Effects of Moringa oleifera Leaf Peptide on Hypoglycemic Activity In Vitro and Postprandial Glycemic Response in Beagle Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Snacks
2.2. α-Amylase and α-Glucosidase Activity Inhibition Assay
2.3. Snack Starch Hydrolysis and eGI Measurement
2.4. Postprandial Glycemic Response Tests In Vivo
2.5. Statistical Analysis
3. Results
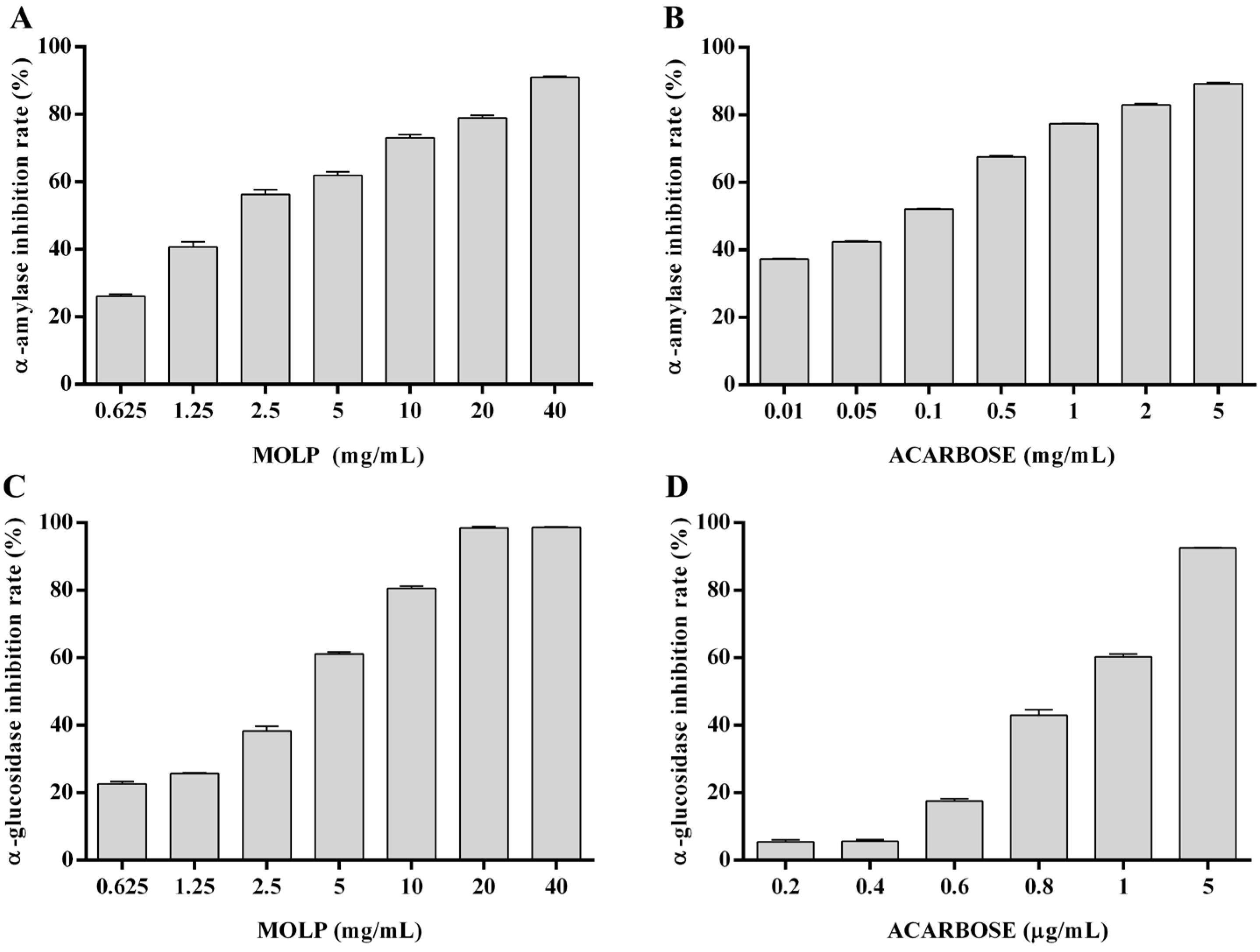
3.1. Enzyme Inhibitory Activity of MOLP In Vitro
3.2. MOLP Reduces Snacks Starch Hydrolysis Rate and eGI In Vitro
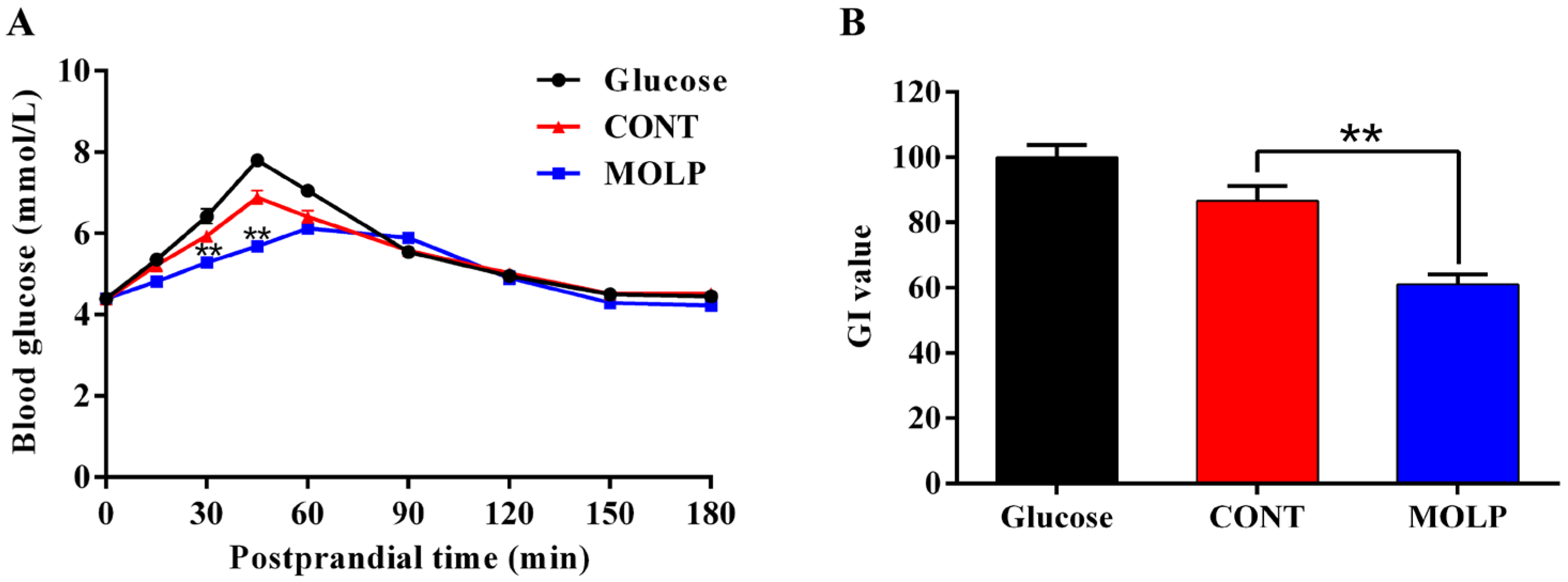
3.3. MOLP Lowers Postprandial Glycemic Response in Dogs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, T.; Wallace, H.; Staats, S. Reasons for companion animal guardianship (pet ownership) from two populations. Soc. Anim. 2008, 16, 279–291. [Google Scholar] [CrossRef]
- Statista. Number of Pet Dogs in Europe from 2010 to 2023. 2024. Available online: https://www.statista.com/statistics/515579/dog-population-europe/ (accessed on 1 July 2024).
- Statista. Household Penetration Rate for Pet-Ownership in the United States from 1988 to 2023. 2024. Available online: https://www.statista.com/statistics/198086/us-household-penetration-rates-for-pet-owning-since-2007/ (accessed on 12 January 2024).
- Statista. Number of Domestic Cats and Dogs Kept as Pets in Urban Households in China from 2017 to 2023 (in Millions). 2024. Available online: https://www.statista.com/statistics/1196715/china-number-of-domestic-dogs-and-cats-kept-as-pets/ (accessed on 23 June 2025).
