Activation of Focal Adhesion Pathway by CIDEA as Key Regulatory Axis in Lipid Deposition in Goat Intramuscular Precursor Adipocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Isolation and Culture of Goat Intramuscular Preadipocytes
2.3. Goat CIDEA Overexpression Vector Construction and siRNA Synthesis
2.4. Oil Red O Staining and Triglyceride Determination
2.5. Cell-Counting Kit-8 (CCK-8) Assay
2.6. Western Blot
2.7. Reverse Transcription–Quantitative PCR (RT-qPCR)
2.8. RNA Sequencing (RNA-seq)
2.9. Statistical Analysis
3. Results
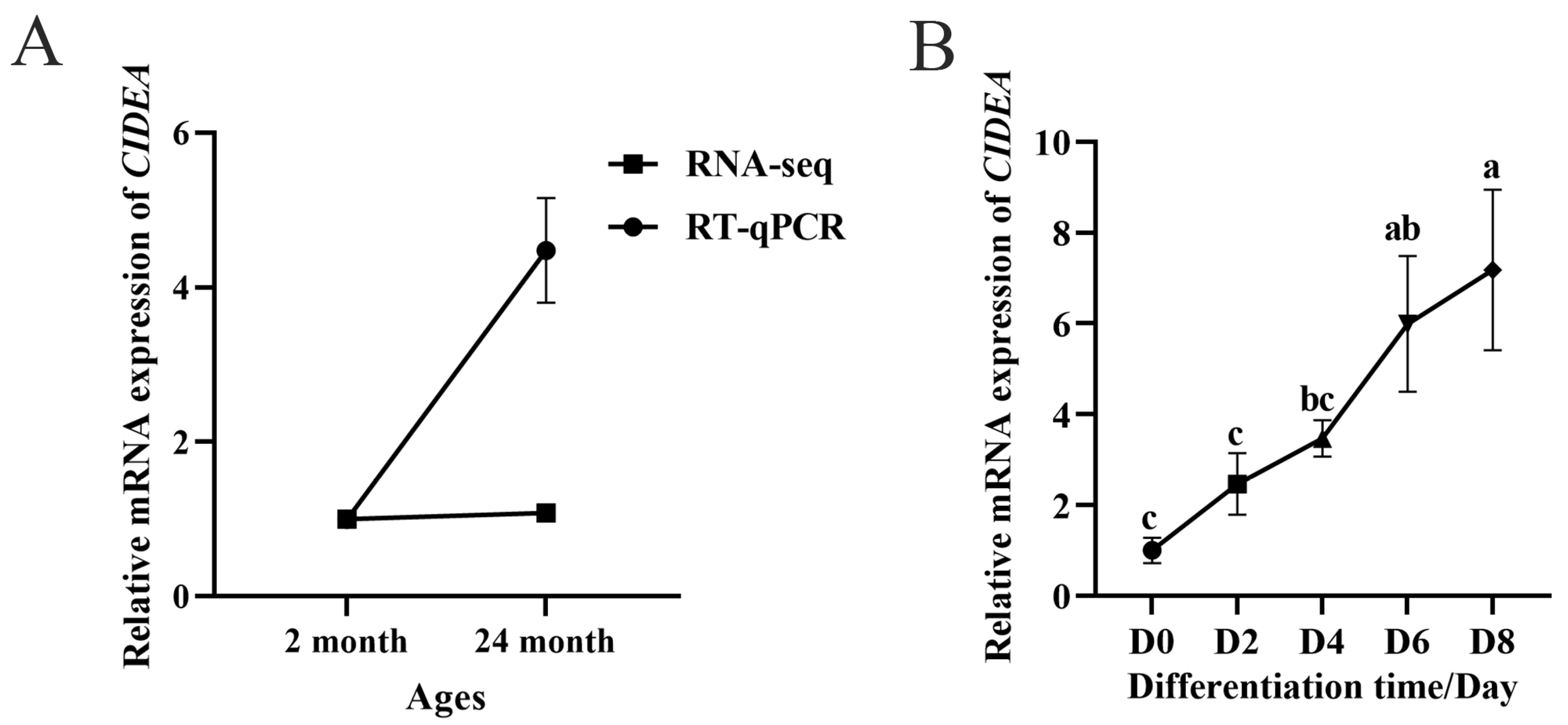
3.1. CIDEA Is Associated with Intramuscular Fat Deposition
3.2. Overexpression of CIDEA Promotes Lipid Deposition in Goat Primary Intramuscular Preadipocytes
3.3. Knockdown of CIDEA Inhibits Adipogenesis in Intramuscular Preadipocytes
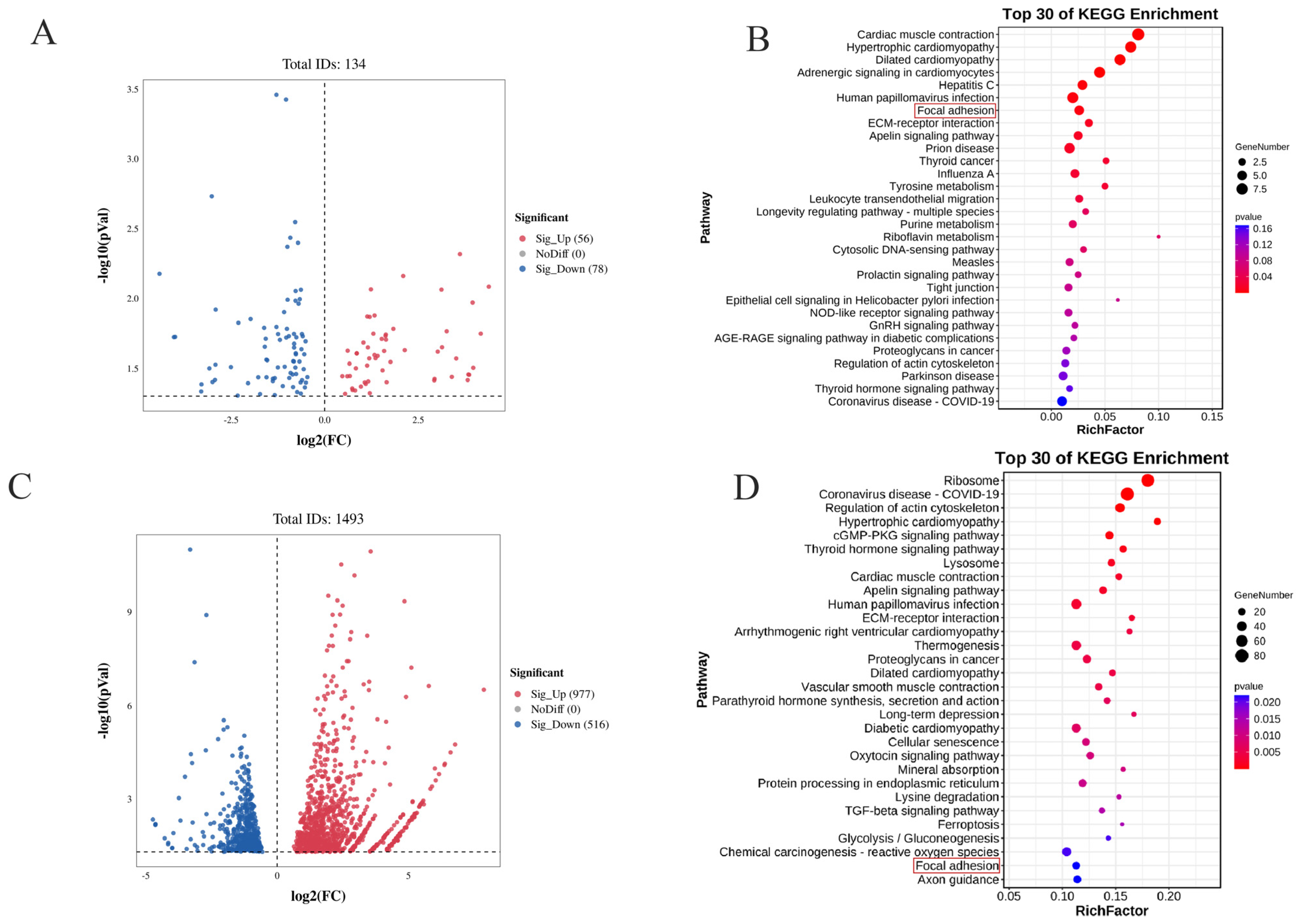
3.4. Screening and Analysis of Differentially Expressed Genes (DEGs) in Intramuscular Preadipocytes with Dysregulated CIDEA Expression
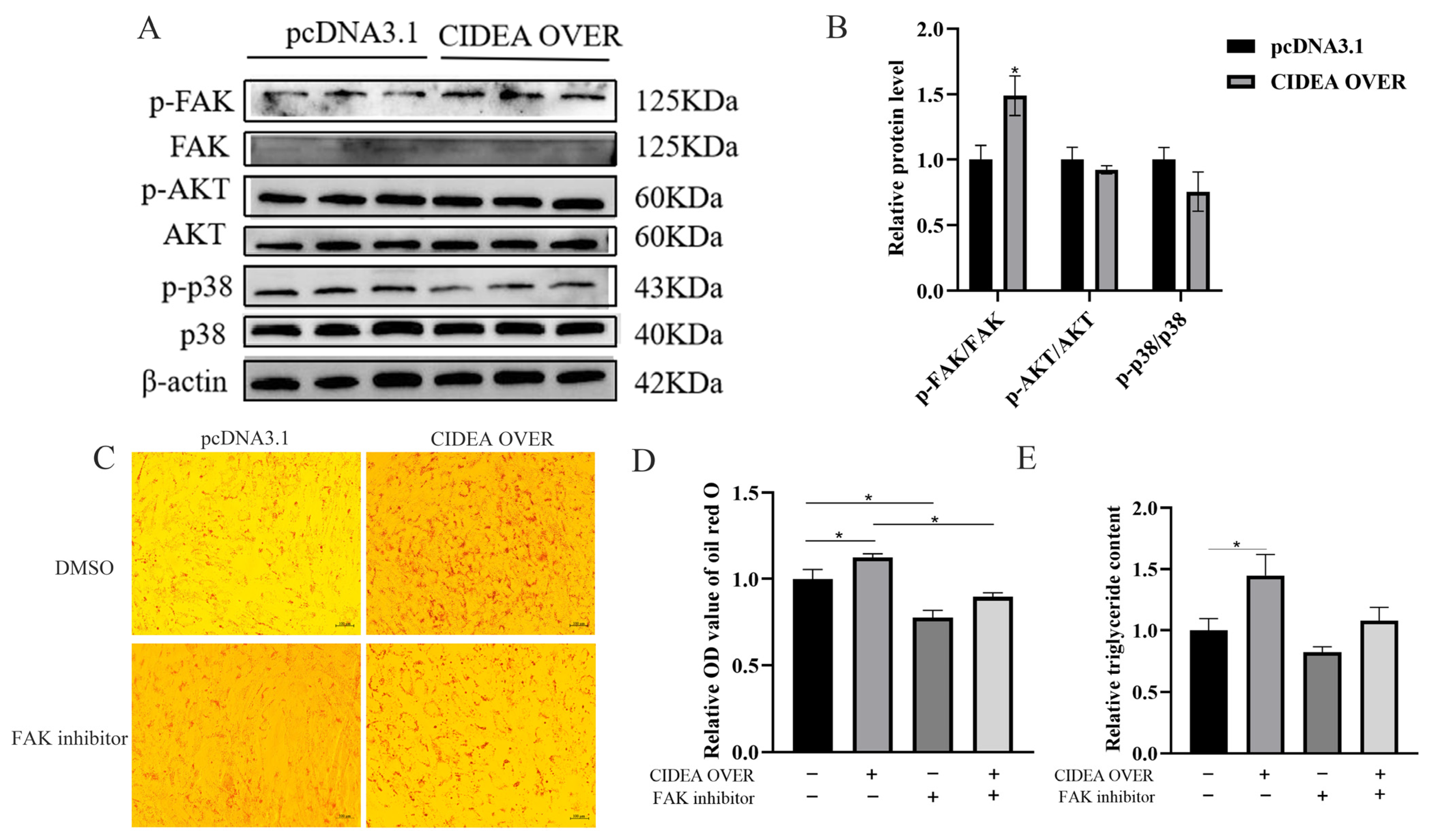
3.5. CIDEA Regulates Lipid Deposition in Goat Intramuscular Preadipocytes via Focal Adhesion Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Sharma, N.; Sharma, S.; Mehta, N.; Verma, A.K.; Chemmalar, S.; Sazili, A.Q. In-vitro meat: A promising solution for sustainability of meat sector. J. Anim. Sci. Technol. 2021, 63, 693–724. [Google Scholar] [CrossRef]
- Baik, M.; Kang, H.J.; Park, S.J.; Na, S.W.; Piao, M.; Kim, S.Y.; Fassah, D.M.; Moon, Y.S. TRIENNIAL GROWTH AND DEVELOPMENT SYMPOSIUM: Molecular mechanisms related to bovine intramuscular fat deposition in the longissimus muscle. J. Anim. Sci. 2017, 95, 2284–2303. [Google Scholar] [CrossRef]
- Tan, Z.; Jiang, H. Molecular and Cellular Mechanisms of Intramuscular Fat Development and Growth in Cattle. Int. J. Mol. Sci. 2024, 25, 2520. [Google Scholar] [CrossRef]
- Chen, F.J.; Yin, Y.; Chua, B.T.; Li, P. CIDE family proteins control lipid homeostasis and the development of metabolic diseases. Traffic 2020, 21, 94–105. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, L.K.; Li, P. CIDE Proteins and Lipid Metabolism. Arterioscler. Thromb. Vasc. 2012, 32, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Gong, J.; Zhao, T.; Zhao, J.; Lam, P.; Ye, J.; Li, J.Z.; Wu, J.; Zhou, H.M.; Li, P. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. EMBO J. 2008, 27, 1537–1548. [Google Scholar] [CrossRef]
- Christianson, J.L.; Boutet, E.; Puri, V.; Chawla, A.; Czech, M.P. Identification of the lipid droplet targeting domain of the Cidea protein. J. Lipid Res. 2010, 51, 3455–3462. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nakajima, S.; Tateya, S.; Saito, M.; Ogawa, W.; Tamori, Y. Cell death-inducing DNA fragmentation factor A-like effector A and fat-specific protein 27β coordinately control lipid droplet size in brown adipocytes. J. Biol. Chem. 2017, 292, 10824–10834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shui, G.; Wang, G.; Wang, C.; Sun, S.; Zouboulis, C.C.; Xiao, R.; Ye, J.; Li, W.; Li, P. Cidea control of lipid storage and secretion in mouse and human sebaceous glands. Mol. Cell Biol. 2014, 34, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yon Toh, S.; Chen, Z.; Guo, K.; Ng, C.P.; Ponniah, S.; Lin, S.C.; Hong, W.; Li, P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 2003, 35, 49–56. [Google Scholar] [CrossRef]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De Matteis, R.; Cinti, S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1244–E1253. [Google Scholar] [CrossRef]
- Do, G.M.; Oh, H.Y.; Kwon, E.Y.; Cho, Y.Y.; Shin, S.K.; Park, H.J.; Jeon, S.M.; Kim, E.; Hur, C.G.; Park, T.S.; et al. Long-term adaptation of global transcription and metabolism in the liver of high-fat diet-fed C57BL/6J mice. Mol. Nutr. Food Res. 2011, 55 (Suppl. 2), S173–S185. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, L.; Ye, J.; Li, D.; Wang, W.; Li, X.; Wu, L.; Wang, H.; Guan, F.; Li, P. Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology 2012, 56, 95–107. [Google Scholar] [CrossRef]
