A Functional Regulatory Variant of FGF9 Gene Affected the Body Weight in Hu Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Phenotypic Data and Samples
2.2. DNA, RNA Extraction and Quality Control
2.3. SNP Identification and Genotyping
2.4. Quantitative Real-Time PCR Analysis
2.5. ATAC-Seq and CUT&Tag Library Construction and Sequencing
2.6. ATAC-Seq and CUT&Tag Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Descriptive Statistics for Body Weight Traits
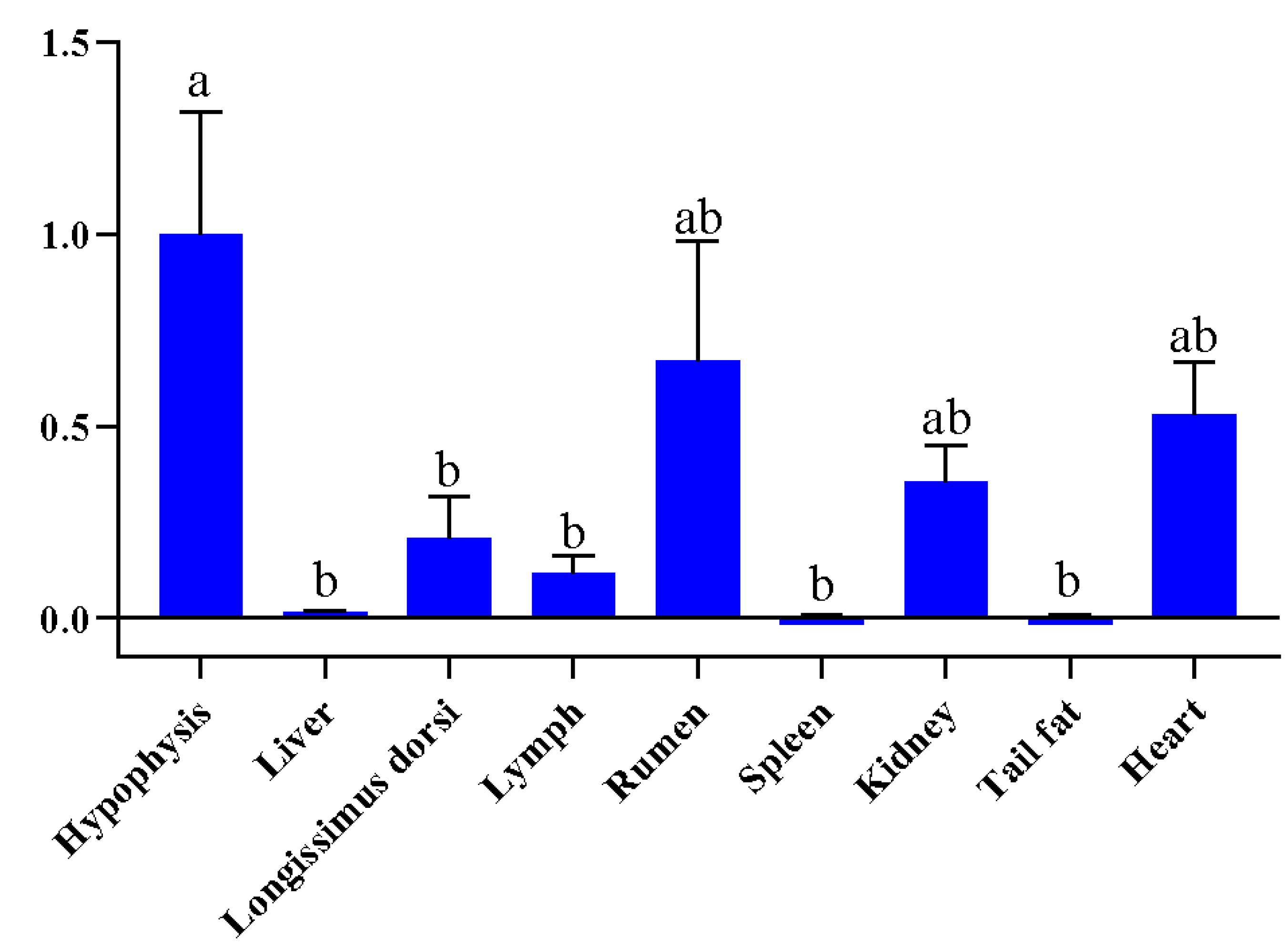
3.2. Expression Features Analysis of FGF9 Gene in Sheep
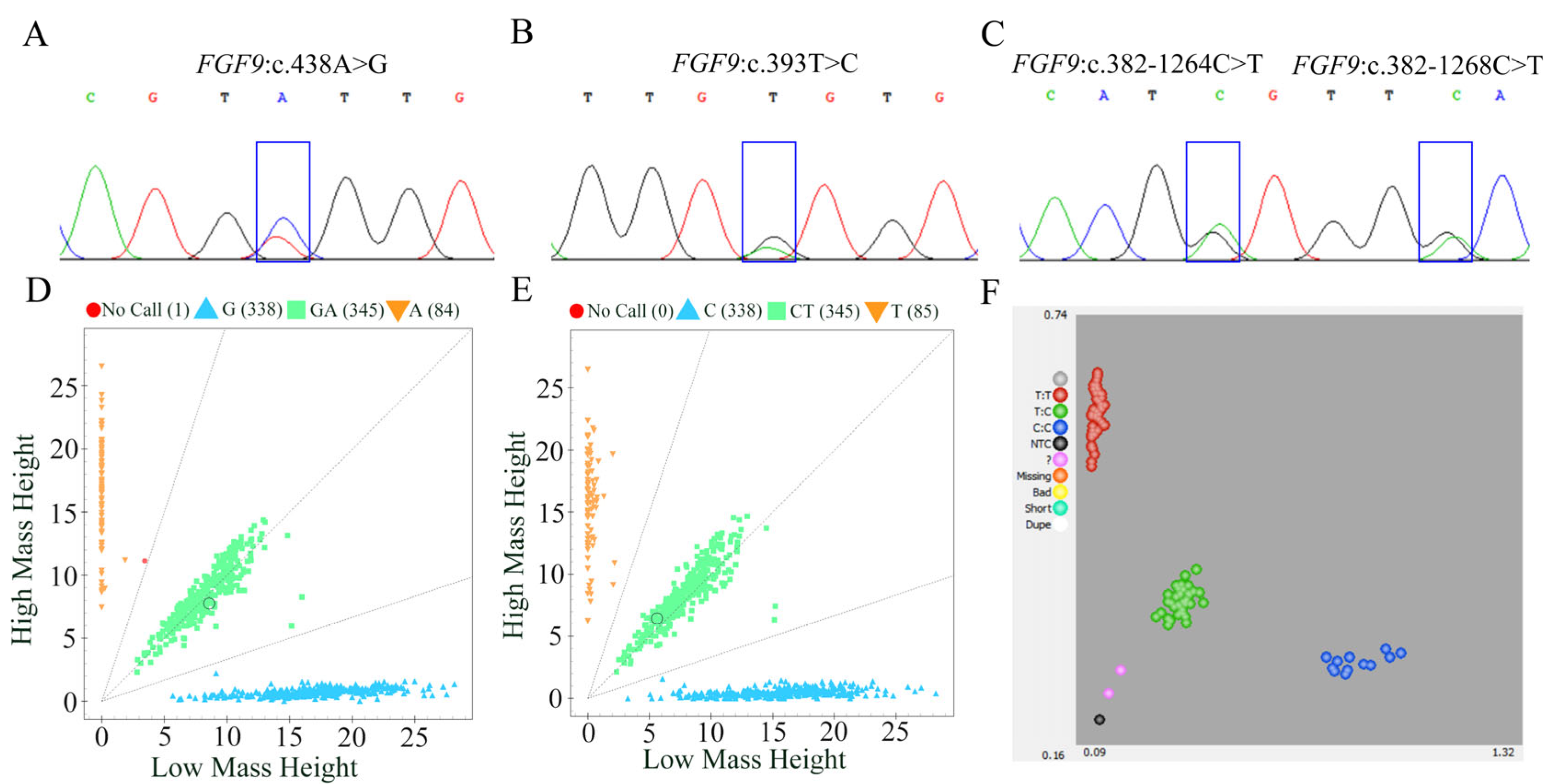
3.3. Detection and Genotyping of FGF9 Polymorphism in Hu Sheep
3.4. Population Genetic Parameter Analysis of SNPs in FGF9 Gene
3.5. Association of Polymorphisms in FGF9 with Body Weight in Hu Sheep
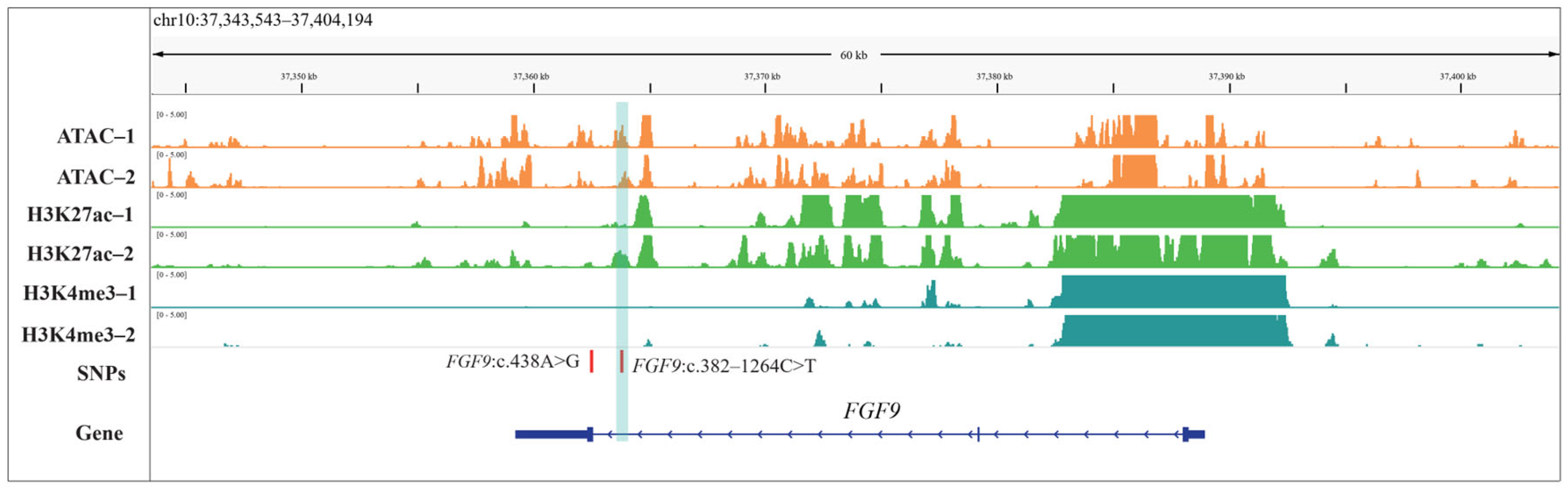
3.6. Epigenetic Data (ATAC-seq, H3K4me3, and H3K427ac) Quality Control of Hypophysis Tissue in Sheep
3.7. Comprehensive Profiling of Genetic Variants and Epigenomic Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATAC-seq | Assay for transposase-accessible chromatin-sequencing |
| BW | Body weight |
| CV | Coefficient of variation |
| Cut&Tag | Cleavage under targets and tagmentation |
| FGF9 | Fibroblast growth factor 9 |
| H3K27ac | Histone H3 lysine 27 acetylation |
| H3K4me3 | Histone H3 lysine 4 trimethylation |
| OCR | Open chromatin region |
| qRT-PCR | Quantitative real-time PCR |
| SNPs | Single nucleotide polymorphisms |
References
- Mohamadipoor Saadatabadi, L.; Mohammadabadi, M.; Amiri Ghanatsaman, Z.; Babenko, O.; Stavetska, R.; Kalashnik, O.; Kucher, D.; Kochuk-Yashchenko, O.; Asadollahpour Nanaei, H. Signature selection analysis reveals candidate genes associated with production traits in Iranian sheep breeds. BMC Vet. Res. 2021, 17, 369. [Google Scholar] [CrossRef]
- Tao, L.; He, X.; Pan, L.; Wang, J.; Gan, S.; Chu, M. Genome-wide association study of body weight and conformation traits in neonatal sheep. Anim. Genet. 2020, 51, 336–340. [Google Scholar] [CrossRef]