- Courcier, E.A.; Thomson, R.M.; Mellor, D.J.; Yam, P.S. An epidemiological study of environmental factors associated with canine obesity. J. Small. Anim. Pract. 2010, 51, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P. Understanding and managing obesity in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1283–1295, vii. [Google Scholar] [CrossRef] [PubMed]
- Corbee, R.J. Obesity in show dogs. J. Anim. Physiol. An. N. 2013, 97, 904–910. [Google Scholar] [CrossRef]
- Watson, P.J.; Herrtage, M.E. Use of glucagon stimulation tests to assess beta-cell function in dogs with chronic pancreatitis. J. Nutr. 2004, 134, 2081S–2083S. [Google Scholar] [CrossRef]
- Cridge, H.; Parker, V.J.; Kathrani, A. Nutritional management of pancreatitis and concurrent disease in dogs and cats. J. Am. Vet. Med. Assoc. 2024, 262, 834–840. [Google Scholar] [CrossRef]
- Chandler, M.; Cunningham, S.; Lund, E.M.; Khanna, C.; Naramore, R.; Patel, A.; Day, M.J. Obesity and associated comorbidities in people and companion animals: A one health perspective. J. Comp. Pathol. 2017, 156, 296–309. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary glycemic index and load and the risk of type 2 diabetes: A systematic review and updated meta-analyses of prospective cohort studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef]
- Brand-Miller, J.; McMillan-Price, J.; Steinbeck, K.; Caterson, I. Dietary glycemic index: Health implications. J. Am. Coll. Nutr. 2009, 28, 446S–449S. [Google Scholar] [CrossRef]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Riccardi, G.; Rivellese, A.A.; Giacco, R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am. J. Clin. Nutr. 2008, 87, 269S–274S. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Minaiyan, M.; Asghari, G.; Taheri, D.; Saeidi, M.; Nasr-Esfahani, S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna. J. Phytomed. 2014, 4, 127–136. [Google Scholar] [PubMed]
- Mwamatope, B.; Tembo, D.; Chikowe, I.; Kampira, E.; Nyirenda, C. Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci. Afr. 2020, 9, e00481. [Google Scholar] [CrossRef]
- Suresh, M.; Mukundan, S.K.; Rajasekar, S.; Gokulakrishnan, S.; Purushothaman, N.; Sethuraman, S.P. Development and assessment of a multipurpose herbal cream with Moringa oleifera lam. Cureus 2024, 16, e69982. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, E.A.; Ahmed, S.M.; Masoud, M.A.; Mohamed, F.A.; Mohammed, H.S. Cardioprotective potential of Moringa oleifera leaf extract loaded niosomes nanoparticles-against doxorubicin toxicity in rats. Curr. Pharm. Biotechnol. 2025, 26, 289–301. [Google Scholar] [CrossRef]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Lorenzo, J.M.; Afolayan, A.J.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef]
- Asare, G.A.; Gyan, B.; Bugyei, K.; Adjei, S.; Mahama, R.; Addo, P.; Otu-Nyarko, L.; Wiredu, E.K.; Nyarko, A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012, 139, 265–272. [Google Scholar] [CrossRef]
- Toma, A.; Makonnen, E.; Mekonnen, Y.; Debella, A.; Addisakwattana, S. Intestinal alpha-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complement. Altern. Med. 2014, 14, 180. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Lin, L.Z.; Zhao, M.M.; Yang, X.Y. The hypoglycemic and hypolipemic potentials of Moringa oleifera leaf polysaccharide and polysaccharide-flavonoid complex. Int. J. Biol. Macromol. 2022, 210, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Toma, A. Antidiabetic activity of hot tea infusion of leaves of Moringa stenopetala in streptozotocin-induced diabetic rats. J. Exp. Pharmacol. 2022, 14, 309–316. [Google Scholar] [CrossRef]
- Villarruel-Lopez, A.; Lopez-de la Mora, D.A.; Vazquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuno, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Rios-Silva, M.; Huerta, M.; Cardenas, Y.; Bricio-Barrios, J.A.; Diaz-Reval, M.I.; Urzua, Z.; Huerta-Trujillo, M.; Lopez-Quezada, K.; Trujillo, X. Effects of Moringa oleifera leaf powder on metabolic syndrome induced in male Wistar rats: A preliminary study. J. Int. Med. Res. 2018, 46, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Ndong, M.; Uehara, M.; Katsumata, S.; Suzuki, K. Effects of oral administration of Moringa oleifera lam on glucose tolerance in goto-kakizaki and wistar rats. J. Clin. Biochem. Nutr. 2007, 40, 229–233. [Google Scholar] [CrossRef]