- Abreu-Vieira, G.; Fischer, A.W.; Mattsson, C.; de Jong, J.M.; Shabalina, I.G.; Rydén, M.; Laurencikiene, J.; Arner, P.; Cannon, B.; Nedergaard, J.; et al. Cidea improves the metabolic profile through expansion of adipose tissue. Nat. Commun. 2015, 6, 7433. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Ranjit, S.; Konda, S.; Nicoloro, S.M.; Straubhaar, J.; Chawla, A.; Chouinard, M.; Lin, C.; Burkart, A.; Corvera, S.; et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 7833–7838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liao, Y.; Shao, P.; Yang, Y.; Huang, L.; Du, Z.; Zhang, C.; Wang, Y.; Lin, Y.; Zhu, J. Integrated analysis of differently expressed microRNAs and mRNAs at different postnatal stages reveals intramuscular fat deposition regulation in goats (Capra hircus). Anim. Genet. 2024, 55, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; Cheng, Y.; Foh, L.C.; Leow, M.K.; Lim, S.C.; Chuah, Y.J.; Kang, Y.; Chen, P. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell Endocrinol. 2012, 362, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q.; Hu, X.; Fang, Z.; Huang, F.; Tang, L.; Zhou, S. Apelin/APJ signaling promotes hypoxia-induced proliferation of endothelial progenitor cells via phosphoinositide-3 kinase/Akt signaling. Mol. Med. Rep. 2015, 12, 3829–3834. [Google Scholar] [CrossRef]
- Acebrón, I.; Righetto, R.D.; Schoenherr, C.; de Buhr, S.; Redondo, P.; Culley, J.; Rodríguez, C.F.; Daday, C.; Biyani, N.; Llorca, O.; et al. Structural basis of Focal Adhesion Kinase activation on lipid membranes. EMBO J. 2020, 39, e104743. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 250. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, J.; Wang, Y.; Li, Q.; Lin, S. Identification of differentially expressed genes through RNA sequencing in goats (Capra hircus) at different postnatal stages. PLoS ONE 2017, 12, e0182602. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gou, X.; Deng, J.; Dong, Z.; Ye, P.; Hu, Z. FAK and BMP-9 synergistically trigger osteogenic differentiation and bone formation of adipose derived stem cells through enhancing Wnt-β-catenin signaling. Biomed. Pharmacother. 2018, 105, 753–757. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Xu, Q.; Li, A.; Yue, Y.; Ma, Y.; Lin, Y. LKB1 Regulates Goat Intramuscular Adipogenesis Through Focal Adhesion Pathway. Front. Physiol. 