- Al-Mamun, H.A.; Kwan, P.; Clark, S.A.; Ferdosi, M.H.; Tellam, R.; Gondro, C. Genome-wide association study of body weight in Australian Merino sheep reveals an orthologous region on OAR6 to human and bovine genomic regions affecting height and weight. Genet. Sel. Evol. 2015, 47, 66. [Google Scholar] [CrossRef]
- Lu, Z.; Yue, Y.; Yuan, C.; Liu, J.; Yang, B. Genome-Wide Association Study of Body Weight Traits in Chinese Fine-Wool Sheep. Animals 2020, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- FAANG Consortium; Andersson, L.; Archibald, A.L.; Bottema, C.D.; Brauning, R.; Burgess, S.C.; Burt, D.W.; Casas, E.; Cheng, H.H.; Clarke, L.; et al. Coordinated international action to accelerate genome-to-phenome with FAANG, the Functional Annotation of Animal Genomes project. Genome Biol. 2015, 16, 4–9. [Google Scholar]
- Liu, S.; Gao, Y.; Canela-Xandri, O.; Wang, S.; Yu, Y.; Cai, W.; Li, B.; Xiang, R.; Chamberlain, A.J.; Pairo-Castineira, E. A multi-tissue atlas of regulatory variants in cattle. Nat. Genet. 2022, 54, 1438–1447. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Li, F.; Yuan, L.; Li, X.; Zhang, Y.; Zhao, Y.; Zhao, L.; Wang, J.; Xu, D.; et al. Whole-genome resequencing reveals molecular imprints of anthropogenic and natural selection in wild and domesticated sheep. Zool. Res. 2022, 43, 695–705. [Google Scholar] [CrossRef]
- He, P.; Zhong, S.; Lin, S.; Xia, Z.; Wang, L.; Han, Y.; Xu, D.; Hu, S.; Li, X.; Li, P.; et al. FGF9 is required for Purkinje cell development and function in the cerebellum. iScience 2024, 27, 109039. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y.A.-O.X.; Zhu, J.; Xiong, Y.A.-O.; Lin, Y.A.-O. Regulation of fibroblast growth factor 9 on the differentiation of goat intramuscular adipocytes. Anim. Sci. J. 2021, 92, e13627. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Jiang, C.; Tian, H. Fibroblast growth factor 9 subfamily and the heart. Appl. Microbiol. Biotechnol. 2018, 102, 605–613. [Google Scholar] [CrossRef]
- Wang, L.; Roth, T.; Abbott, M.; Ho, L.; Wattanachanya, L.; Nissenson, R.A. Osteoblast-derived FGF9 regulates skeletal homeostasis. Bone 2017, 98, 18–25. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Zhao, S.; Li, W.; Liu, W.; Tang, L.; Wang, Z.; Wang, W.; Liu, R.; Ning, G.; et al. FGF9 inhibits browning program of white adipocytes and associates with human obesity. J. Mol. Endocrinol. 2019, 62, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, F.; Xue, R.; Huang, T.L.; Lundh, M.; Liu, Y.; Leiria, L.O.; Lynes, M.D.; Kempf, E.; Wang, C.-H.; Sugimoto, S.; et al. FGF6 and FGF9 regulate UCP1 expression independent of brown adipogenesis. Nat. Commun. 2020, 11, 1421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, F.; Cheng, X.; Yang, M.; Ning, Z. Identification of candidate genomic regions for egg yolk moisture content based on a genome-wide association study. BMC Genom. 2023, 24, 110. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Wang, Y.; Chen, Q.; Sun, Y.; Kang, L.; Jiang, Y. Phosphorylation of LSD1 at serine 54 regulates genes involved in follicle selection by enhancing demethylation activity in chicken ovarian granulosa cells. Poult. Sci. 2024, 103, 103850. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, T.; Wu, L.; Liu, X.; Xue, S.; Lei, M. Identification of non-coding and coding RNAs in porcine endometrium. Genomics 2017, 109, 43–50. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Yang, H.; Deng, K.; Guo, Y.; Zhang, T.; Zhang, G.; Wang, F. Role of FGF9 in sheep testis steroidogenesis during sexual maturation. Anim. Reprod. Sci. 2018, 197, 177–184. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, S.; Wang, D.; Liu, J.; Luo, X.; Liu, Y.; Li, X.; Sun, F.; Xia, G.; Zhang, L. Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep. Genes 2023, 14, 1106. [Google Scholar] [CrossRef]
- Li, T.; Xing, F.; Zhang, N.; Chen, J.; Zhang, Y.; Yang, H.; Peng, S.; Ma, R.; Liu, Q.; Gan, S.; et al. Genome-Wide Association Analysis of Growth Traits in Hu Sheep. Genes 2024, 15, 1637. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Li, F.; Yuan, L.; Zhang, Y.; Li, X.; Zhao, Y.; Song, Q.; Li, G.; Wang, W. Polymorphisms in ovine ME1 and CA1 genes and their association with feed efficiency in Hu sheep. J. Anim. Breed. Genet. 2021, 138, 589–599. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Li, F.; Li, C.; La, Y.; Mo, F.; Li, G.; Zhang, Y.; Li, X.; Song, Q.; et al. Transcriptome Analysis Identifies Candidate Genes and Pathways Associated with Feed Efficiency in Hu Sheep. Front. Genet. 2019, 10, 1183. [Google Scholar] [CrossRef]
- Leslie, F.; Cécile, G.; Jean-François, A. Development of Nuclear Microsatellite Loci and Mitochondrial Single Nucleotide Polymorphisms for the Natterjack Toad, Bufo (Epidalea) calamita (Bufonidae), Using Next Generation Sequencing and Competitive Allele Specific PCR (KASPar). J. Hered. 2016, 107, 660–665. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.; Xu, Y.; Luan, Y.; Zhou, H.; Qi, X.; Hu, M.; Wang, D.; Wang, Z.; Fu, Y.; et al. A compendium and comparative epigenomics analysis of cis-regulatory elements in the pig genome. Nat. Commun. 2021, 12, 2217. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, J.; Li, X.; Huang, K.; Yuan, L.; Zhao, Y.; Xu, D.; Zhang, Y.; Zhao, L.; Yang, X.; et al. Comprehensive multi-tissue epigenome atlas in sheep: A resource for complex traits, domestication, and breeding. Imeta 2024, 3, e254. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; He, Q.Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 2015, 31, 2382–2383. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Song, X.; Shan, H.; Jiang, J.; Xiong, P.; Wu, J.; Shi, F.; Jiang, Y. Genome-Wide Association Study of Body Weights in Hu Sheep and Population Verification of Related Single-Nucleotide Polymorphisms. Front. Genet. 2020, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Zhang, C.; Gao, Y.; Zhao, L.; Guo, J.; Yang, Y.; Yu, Q.; Li, Y.; Wang, Z.; Yang, H. Genome-wide unraveling SNP pairwise epistatic effects associated with sheep body weight. Anim. Biotechnol. 2023, 34, 3416–3427. [Google Scholar] [CrossRef]
- Pasandideh, M.A.-O.; Gholizadeh, M.; Rahimi-Mianji, G. A genome-wide association study revealed five SNPs affecting 8-month weight in sheep. Anim. Genet. 2020, 51, 973–976. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Q.; Wu, P.; Chen, H.; Wang, S.; Chen, D.; Yu, Y.; Guo, Z.; Wang, J.; Tang, G. Pituitary-Gland-Based Genes Participates in Intrauterine Growth Restriction in Piglets. Genes 2022, 13, 2141. [Google Scholar] [CrossRef]
- Zhang, S.A.-O.; Cui, Y.; Ma, X.A.-O.; Yong, J.; Yan, L.A.-O.; Yang, M.; Ren, J.; Tang, F.A.-O.; Wen, L.A.-O.; Qiao, J.A.-O. Single-cell transcriptomics identifies divergent developmental lineage trajectories during human pituitary development. Nat. Commun. 2020, 11, 5275. [Google Scholar] [CrossRef]
- Tang, L.A.-O.; Wu, M.; Lu, S.; Zhang, H.; Shen, Y.; Shen, C.; Liang, H.; Ge, H.; Ding, X.; Wang, Z.A.-O. Fgf9 Negatively Regulates Bone Mass by Inhibiting Osteogenesis and Promoting Osteoclastogenesis Via MAPK and PI3K/AKT Signaling. J. Bone Miner. Res. 2020, 36, 779–791. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Shiflett, L.A.; Brotto, L.; Bonewald, L.F.; Wacker, M.J.; Dallas, S.A.-O.X.; Brotto, M. Fibroblast growth factor 9 (FGF9) inhibits myogenic differentiation of C2C12 and human muscle cells. Cell Cycle 2019, 18, 3562–3580. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yao, Y.; Yin, H.; Cai, Z.; Wang, Y.; Bai, L.; Kern, C.; Halstead, M.; Chanthavixay, G.; Trakooljul, N.; et al. Pig genome functional annotation enhances the biological interpretation of complex traits and human disease. Nat. Commun. 2021, 12, 5848. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Li, C.; Wu, H.; Zhang, R.; Hu, X. Chicken chromatin accessibility atlas accelerates epigenetic annotation of birds and gene fine-mapping associated with growth traits. Zool. Res. 2023, 44, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Smemo, S.; Tena, J.J.; Kim, K.H.; Gamazon, E.R.; Sakabe, N.J.; Gómez-Marín, C.; Aneas, I.; Credidio, F.L.; Sobreira, D.R.; Wasserman, N.F.; et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 2014, 507, 371–375. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, Y.; Wan, S.; Mei, Q.; Wang, H.; Fu, C.; Li, X.; Zhao, S.; Xu, X.; Xiang, T. Integrated analysis of genome-wide association studies and 3D epigenomic characteristics reveal the BMP2 gene regulating loin muscle depth in Yorkshire pigs. PLoS Genet. 2023, 19, e1010820. [Google Scholar] [CrossRef]
- Raisner, R.; Kharbanda, S.; Jin, L.; Jeng, E.; Chan, E.; Merchant, M.; Haverty, P.M.; Bainer, R.; Cheung, T.; Arnott, D.; et al. Enhancer Activity Requires CBP/P300 Bromodomain-Dependent Histone H3K27 Acetylation. Cell Rep. 2018, 24, 1722–1729. [Google Scholar] [CrossRef]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence (5′-3′) | Annealing Temperature (°C) | Amplicon Size (bp) |
|---|---|---|---|
| FGF9-expression-F | GAAGCTGCATTTAATCCCAAG | 60 | 204 bp |
| FGF9-expression-R | GATCACTTTTGGCTGTCTCC | ||
| β-actin-F | TCCGTGACATCAAGGAGAAGC | 60 | 256 bp |
| β-actin-R | CCGTGTTGGCGTAGAGGT |
| Traits | Mean | SD | Median | Min | Max | Skew | Kurtosis | CV (%) |
|---|---|---|---|---|---|---|---|---|
| BW80 | 19.42 | 4.02 | 19.30 | 9.50 | 34.40 | 0.245 | −0.061 | 20.73% |
| BW100 | 24.55 | 4.83 | 24.50 | 9.78 | 42.40 | 0.052 | 0.073 | 19.68% |
| BW120 | 30.37 | 5.35 | 30.30 | 13.80 | 49.40 | 0.013 | 0.133 | 17.62% |
| BW140 | 36.07 | 5.74 | 36.10 | 19.55 | 56.30 | 0.029 | 0.016 | 15.91% |
| BW160 | 41.87 | 6.13 | 41.60 | 25.10 | 63.60 | 0.070 | 0.122 | 14.64% |
| BW180 | 46.80 | 6.41 | 46.80 | 24.55 | 70.20 | −0.009 | 0.242 | 13.70% |
| Locus | Genotype | No. | BW80 | BW100 | BW120 | BW140 | BW160 | BW180 |
|---|---|---|---|---|---|---|---|---|
| FGF9:c.438A>G | GG | 471 | 19.50 ± 0.19 | 24.61 ± 0.22 | 30.44 ± 0.25 | 36.20 ± 0.27 | 42.05 ± 0.28 | 46.88 ± 0.30 |
| GA | 478 | 19.44 ± 0.18 | 24.52 ± 0.22 | 30.34 ± 0.25 | 35.96 ± 0.26 | 41.75 ± 0.28 | 46.79 ± 0.29 | |
| AA | 118 | 19.11 ± 0.37 | 24.58 ± 0.45 | 30.27 ± 0.49 | 36.02 ± 0.53 | 41.64 ± 0.57 | 46.50 ± 0.59 | |