- Jaiswal, D.; Kumar Rai, P.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, J.Z.; Song, C.R.; Ruan, H.J.; Liu, J.H.; Liu, Y.Y.; Sui, Y.L. Effects of Moringa oleifera leaf extract plus rosiglitazone on serum leptin and glucose and lipid metabolism in type 2 diabetic rats. Altern. Ther. Health. Med. 2023, 29, 650–655. [Google Scholar]
- Yassa, H.D.; Tohamy, A.F. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced diabetes mellitus in adult rats. Acta. Histochem. 2014, 116, 844–854. [Google Scholar] [CrossRef]
- Amina, E.E.; Adisa, J.O.; Gamde, S.M.; Omoruyi, E.B.; Kwaambwa, H.M.; Mwapagha, L.M. Hypoglycemic assessment of aqueous leaf extract of Moringa oleifera on diabetic wistar rats. Biochem. Res. Int. 2024, 2024, 9779021. [Google Scholar] [CrossRef]
- Khan, W.; Parveen, R.; Chester, K.; Parveen, S.; Ahmad, S. Hypoglycemic potential of aqueous extract of Moringa oleifera leaf and in vivo GC-MS metabolomics. Front. Pharmacol. 2017, 8, 577. [Google Scholar] [CrossRef]
- Olayaki, L.A.; Irekpita, J.E.; Yakubu, M.T.; Ojo, O.O. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 585–593. [Google Scholar] [CrossRef]
- Anthanont, P.; Lumlerdkij, N.; Akarasereenont, P.; Vannasaeng, S.; Sriwijitkamol, A. Moringa oleifera leaf increases insulin secretion after single dose administration: A preliminary study in healthy subjects. J. Med. Assoc. Thai. 2016, 99, 308–313. [Google Scholar]
- Fombang, E.N.; Saa, R.W. Antihyperglycemic activity of Moringa oleifera lam leaf functional tea in rat models and human subjects. Food. Nutr. Sci. 2016, 7, 1020–1032. [Google Scholar] [CrossRef]
- Paula, P.C.; Sousa, D.O.; Oliveira, J.T.; Carvalho, A.F.; Alves, B.G.; Pereira, M.L.; Farias, D.F.; Viana, M.P.; Santos, F.A.; Morais, T.C.; et al. A protein isolate from Moringa oleifera leaves has hypoglycemic and antioxidant effects in alloxan-induced diabetic mice. Molecules 2017, 22, 271. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.F.; Shi, Y.; Wu, Y.J.; Wei, C.H.; Cao, L.K. Preparation, structure, and in-vitro hypoglycemic potential of debranched millet starch-fatty acid composite resistant starch. Food Chem. X 2023, 20, 100929. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Guo, W.X.; Yang, L.Y.; Shi, X.Y.; Cong, X.; Cheng, S.Y.; Li, L.L.; Cheng, H. Effects of color protection and enzymatic hydrolysis on the microstructure, digestibility, solubility and swelling degree of chestnut flour. Food Chem. X 2024, 23, 101770. [Google Scholar] [CrossRef]
- Shumoy, H.; Raes, K. In vitro starch hydrolysis and estimated glycemic index of tef porridge and injera. Food. Chem. 2017, 229, 381–387. [Google Scholar] [CrossRef]
- Laflamme, D. Development and validation of a body condition score system for dogs. Canine. Pract. 1997, 22, 10–15. [Google Scholar]
- Rankovic, A.; Adolphe, J.L.; Ramdath, D.D.; Shoveller, A.K.; Verbrugghe, A. Glycemic response in nonracing sled dogs fed single starch ingredients and commercial extruded dog foods with different carbohydrate sources. J. Anim. Sci. 2020, 98, skaa241. [Google Scholar] [CrossRef]
- Nguyen, P.; Dumon, H.; Biourge, V.; Pouteau, E. Glycemic and insulinemic responses after ingestion of commercial foods in healthy dogs: Influence of food composition. J. Nutr. 1998, 128, 2654S. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Kim, D.H.; Jeong, I.S.; Choi, G.C.; Park, H.M. Evaluation of four portable blood glucose meters in diabetic and non-diabetic dogs and cats. Vet. Q. 2016, 36, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Chlup, R.; Seckar, P.; Zapletalova, J.; Langova, K.; Kudlova, P.; Chlupova, K.; Bartek, J.; Jelenova, D. Automated computation of glycemic index for foodstuffs using continuous glucose monitoring. J. