Registered with the Federal Inspection Service (SIF), in Order to Reduce the Prevalence of This Agent and Establish an Adequate Level of Consumer Protection. 2016. Available online: https://www.gov.br/agricultura/pt-br/assuntos/sanidade-animal-e-vegetal/saude-animal/programas-de-saude-animal/pnsa/in20-2016-en-atualizada2017.pdf (accessed on 10 December 2017).
- Ministry of Agriculture; Livestock and Food Supply; MAPA Ordinance No. 17. Establishes, within the Department of Inspection of Animal Products of the Secretariat of Agricultural Defense of the Ministry of Agriculture; Livestock and Food Supply (DIPOA/SDA/MAPA); the Advisory Scientific Committee on Microbiology of Products of Animal Origin. 2013. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/controle-de-patogenos/arquivos-controle-de-patogenos/por00000017.pdf (accessed on 2 January 2018).
- Dargatz, D.A.; Hill, G.W. Analysis of Survey Data. Prev. Vet. Med. 1996, 28, 225–237. [Google Scholar] [CrossRef]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Chalottetown, PEI, Canada, 2009; ISBN 978-0-919013-60-5. [Google Scholar]
- Microbiological Specifications For, I.C.O. Microorganisms in Foods 8: Use of Data for Assessing Process Control and Product Acceptance; Springer: Boston, MA, USA, 2011; ISBN 978-1-4419-9373-1. [Google Scholar]
- Ministry of Agriculture, Livestock and Food Supply—MAPA National Establishment Consultation. Available online: https://sigsif.agricultura.gov.br/sigsif_cons/%21ap_estabelec_nacional_cons (accessed on 10 January 2025).
- ISO 6579-3; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. International Standard ISO: Geneva, Switzerland, 2014.
- Mooijman, K.A. The New ISO 6579-1: A Real Horizontal Standard for Detection of Salmonella, at Last! Food Microbiol. 2018, 71, 2–7. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025:2017(E); ISO General Requirements for the Competence of Testing and Calibration Laboratories. International Standards Organization: Geneva, Switzerland, 2017.
- Codex Alimentarius Commission Procedural Manual; FAO; WHO: Rome, Italy, 2025; ISBN 978-92-5-139597-4.
- Esri ArcGIS Pro 2025. Available online: https://www.arcgis.com/index.html (accessed on 25 July 2025).
- Zeng, H.; De Reu, K.; Gabriël, S.; Mattheus, W.; De Zutter, L.; Rasschaert, G. Salmonella Prevalence and Persistence in Industrialized Poultry Slaughterhouses. Poult. Sci. 2021, 100, 100991. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, H.; Xu, X. Investigation of Microbial Contamination in a Chicken Slaughterhouse Environment. J. Food Sci. 2021, 86, 3598–3610. [Google Scholar] [CrossRef]
- Food, E.; Authority, S. Analysis of the Baseline Survey on the Prevalence of Campylobacter in Broiler Batches and of Campylobacter and Salmonella on Broiler Carcasses, in the EU, 2008—Part B: Analysis of Factors Associated with Salmonella Contamination of Broiler Carcasses. EFSA J. 2011, 9, 2017. [Google Scholar] [CrossRef]
- USDA Food Safety and Inspection Service (FSIS). FSIS Directive 10250.1 Establishment Eligibility Criteria for the Salmonella Verification and FSIS Scheduling Algorithm for the Salmonella Verification Sampling Program for Raw Meat and Poultry Introduction Eligibility Criteria and the Sampling Frame; US Department of Agriculture: Washington, DC, USA, 2011; Available online: https://www.fsis.usda.gov/sites/default/files/import/Salmonella_Scheduling_Algorithm_Functions.pdf (accessed on 5 October 2017).
- Secretariat of Agricultural Defense; Department of Inspection of Products of Animal Origin; General Coordination of Special Programs; MAPA. Yearbook of the Control Programs of Food of Animal Origin of DIPOA; MAPA: Brasília, Brazil, 2023. [Google Scholar]
- Hue, O.; Le Bouquin, S.; Lalande, F.; Allain, V.; Rouxel, S.; Petetin, I.; Quesne, S.; Laisney, M.-J.; Gloaguen, P.-Y.; Picherot, M.; et al. Prevalence of Salmonella Spp. on Broiler Chicken Carcasses and Risk Factors at the Slaughterhouse in France in 2008. Food Control 2011, 22, 1158–1164. [Google Scholar] [CrossRef]
- Mezali, L.; Mebkhout, F.; Nouichi, S.; Boudjellaba, S.; Hamdi, T.-M. Serotype Diversity and Slaughterhouse-Level Risk Factors Related to Salmonella Contamination on Poultry Carcasses in Algiers. J. Infect. Dev. Ctries. 2019, 13, 384–393. [Google Scholar] [CrossRef]
- De Lima, M.S.; Isolan, L.W.; Hessel, C.T.; Pessoa, J.P.; Tondo, E.C. Prevalence of Salmonella spp. in Poultry Carcasses Samples Collected in Slaughterhouses of Southern Brazil from 2006 to 2015. J. Infect. Dev. Ctries. 2018, 12, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.L.S.P.; Kanashiro, A.M.I.; Stoppa, G.F.Z.; Castro, A.G.M.; Luciano, R.L.; Tessari, E.N.C. Occurrence of Salmonella spp. in Chicken Carcasses from Abattoirs in the State of São Paulo, Brazil, in the Period from 2000 to 2010. 2015, Número 24. Available online: https://faef.revista.inf.br/imagens_arquivos/arquivos_destaque/k0NDVFIlGwZBWtn_2015-4-28-9-16-30.pdf (accessed on 15 July 2025).
- Siceloff, A.T.; Waltman, D.; Shariat, N.W. Regional Salmonella Differences in United States Broiler Production from 2016 to 2020 and the Contribution of Multiserovar Populations to Salmonella Surveillance. Appl. Environ. Microbiol. 2022, 88, e00204-22. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Cerdà-Cuéllar, M.; González-Bodi, S.; Lorenzo-Rebenaque, L.; Vega, S. Research Note: Persistent Salmonella Problems in Slaughterhouses Related to Clones Linked to Poultry Companies. Poult. Sci. 2022, 101, 101968. [Google Scholar] [CrossRef] [PubMed]
- Rosamilia, A.; Galletti, G.; Casadei, G.; Dell’Orfano, G.; Ferrari, M.; Carlantonio, E.D.; Vergani, F.; Riceputi, N.; Zanchini, F.; Bardasi, L.; et al. Assessment of Process Hygiene Criteria in Poultry Slaughterhouses: A Comparative Analysis of Own-Checks and Official Controls in Northeast Italy (2021–2023). Poult. Sci. 2025, 104, 105465. [Google Scholar] [CrossRef]
- De Witte, L.; De Reu, K.; Van Immerseel, F.; Van Raemdonck, J.; Botteldoorn, N.; Van Der Eycken, M.; Rasschaert, G. Research Note: Gut Instincts: Salmonella Contamination Based on Monitoring Systems in the Broiler Sector. Poult. Sci. 2025, 104, 104848. [Google Scholar] [CrossRef]
- Betiku, E.; Ogundipe, T.T.; Kalapala, T.; Obe, T. A Mini-Review on Multi-Hurdle Control of Salmonella Along Poultry Production Continuum. Animals 2025, 15, 875. [Google Scholar] [CrossRef]
- Meher, M.M.; Sharif, M.A.; Al Bayazid, A. Seroprevalence of Salmonella spp. Infection in Different Types of Poultry and Biosecurity Measures Associated with Salmonellosis. Int. J. Agric. Environ. Food Sci. 2022, 6, 557–567. [Google Scholar] [CrossRef]
- Totton, S.C.; Farrar, A.M.; Wilkins, W.; Bucher, O.; Waddell, L.A.; Wilhelm, B.J.; McEwen, S.A.; Rajić, A. A Systematic Review and Meta-Analysis of the Effectiveness of Biosecurity and Vaccination in Reducing Salmonella spp. in Broiler Chickens. Food Res. Int. 2012, 45, 617–627. [Google Scholar] [CrossRef]
- Oscar, T. Salmonella Prevalence Alone Is Not a Good Indicator of Poultry Food Safety. Risk Anal. 2021, 41, 110–130. [Google Scholar] [CrossRef]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and Antimicrobial Resistance Patterns of Salmonella Isolated from Poultry Farms in Southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef]
- Buncic, S.; Sofos, J. Interventions to Control Salmonella Contamination during Poultry, Cattle and Pig Slaughter. Food Res. Int. 2012, 45, 641–655. [Google Scholar] [CrossRef]
- Santos, L.A.D.; Mion, L.; Marotzki, M.; Parizotto, L.; Rodrigues, L.B.; Nascimento, V.P.D.; Santos, L.R.D. Número Mais Provável Miniaturizado e Microbiologia Convencional Para Isolamento de Salmonella spp. Em Abatedouros de Frangos de Corte. Pesqui. Veterinária Bras. 2015, 35, 223–229. [Google Scholar] [CrossRef]
- Wideman, N.; Bailey, M.; Bilgili, S.F.; Thippareddi, H.; Wang, L.; Bratcher, C.; Sanchez-Plata, M.; Singh, M. Evaluating Best Practices for Campylobacter and Salmonella Reduction in Poultry Processing Plants. Poult. Sci. 2016, 95, 306–315. [Google Scholar] [CrossRef]
- Obe, T.; Nannapaneni, R.; Schilling, W.; Zhang, L.; McDaniel, C.; Kiess, A. Prevalence of Salmonella enterica on Poultry Processing Equipment after Completion of Sanitization Procedures. Poult. Sci. 2020, 99, 4539–4548. [Google Scholar] [CrossRef]
- Souza, A.P.O.; Taconeli, C.A.; Plugge, N.F.; Molento, C.F.M. Broiler Chicken Meat Inspection Data in Brazil: A First Glimpse into an Animal Welfare Approach. Rev. Bras. Cienc. Avicola 2018, 20, 547–554. [Google Scholar] [CrossRef]
- USDA; Food Safety and Ispection Service (FSIS). DRAFT FSIS Compliance Guideline for Controlling Salmonella and Campylobacter in Raw Poultry; US Department of Agriculture: Washington, DC, USA, 2015. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Campylobacter in Broiler Meat Production: Control Options and Performance Objectives and/or Targets at Different Stages of the Food Chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Iannetti, L.; Neri, D.; Santarelli, G.A.; Cotturone, G.; Podaliri Vulpiani, M.; Salini, R.; Antoci, S.; Di Serafino, G.; Di Giannatale, E.; Pomilio, F.; et al. Animal Welfare and Microbiological Safety of Poultry Meat: Impact of Different at-Farm Animal Welfare Levels on at-Slaughterhouse Campylobacter and Salmonella Contamination. Food Control 2020, 109, 106921. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Salmonella Control in Poultry Flocks and Its Public Health Impact. EFSA J. 2019, 17, e05596. [Google Scholar] [CrossRef]
- Iannetti, L.; Neri, D.; Torresi, M.; Acciari, V.A.; Di Marzio, V.; Centorotola, G.; Scattolini, S.; Pomilio, F.; Di Giannatale, E.; Vulpiani, M.P. Can Animal Welfare Have an Impact on Food Safety? A Study in the Poultry Production Chain. Eur. J. Public Health 2020, 30, ckaa166.202. [Google Scholar] [CrossRef]
- Oh, H.; Yoon, Y.; Yoon, J.-W.; Oh, S.-W.; Lee, S.; Lee, H. Salmonella Risk Assessment in Poultry Meat from Farm to Consumer in Korea. Foods 2023, 12, 649. [Google Scholar] [CrossRef]
- Stathas, L.; Aspridou, Z.; Koutsoumanis, K. Quantitative Microbial Risk Assessment of Salmonella in Fresh Chicken Patties. Food Res. Int. 2024, 178, 113960. [Google Scholar] [CrossRef]
- Brazilian Association of Animal Protein-ABPA. Annual Report of the Brazilian Association of Animal Protein; ABPA: São Paulo, Brazil, 2018; p. 176. Available online: https://abpa-br.org/abpa-relatorio-anual/ (accessed on 19 March 2019).
- Ministry of Development, Industry. Trade and Services Comex Stat Official System for Extracting Statistics on Brazilian Foreign Trade of Goods. 2020. Available online: https://comexstat.mdic.gov.br/pt/home (accessed on 5 December 2020).
- Costa, R.G.; Festivo, M.L.; Araújo, M.S.; Reis, E.M.F.; Lázaro, N.S.; Rodrigues, D.P. Antimicrobial Susceptibility and Serovars of Salmonella Circulating in Commercial Poultry Carcasses and Poultry Products in Brazil. J. Food Prot. 2013, 76, 2011–2017. [Google Scholar] [CrossRef]
- Ministry of Agriculture; Livestock and Food Supply; MAPA. Techinical Note—Understand Better—Salmonella in Poultry; Brasília, Brazil. 2018. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/arquivos-publicacoes-dipoa/entenda-melhor-salmonela-em-carne-de-frango (accessed on 5 October 2020).
- Ministry of Agriculture; Livestock and Food Supply; MAPA. National Action Plan for the Prevention and Control of Antimicrobial Resistance in Animal Production; MAPA: Brasília, Brazil, 2018. [Google Scholar]
- Rau, R.B.; Ribeiro, A.R.; Dos Santos, A.; Barth, A.L. Antimicrobial Resistance of Salmonella from Poultry Meat in Brazil: Results of a Nationwide Survey. Epidemiol. Infect. 2021, 149, e228. [Google Scholar] [CrossRef]
- Alikhan, N.-F.; Moreno, L.Z.; Castellanos, L.R.; Chattaway, M.A.; McLauchlin, J.; Lodge, M.; O’Grady, J.; Zamudio, R.; Doughty, E.; Petrovska, L.; et al. Dynamics of Salmonella enterica and Antimicrobial Resistance in the Brazilian Poultry Industry and Global Impacts on Public Health. PLOS Genet. 2022, 18, e1010174. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. CDC National Enteric Disease Surveillance: Salmonella Annual Report; CDC: Sydney, Australia, 2013. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2014. EFSA J. 2015, 13, 4329. [Google Scholar] [CrossRef]
- Cargnel, M.; Filippitzi, M.; Van Cauteren, D.; Mattheus, W.; Botteldoorn, N.; Cambier, L.; Welby, S. Assessing Evidence of a Potential Salmonella Transmission across the Poultry Food Chain. Zoonoses Public Health 2023, 70, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Baptista, D.; Borsoi, A.; Reischak, D.; Nascimento, A.; Montesino, L.; Camillo, S.; Abreu, D.; Pereira, V. Salmonella Serovars Isolated from Poultry Breeding Flocks under the Brazilian Official Control Programme Between 2016 and 2018. Braz. J. Poult. Sci. 2023, 25, eRBCA-2022-1646. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, R.; Wu, C.; Zheng, Z.; Deng, Y.; Chen, K.; Xiang, Y.; Xu, C.; Zou, L.; Liao, M.; et al. Characterization of the Prevalence of Salmonella in Different Retail Chicken Supply Modes Using Genome-Wide and Machine-Learning Analyses. Food Res. Int. 2024, 191, 114654. [Google Scholar] [CrossRef] [PubMed]
| Port | No. of Poultry Slaughtered/Day | No. of Slaughterhouses Analyzed |
|---|---|---|
| Small | <50,000 | 16 |
| Medium | 50,001–100,000 | 30 |
| Large | 100,001–200,000 | 44 |
| Very Large | >200,001 | 25 |
| Slaughterhouse | n | c | No. of Cycles/Year | Collection Frequency |
|---|---|---|---|---|
| Small | 8 | 2 | 2 | 1 sample/3 weeks |