2021, 12, 755598. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, W.; Wang, Y.; Li, H.; Zhang, C.; Wang, Y.; Lin, Y.; Shi, H.; Xiang, H.; Huang, L.; et al. Expression Variation of CPT1A Induces Lipid Reconstruction in Goat Intramuscular Precursor Adipocytes. Int. J. Mol. Sci. 2023, 24, 13415. [Google Scholar] [CrossRef]
- Li, F.; Gu, Y.; Dong, W.; Li, H.; Zhang, L.; Li, N.; Li, W.; Zhang, L.; Song, Y.; Jiang, L.; et al. Cell death-inducing DFF45-like effector, a lipid droplet-associated protein, might be involved in the differentiation of human adipocytes. FEBS J. 2010, 277, 4173–4183. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, S.; Zhou, M.; Hu, H.; Li, J. The role of DGAT1 and DGAT2 in tumor progression via fatty acid metabolism: A comprehensive review. Int. J. Biol. Macromol. 2024, 278 Pt 3, 134835. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Lin, Y.; Wang, Y.; Shi, H.; Xiang, H.; Zhu, J. Diacylglycerol acyltransferase 2 promotes the adipogenesis of intramuscular preadipocytes in goat. Anim. Biotechnol. 2022, 34, 2376–2383. [Google Scholar] [CrossRef]
- Brejchova, K.; Radner, F.P.W.; Balas, L.; Paluchova, V.; Cajka, T.; Chodounska, H.; Kudova, E.; Schratter, M.; Schreiber, R.; Durand, T.; et al. Distinct roles of adipose triglyceride lipase and hormone-sensitive lipase in the catabolism of triacylglycerol estolides. Proc. Natl. Acad. Sci. USA 2021, 118, e2020999118. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.; Macwan, V.; Dixon, C.L.; Li, X.; Liu, S.; Yu, Y.; Xu, P.; Sun, Q.; Hu, Q.; et al. S-acylation of ATGL is required for lipid droplet homoeostasis in hepatocytes. Nat. Metab. 2024, 6, 1549–1565. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wei, G.; Liao, Z.Q.; Wang, F.X.; Zong, C.; Qiu, J.; Le, Y.; Yu, Z.L.; Yang, S.Y.; Wang, H.S.; et al. Design and Synthesis of Novel Indole Ethylamine Derivatives as a Lipid Metabolism Regulator Targeting PPARα/CPT1 in AML12 Cells. Molecules 2023, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xiong, Q.; Tao, H.; Liu, Y.; Zhang, N.; Li, X.F.; Suo, X.J.; Yang, Q.P.; Chen, M.X. ACOX1, regulated by C/EBPα and miR-25-3p, promotes bovine preadipocyte adipogenesis. J. Mol. Endocrinol. 2021, 66, 195–205. [Google Scholar] [CrossRef]
- Wang, W.; Lv, N.; Zhang, S.; Shui, G.; Qian, H.; Zhang, J.; Chen, Y.; Ye, J.; Xie, Y.; Shen, Y.; et al. Cidea is an essential transcriptional coactivator regulating mammary gland secretion of milk lipids. Nat. Med. 2012, 18, 235–243. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Duan, F.; Wang, Y.; Chen, S.; Shi, K.; Xia, Y.; Li, X.; Gao, Y.; Liu, G. DNMT3B Alleviates Liver Steatosis Induced by Chronic Low-grade LPS via Inhibiting CIDEA Expression. Cell Mol. Gastroenterol. Hepatol. 2024, 17, 59–77. [Google Scholar] [CrossRef]
- Tian, H.; Luo, J.; Guo, P.; Li, C.; Zhang, X. C/EBPα promotes triacylglycerol synthesis via regulating PPARG promoter activity in goat mammary epithelial cells. J. Anim. Sci. 2023, 101, skac412. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, D.; Chen, L.; Guo, W.; Hu, G.; Liu, J.; Fu, S. CIDEA Regulates De Novo Fatty Acid Synthesis in Bovine Mammary Epithelial Cells by Targeting the AMPK/PPARγ Axis and Regulating SREBP1. J. Agric. Food Chem. 2022, 70, 11324–11335. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Long, X.; Zhang, J.; Huang, X.; Aa, J.; Yang, H.; Chen, Z.; Xing, J. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J. Hepatol. 2015, 63, 1378–1389. [Google Scholar] [CrossRef]
- Ho, T.C.; Wan, H.T.; Lee, W.K.; Lam, T.K.Y.; Lin, X.; Chan, T.F.; Lai, K.P.; Wong, C.K.C. Effects of In Utero PFOS Exposure on Epigenetics and Metabolism in Mouse Fetal Livers. Environ. Sci. Technol. 2023, 57, 14892–14903. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; George, S.R.; O’Dowd, B.F. Unravelling the roles of the apelin system: Prospective therapeutic applications in heart failure and obesity. Trends Pharmacol. Sci. 2006, 27, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Lv, Y.-C.; Zhang, M.; Xie, W.; Tan, Y.-L.; Gong, D.; Cheng, H.-P.; Liu, D.; Li, L.; Liu, X.-Y.; et al. Apelin-13 impedes foam cell formation by activating Class III PI3K/Beclin-1-mediated autophagic pathway. Biochem. Biophys. Res. Commun. 