| FGF9:c.382-1264C>T | TT | 481 | 19.40 ± 0.18 a | 24.56 ± 0.22 a | 30.29 ± 0.24 a | 36.04 ± 0.26 a | 41.54 ± 0.27 a | 46.37 ± 0.29 ab |
| TC | 468 | 19.33 ± 0.19 a | 24.53 ± 0.22 a | 30.40 ± 0.25 a | 36.15 ± 0.27 a | 41.69 ± 0.28 a | 46.65 ± 0.30 a | |
| CC | 118 | 18.36 ± 0.37 b | 23.49 ± 0.44 b | 29.20 ± 0.48 b | 34.92 ± 0.53 b | 40.44 ± 0.55 b | 45.41 ± 0.59 b |
| Assay | Raw Reads | Clean Reads | Clean Q20 (%) | Clean Q30 (%) | Mapping Rate (%) | RSC | FRiP |
|---|---|---|---|---|---|---|---|
| ATAC-seq_1 | 355,833,444 | 351,268,398 | 97.52 | 93.33 | 99.49 | 1.56 | 0.38 |
| ATAC-seq_2 | 347,033,728 | 342,040,172 | 97.35 | 92.95 | 99.64 | 1.87 | 0.36 |
| H3K4me3_1 | 71,235,594 | 66,164,158 | 98.06 | 93.51 | 97.80 | 1.24 | 0.79 |
| H3K4me3_2 | 130,505,626 | 115,742,448 | 97.33 | 91.53 | 97.40 | 1.18 | 0.86 |
| H3K27ac_1 | 68,907,382 | 64,771,048 | 98.49 | 94.55 | 98.30 | 1.42 | 0.23 |
| H3K27ac_2 | 91,169,150 | 84,833,346 | 98.01 | 93.19 | 98.00 | 1.33 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, D.; Li, F.; Xu, D.; Cheng, J.; Li, X.; Zhao, Y.; Zhang, Y.; Zhao, L.; Cao, P.; et al. A Functional Regulatory Variant of FGF9 Gene Affected the Body Weight in Hu Sheep. Animals 2025, 15, 2375. https://doi.org/10.3390/ani15162375
Zhang X, Zhang D, Li F, Xu D, Cheng J, Li X, Zhao Y, Zhang Y, Zhao L, Cao P, et al. A Functional Regulatory Variant of FGF9 Gene Affected the Body Weight in Hu Sheep. Animals. 2025; 15(16):2375. https://doi.org/10.3390/ani15162375
Chicago/Turabian StyleZhang, Xiaoxue, Deyin Zhang, Fadi Li, Dan Xu, Jiangbo Cheng, Xiaolong Li, Yuan Zhao, Yukun Zhang, Liming Zhao, Peiliang Cao, and et al. 2025. "A Functional Regulatory Variant of FGF9 Gene Affected the Body Weight in Hu Sheep" Animals 15, no. 16: 2375. https://doi.org/10.3390/ani15162375
APA StyleZhang, X., Zhang, D., Li, F., Xu, D., Cheng, J., Li, X., Zhao, Y., Zhang, Y., Zhao, L., Cao, P., Tian, H., Wu, W., & Wang, W. (2025). A Functional Regulatory Variant of FGF9 Gene Affected the Body Weight in Hu Sheep. Animals, 15(16), 2375. https://doi.org/10.3390/ani15162375






