Effects of Light Wavelength on Broiler Performance, Blood Cell Profiles, Stress Levels, and Tibiotarsi Morphology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
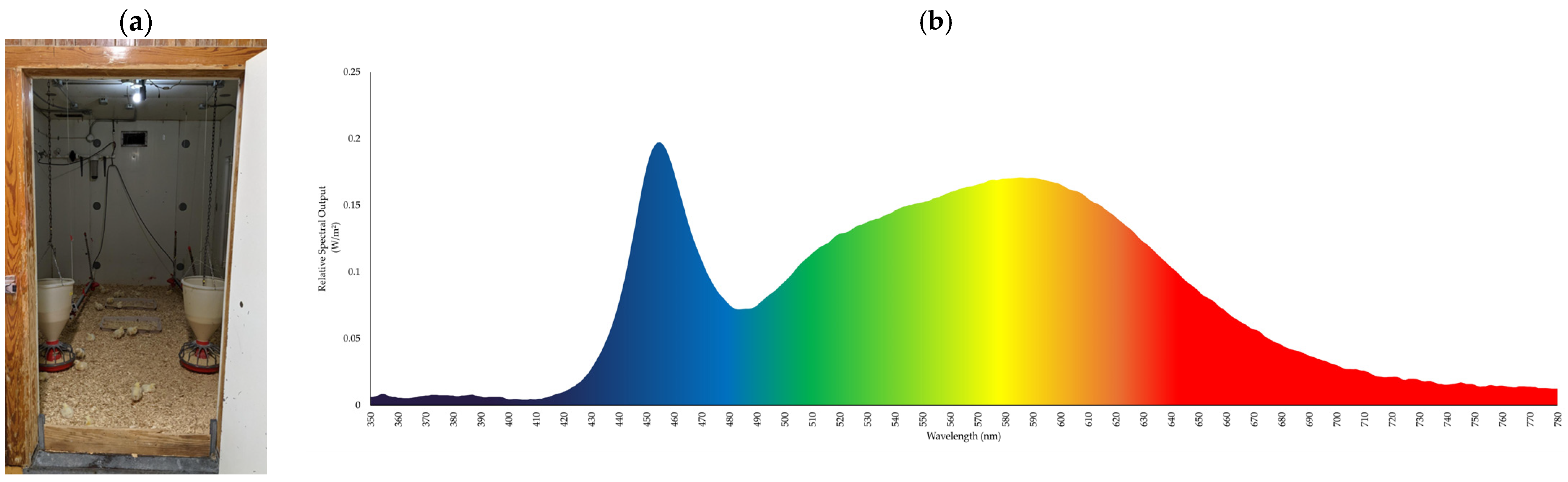
2.1. Housing and Facilities
2.2. Experimental Design
2.3. Data Collection and Sampling
2.3.1. Production
2.3.2. Tonic Immobility
2.3.3. Stress
2.3.4. Activity
2.3.5. Tibiotarsus Morphology
2.3.6. Processing
2.4. Statistical Analysis
3. Results
3.1. Performance
3.2. Tonic Immobility
3.3. Blood Cell Profiles
3.4. Stress Physiology
3.5. Activity
3.6. Tibia Morphology
3.7. Processing Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bessei, W. Impact of Animal Welfare on Worldwide Poultry Production. Worlds Poult. Sci. J. 2018, 74, 211–224. [Google Scholar] [CrossRef]
- Sakata, A.J.; Siqueira, J.A.C. Lighting in Broiler Poultry Houses: Optimization for Growth, Welfare and Sustainability. Rev. Caribeña Cienc. Soc. 2024, 13, e4377. [Google Scholar] [CrossRef]
- Senaratna, D.; Samarakone, T.; Atapattu, N.S.B.M.; Nayanarasi, H.A.D. Effect of the Colour of Light on Growth Performance, Behaviour and Bone Parameters of Broiler Chicken. Trop. Agric. 2008, 20, 185–192. [Google Scholar]
- Cao, J.; Wang, Z.; Dong, Y.; Zhang, Z.; Li, J.; Li, F.; Chen, Y. Effect of Combinations of Monochromatic Lights on Growth and Productive Performance of Broilers. Poult. Sci. 2012, 91, 3013–3018. [Google Scholar] [CrossRef]
- Franco, B.; Shynkaruk, T.; Crowe, T.; Fancher, B.; French, N.; Gillingham, S.; Schwean-Lardner, K. Light Color and the Commercial Broiler: Effect on Behavior, Fear, and Stress. Poult. Sci. 2022, 101, 102052. [Google Scholar] [CrossRef]
- Lewis, P.; Morris, T. Chapter 1: Light. In Poultry Lighting: The Theory and Practice; Nottingham University Press: Nottingham, UK, 2006; pp. 1–6. ISBN 0-9552104-0-2. [Google Scholar]
- Kang, S.W.; Christensen, K.D.; Kidd, M.T.; Orlowski, S.K.; Clark, J. Effects of a Variable Light Intensity Lighting Program on the Welfare and Performance of Commercial Broiler Chickens. Front. Physiol. 2023, 14, 1059055. [Google Scholar] [CrossRef]
- Lewis, P.; Morris, T. Chapter 2: Physiology and Mechanisms. In Poultry Lighting: The Theory and Practice; Nottingham University Press: Nottingham, UK, 2006; pp. 7–22. ISBN 0-9552104-0-2. [Google Scholar]
- Benoit, J. Opto-sexual reflex in the duck: Physiological and histological aspects. Yale J. Biol. Med. 1961, 34, 97. [Google Scholar]
- Benoit, J. The Role of the Eye and of the Hypothalamus in the Photostimulation of Gonads in the Duck. Ann. N. Y. Acad. Sci. 1964, 117, 204–215. [Google Scholar] [CrossRef]
- Prayitno, D.; Phillips, C.; Omed, H. The Effects of Color of Lighting on the Behavior and Production of Meat Chickens. Poult. Sci. 1997, 76, 452–457. [Google Scholar] [CrossRef]
- Rozenboim, I.; Biran, I.; Uni, Z.; Robinzon, B.; Halevy, O. The Effect of Monochromatic Light on Broiler Growth and Development. Poult. Sci. 1999, 78, 135–138. [Google Scholar] [CrossRef]
- Oke, O.E.; Oso, O.; Iyasere, O.; Oni, A.; Bakre, O.; Rahman, S. Evaluation of Light Color Manipulation on Behavior and Welfare of Broiler Chickens. J. Appl. Anim. Welf. Sci. 2023, 26, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Hesham, M.H.; El Shereen, A.H.; Enas, S.N. Impact of Different Light Colors in Behavior, Welfare Parameters and Growth Performance of Fayoumi Broiler Chickens Strain. J. Hell. Vet. Med. Soc. 2018, 69, 951–958. [Google Scholar] [CrossRef]
- Cao, J.; Liu, W.; Wang, Z.; Xie, D.; Jia, L.; Chen, Y. Green and Blue Monochromatic Lights Promote Growth and Development of Broilers via Stimulating Testosterone Secretion and Myofiber Growth. J. Appl. Poult. Res. 2008, 17, 211–218. [Google Scholar] [CrossRef]
- Ke, Y.Y.; Liu, W.J.; Wang, Z.X.; Chen, Y.X. Effects of Monochromatic Light on Quality Properties and Antioxidation of Meat in Broilers. Poult. Sci. 2011, 90, 2632–2637. [Google Scholar] [CrossRef]
- Pan, J.; Yang, Y.; Yang, B.; Yu, Y. Artificial Polychromatic Light Affects Growth and Physiology in Chicks. PLoS ONE 2014, 9, e113595. [Google Scholar] [CrossRef]
- De Santana, M.R.; Garcia, R.G.; De, I.; Naas, A.; De, I.C.; Paz, L.A.; Caldara, F.R.; Barreto, B. Light Emitting Diode (LED) Use in Artificial Lighting for Broiler Chicken Production. Aprovado pelo Conselho Editorial 2014, 34, 422–427. [Google Scholar] [CrossRef]
- Mosa, R.K.; Abbas, R.J.; Tabeekh, M.A.S.A. The Effect of Color Light and Stocking Density on Some Traits of broiler Carcasses. MRVSA 2014, 3, 24–35. [Google Scholar]
- Sultana, S.; Hassan, M.R.; Choe, H.S.; Ryu, K.S. The Effect of Monochromatic and Mixed LED Light Colour on the Behaviour and Fear Responses of Broiler Chicken. Avian Biol. Res. 2013, 6, 207–214. [Google Scholar] [CrossRef]
- Abdel-Moneim, E.A.-M.; Siddiqui, S.A.; Shehata, A.M.; Biswas, A.; Abougabal, M.S.; Kamal, A.M.; Mesalam, N.M.; Elsayed, M.A.; Yang, B.; Ebeid, T.A.; et al. Impact of Light Wavelength on Growth and Welfare of Broilers Chickens: Overview and Future Perspective. Ann. Anim. Sci. 2024, 24, 731–748. [Google Scholar] [CrossRef]
- Moura, D.J.; Nääs, I.; Pereira, D.; Silva, R.; Camargo, G. Animal Welfare Concepts and Strategy for poultry Production: A Review. Braz. J. Poult. Sci. 2006, 8, 137–148. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Effect of a Combination of Green and Blue Monochromatic Light on Broiler Immune Response. J. Photochem. Photobiol. B 2014, 138, 118–123. [Google Scholar] [CrossRef]
- Xie, D.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Wang, J.F.; Chen, J.L.; Chen, Y.X. Effects of Monochromatic Light on Immune Response of Broilers. Poult. Sci. 2008, 87, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2022. [Google Scholar]
- Cobb-Vantress Cobb Broiler Management Guide. Available online: www.cobb-vantress.com (accessed on 8 July 2025).
- Santamaria, J.M.; Beck, C.N.; Orlowski, S.K.; Maqueda, M.; Bottje, W.G.; Erf, G.F. Selection for Improved Water Efficiency in Broiler Breeder Lines Does Not Negatively Impact Immune Response Capabilities to Gram− and Gram+ Bacterial Components and a Killed-Salmonella Enteritidis Vaccine. Vet. Sci. 2025, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Brage, S. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef] [PubMed]
- Magnaterra, A.; Mitchell, R.; Angel, C.R.; Khong, M.; McMillian, Z.; Snyder, A.; Weimer, S. Research Note: Comparison of Two Methods to Measure Broiler Tibia Morphology. Poult. Sci. 2023, 102, 102245. [Google Scholar] [CrossRef]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration with Fibrosis and Regeneration in the Pectoralis Major Muscle of Broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Tijare, V.V.; Yang, F.L.; Kuttappan, V.A.; Alvarado, C.Z.; Coon, C.N.; Owens, C.M. Meat Quality of Broiler Breast Fillets with White Striping and Woody Breast Muscle Myopathies. Poult. Sci. 2016, 95, 2167–2173. [Google Scholar] [CrossRef]
- Halevy, O.; Biran, I.; Rozenboim, I. Various Light Source Treatments Affect Body and Skeletal Muscle Growth by Affecting Skeletal Muscle Satellite Cell Proliferation in Broilers. Comp. Biochem. Physiol. A 1998, 120, 317–323. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, X.; Wang, M.; Wang, W.; Yang, Y. Effect of light intensity on growth performance and bone development of tibia in broilers. J. Anim. Physiol. Anim. Nutr. 2023, 107, 192–199. [Google Scholar] [CrossRef]
- Prayitno, D.S.; Phillips, C.J.C.; Stokes, D.K. The Effects of Color and Intensity of Light on Behavior and Leg Disorders in Broiler Chickens. Poult. Sci. 1997, 76, 1674–1681. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Zamiri, M.J.; Akhlaghi, A.; Ghovvati, S.; Kargar, S.; Amini, J. Influence of LED Light Spectra and Photoperiods on Performance, Bone Characteristics and Related Genes Expression in Broiler Breeders. Br. Poult. Sci. 2025, 66, 315–323. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Pillai, P.B.; Cavitt, L.C.; Owens, C.M.; Emmert, J.L. Evaluation of Slower-Growing Broiler Genotypes Grown with and without Outdoor Access: Growth Performance and Carcass Yield. Poult. Sci. 2005, 84, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Lien, R.J.; Hess, J.B.; Mckee, S.R.; Bilgili, S.F.; Townsend, J.C. Effect of Light Intensity and Photoperiod on Live Performance, Heterophil-to-Lymphocyte Ratio, and Processing Yields of Broilers. Poult. Sci. 2007, 86, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Charles, R.G.; Robinson, F.E.; Hardin, R.T.; Yu, M.W.; Feddes, J.; Classen, H.L. Growth, Body Composition, and Plasma Androgen Concentration of Male Broiler Chickens Subjected to Different Regimens of Photoperiod and Light Intensity. Poult. Sci. 1992, 71, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Weimer, S.L.; Zuelly, S.; Davis, M.; Karcher, D.M.; Erasmus, M.A. Differences in carcass composition and meat quality of conventional and slow-growing broiler chickens raised at 2 stocking densities. Poult. Sci. 2022, 101, 101833. [Google Scholar] [CrossRef]
- Khosravinia, H. Preference of Broiler Chicks for Color of Lighting and Feed. J. Poult. Sci. 2007, 44, 213–219. [Google Scholar] [CrossRef]
- Oishi, T.; Ohashi, K. Effects of wavelengths of light on the photoperiodic gonadal response of blinded-pinealectomized Japanese quail. Zool. Sci. 1993, 10, 757–762. [Google Scholar]
- Manser, C. Effects of Lighting on the Welfare of Domestic Poultry a Review. Anim. Welfare 1996, 5, 341–360. [Google Scholar] [CrossRef]
- Olanrewaju, H.A.; Thaxton, J.P.; Dozier, W.A.; Purswell, J.; Roush, W.B.; Branton, S.L. A Review of Lighting Programs for Broiler Production. Int. J. Poult. Sci. 2006, 5, 301–308. [Google Scholar] [CrossRef]
- Egbuniwe, I.C.; Ayo, J.O. Physiological Roles of Avian Eyes in Light Perception and Their Responses to Photoperiodicity. Worlds Poult. Sci. J. 2016, 72, 605–614. [Google Scholar] [CrossRef]
- Soliman, F.N.K.; El-Sabrout, K. Light Wavelengths/Colors: Future Prospects for Broiler Behavior and Production. J. Vet. Behav. 2020, 36, 34–39. [Google Scholar] [CrossRef]
- Linhoss, J.E.; Falana, O.B.; Davis, J.D.; Purswell, J.L.; Edge, C.M.; Olanrewaju, H.A.; Baker-Cook, B.I.; Hanlon, C. An Updated Review on the Effect of Lighting on Broilers and the Environment of Commercial Houses. Worlds Poult. Sci. J. 2025, 81, 373–401. [Google Scholar] [CrossRef]
- Boshouwers, F.M.G.; Nicaise, E. Responses of broiler chickens to high-frequency and low-frequency fluorescent light. Br. Poult. Sci. 1992, 33, 711–717. [Google Scholar] [CrossRef]



| Measures | White | Blue | Green | SEM | p-Value |
|---|---|---|---|---|---|
| BW (kg) 1 | |||||
| Starter (D14) | 0.49 | 0.47 | 0.48 | 0.01 | 0.12 |
| Grower (D28) | 1.90 | 1.86 | 1.88 | 0.01 | 0.06 * |
| Finisher (D41) | 3.30 | 3.32 | 3.32 | 0.03 | 0.57 |
| FCR 2 | |||||
| Starter (D14) | 1.20 | 1.23 | 1.22 | 0.01 | 0.32 |
| Grower (D28) | 1.41 | 1.43 | 1.44 | 0.02 | 0.54 |
| Finisher (D41) | 1.89 | 1.86 | 1.88 | 0.03 | 0.75 |
| Cumulative | 1.57 | 1.58 | 1.59 | 0.01 | 0.69 |
| HLR 1,2 | Heterophils (103/uL) | Lymphocytes (103/uL) | TBR 3 | T Cells (103/uL) | B Cells (103/uL) | Thrombocytes (103/uL) | WBC (103/uL) | RBC (103/uL) | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | |||||||||
| White | 0.43 | 4.28 | 10.28 a | 5.65 | 8.64 a | 1.64 | 39.83 | 19.39 | 2426.80 |
| Blue | 0.49 | 4.03 | 8.62 b | 5.48 | 7.16 b | 1.46 | 33.41 | 18.86 | 2410.63 |
| Green | 0.45 | 4.32 | 9.83 ab | 5.27 | 8.16 ab | 1.67 | 34.11 | 19.78 | 2390.36 |
| SEM | 0.03 | 0.26 | 0.40 | 0.30 | 0.33 | 0.10 | 2.31 | 0.70 | 37.43 |
| Age (Days) | |||||||||
| 21 | 0.46 | 3.24 b | 7.38 b | 5.83 a | 6.23 a | 1.15 a | 35.80 | 16.17 b | 2194.70 b |
| 41 | 0.45 | 5.17 a | 11.77 a | 5.09 b | 9.74 b | 2.02 b | 35.76 | 22.52 a | 2623.83 a |
| SEM | 0.02 | 0.21 | 0.33 | 0.25 | 0.27 | 0.08 | 1.92 | 0.57 | 30.50 |
| Treatment × Age | |||||||||
| White × 21 | 0.41 | 3.10 | 7.75 | 5.95 | 6.61 | 1.15 | 40.49 | 16.26 | 2190.27 |
| White × 41 | 0.46 | 5.45 | 12. | 5.35 | 10.68 | 2.13 | 39.17 | 22.52 | 2663.34 |
| Blue × 21 | 0.49 | 3.30 | 6.93 | 5.99 | 5.87 | 1.06 | 33.14 | 15.96 | 2222.91 |
| Blue × 41 | 0.49 | 4.77 | 10.32 | 4.97 | 8.45 | 1.86 | 33.67 | 21.76 | 2598.36 |
| Green × 21 | 0.46 | 3.35 | 7.48 | 5.56 | 6.22 | 1.25 | 33.78 | 16.28 | 2170.93 |
| Green × 41 | 0.44 | 5.28 | 12.18 | 4.98 | 10.09 | 2.08 | 34.44 | 23.28 | 2609.80 |
| SEM | 0.04 | 0.37 | 0.57 | 0.43 | 0.47 | 0.14 | 3.22 | 0.99 | 52.88 |
| p-values | |||||||||
| Treatment | 0.44 | 0.72 | 0.01 | 0.69 | 0.009 | 0.32 | 0.11 | 0.64 | 0.79 |
| Age | 0.73 | 0.0001 | 0.0001 | 0.04 | 0.0001 | 0.0001 | 0.98 | 0.0001 | 0.0001 |
| Treatment × Age | 0.71 | 0.54 | 0.32 | 0.85 | 0.25 | 0.81 | 0.94 | 0.83 | 0.65 |
| Temperature (°C) 1 | White | Blue | Green | SEM | p-Value |
|---|---|---|---|---|---|
| Eye 2 | |||||
| Minimum | 30.10 | 30.59 | 30.94 | 0.28 | 0.11 |
| Average | 30.88 | 31.30 | 31.76 | 0.27 | 0.09 * |
| Maximum | 31.74 | 32.20 | 32.67 | 0.28 | 0.07 * |
| Beak | |||||
| Minimum | 31.39 | 30.09 | 32.10 | 0.76 | 0.18 |
| Average | 33.05 | 31.50 | 33.49 | 0.78 | 0.18 |
| Maximum | 34.41 | 32.67 | 34.67 | 0.80 | 0.18 |
| Measure 1 | White | Blue | Green | SEM | p-Value |
|---|---|---|---|---|---|