2015, 466, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Liu, W.; Feng, F.; Li, X.; He, L.; Lv, D.; Qin, X.; Li, L.; Li, L.; Chen, L. Apelin-13 promotes cardiomyocyte hypertrophy via PI3K-Akt-ERK1/2-p70S6K and PI3K-induced autophagy. Acta Biochim. Biophys. Sin. 2015, 47, 969–980. [Google Scholar] [CrossRef]
- Chen, H.J.; Yan, X.Y.; Sun, A.; Zhang, L.; Zhang, J.; Yan, Y.E. High-Fat-Diet-Induced Extracellular Matrix Deposition Regulates Integrin-FAK Signals in Adipose Tissue to Promote Obesity. Mol. Nutr. Food Res. 2022, 66, e2101088. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhuang, S.; Chen, X.; Du, J.; Zhong, L.; Ding, J.; Wang, L.; Yi, J.; Hu, G.; Tang, G.; et al. lncRNA ITGB8-AS1 functions as a ceRNA to promote colorectal cancer growth and migration through integrin-mediated focal adhesion signaling. Mol. Ther. 2022, 30, 688–702. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, P.; Li, Q.; Liao, Y.; Wang, Y.; Lin, Y.; Xiang, H.; Du, Z.; Zhang, C.; Zhu, J.; Huang, L. Activation of Focal Adhesion Pathway by CIDEA as Key Regulatory Axis in Lipid Deposition in Goat Intramuscular Precursor Adipocytes. Animals 2025, 15, 2374. https://doi.org/10.3390/ani15162374
Shao P, Li Q, Liao Y, Wang Y, Lin Y, Xiang H, Du Z, Zhang C, Zhu J, Huang L. Activation of Focal Adhesion Pathway by CIDEA as Key Regulatory Axis in Lipid Deposition in Goat Intramuscular Precursor Adipocytes. Animals. 2025; 15(16):2374. https://doi.org/10.3390/ani15162374
Chicago/Turabian StyleShao, Peng, Qi Li, Yu Liao, Yong Wang, Yaqiu Lin, Hua Xiang, Zhanyu Du, Changhui Zhang, Jiangjiang Zhu, and Lian Huang. 2025. "Activation of Focal Adhesion Pathway by CIDEA as Key Regulatory Axis in Lipid Deposition in Goat Intramuscular Precursor Adipocytes" Animals 15, no. 16: 2374. https://doi.org/10.3390/ani15162374
APA StyleShao, P., Li, Q., Liao, Y., Wang, Y., Lin, Y., Xiang, H., Du, Z., Zhang, C., Zhu, J., & Huang, L. (2025). Activation of Focal Adhesion Pathway by CIDEA as Key Regulatory Axis in Lipid Deposition in Goat Intramuscular Precursor Adipocytes. Animals, 15(16), 2374. https://doi.org/10.3390/ani15162374