| Medium | 8 | 2 | 2 | 1 sample/3 weeks |
| Large | 8 | 2 | 2 | 1 sample/2 weeks |
| Very Large | 8 | 2 | 2 | 1 sample/2 weeks |
| Marketplace | No. of Slaughtering Establishments | No. of Slaughter Establishments Sampled | No. of Samples Analyzed |
|---|---|---|---|
| External (EM) | 116 | 95 | (1267) |
| Internal (IM) | 24 | 20 | (167) |
| Total | 140 | 115 | (1434) |
| Slaughterhouse Size | % Salmonella | Number of Positive Samples | CI (95%) |
|---|---|---|---|
| Small | 25.18 | 25 (124) | 14.41–40.20 |
| Medium | 17.15 | 42 (256) | 9.8–28.35 |
| Large | 18.7 | 114 (633) | 13.75–24.97 |
| Very Large | 17.13 | 68 (421) | 11.93–23.97 |
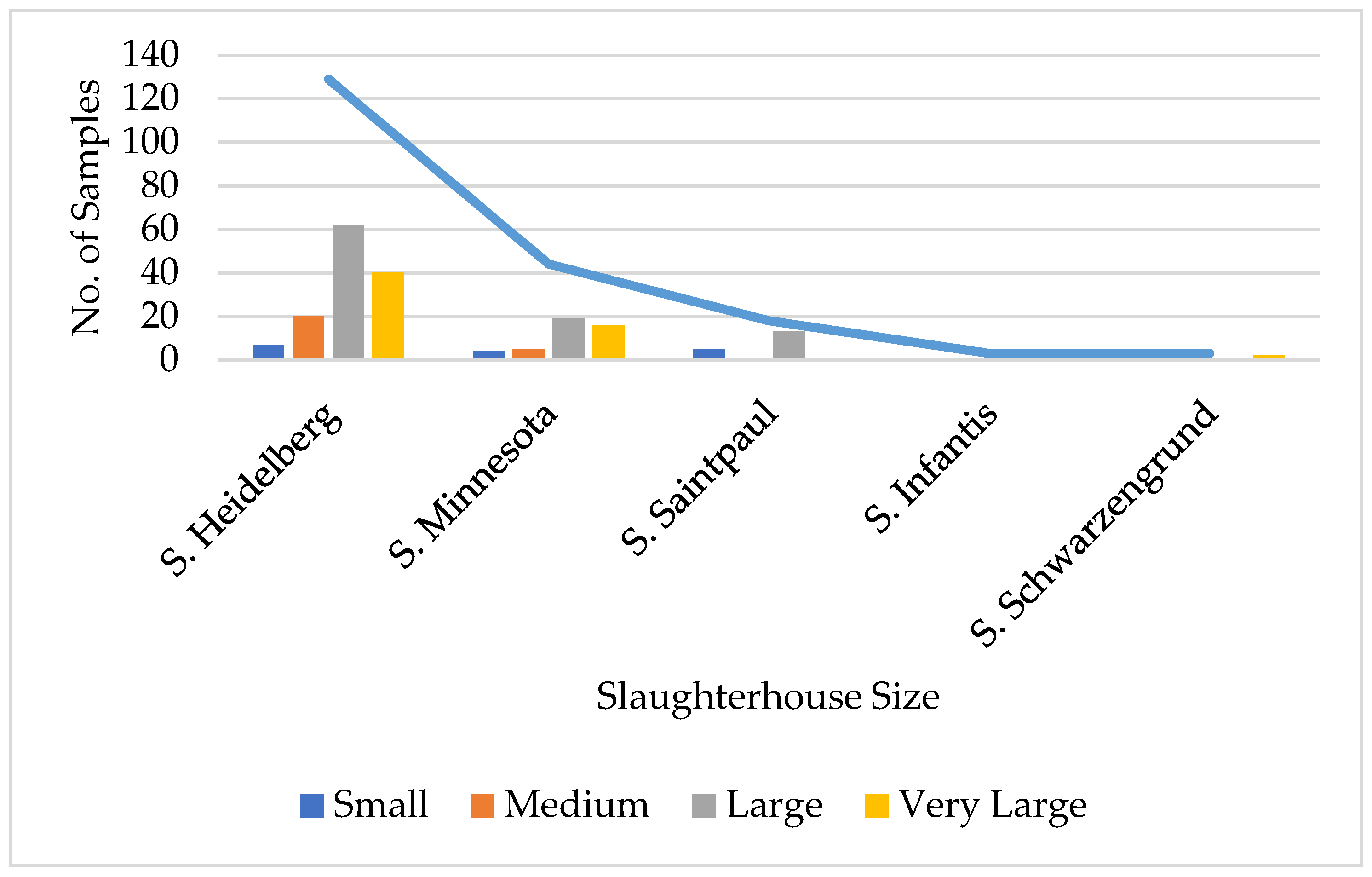
| Salmonella Serovars | Number of Isolates | % |
|---|---|---|
| Salmonella Heidelberg | 129 | 9.0 |
| Salmonella Minnesota | 44 | 3.07 |
| Salmonella Saintpaul | 18 | 1.26 |
| Salmonella Infantis | 3 | 0.21 |
| Salmonella Schwarzengrund | 3 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brasileiro, A.C.M.; Sá, C.V.G.C.d.; Rodrigues, C.S.; Oliveira, A.; Nicolino, R.; Haddad, J.P.A. Risk Factors and Prevalence of Salmonella spp. in Poultry Carcasses in Slaughterhouses Under Official Veterinary Inspection Service in Brazil. Animals 2025, 15, 2377. https://doi.org/10.3390/ani15162377
Brasileiro ACM, Sá CVGCd, Rodrigues CS, Oliveira A, Nicolino R, Haddad JPA. Risk Factors and Prevalence of Salmonella spp. in Poultry Carcasses in Slaughterhouses Under Official Veterinary Inspection Service in Brazil. Animals. 2025; 15(16):2377. https://doi.org/10.3390/ani15162377
Chicago/Turabian StyleBrasileiro, Anna Carolina Massara, Cláudia Valéria Gonçalves Cordeiro de Sá, Carla Susana Rodrigues, Adriana Oliveira, Rafael Nicolino, and João Paulo Amaral Haddad. 2025. "Risk Factors and Prevalence of Salmonella spp. in Poultry Carcasses in Slaughterhouses Under Official Veterinary Inspection Service in Brazil" Animals 15, no. 16: 2377. https://doi.org/10.3390/ani15162377
APA StyleBrasileiro, A. C. M., Sá, C. V. G. C. d., Rodrigues, C. S., Oliveira, A., Nicolino, R., & Haddad, J. P. A. (2025). Risk Factors and Prevalence of Salmonella spp. in Poultry Carcasses in Slaughterhouses Under Official Veterinary Inspection Service in Brazil. Animals, 15(16), 2377. https://doi.org/10.3390/ani15162377