| Length (mm) | 99.5 | 105.63 | 101.75 | 1.58 | 0.06 * |
| PHW (mm) | 28.26 | 28.38 | 28.62 | 0.48 | 0.87 |
| Angle (°) | 39.49 | 38.92 | 36.83 | 0.77 | 0.08 * |
| DHW (mm) | 21.3 | 21.59 | 21.78 | 0.44 | 0.75 |
| 90% (mm) | 25.14 | 25.33 | 25.57 | 0.72 | 0.92 |
| 75% (mm) | 14.08 | 13.96 | 14.54 | 0.45 | 0.65 |
| 50% (mm) | 10.21 | 9.77 | 10.70 | 0.35 | 0.23 |
| 25% (mm) | 12.71 | 12.98 | 13.16 | 0.38 | 0.71 |
| 10% (mm) | 18.89 | 18.84 | 19.12 | 0.56 | 0.93 |
| MID (mm) | 1.34 | 1.70 | 1.64 | 0.14 | 0.20 |
| LID (mm) | 1.52 | 1.74 | 1.75 | 0.15 | 0.49 |
| DID (mm) | 5.88 | 5.95 | 6.02 | 0.15 | 0.81 |
| Ash (g) | 3.62 | 3.68 | 3.85 | 0.22 | 0.75 |
| Ash (%) 2 | 43.90 | 45.72 | 44.67 | 0.53 | 0.31 |
| Yield (%) 1 | White | Blue | Green | SEM | p-Value |
|---|---|---|---|---|---|
| Carcass | 72.85 a | 72.34 b | 72.95 a | 0.17 | 0.02 |
| Breast | 26.89 a | 26.41 b | 26.39 b | 0.13 | 0.01 |
| Tenders 2 | 5.00 a | 4.87 b | 4.94 a | 0.02 | 0.003 |
| White Meat 2 | 31.89 a | 31.21 b | 31.33 b | 0.14 | 0.001 |
| Wings | 10.28 | 10.36 | 10.25 | 0.03 | 0.07 * |
| Leg Quarters | 30.62 ab | 30.48 b | 30.90 a | 0.10 | 0.01 |
| Rack + Skin | 26.45 b | 27.02 a | 26.67 b | 0.09 | <0.0001 |
| Fat Pad | 1.41 a | 1.43 a | 1.33 b | 0.02 | 0.02 |
| Body Weight (g) 3 | 3287.2 | 3299.6 | 3288.3 | 27.81 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perretti, A.; Oyeniran, V.J.; Cherry, J.M.; Whittle, R.H.; Grider, Z.; Nelson, A.H.; Kang, S.W.; Erf, G.F.; Weimer, S.L. Effects of Light Wavelength on Broiler Performance, Blood Cell Profiles, Stress Levels, and Tibiotarsi Morphology. Animals 2025, 15, 2372. https://doi.org/10.3390/ani15162372
Perretti A, Oyeniran VJ, Cherry JM, Whittle RH, Grider Z, Nelson AH, Kang SW, Erf GF, Weimer SL. Effects of Light Wavelength on Broiler Performance, Blood Cell Profiles, Stress Levels, and Tibiotarsi Morphology. Animals. 2025; 15(16):2372. https://doi.org/10.3390/ani15162372
Chicago/Turabian StylePerretti, Angela, Victor J. Oyeniran, Jaelen M. Cherry, Rosemary H. Whittle, Zachary Grider, Alexander H. Nelson, Seong W. Kang, Gisela F. Erf, and Shawna L. Weimer. 2025. "Effects of Light Wavelength on Broiler Performance, Blood Cell Profiles, Stress Levels, and Tibiotarsi Morphology" Animals 15, no. 16: 2372. https://doi.org/10.3390/ani15162372
APA StylePerretti, A., Oyeniran, V. J., Cherry, J. M., Whittle, R. H., Grider, Z., Nelson, A. H., Kang, S. W., Erf, G. F., & Weimer, S. L. (2025). Effects of Light Wavelength on Broiler Performance, Blood Cell Profiles, Stress Levels, and Tibiotarsi Morphology. Animals, 15(16), 2372. https://doi.org/10.3390/ani15162372





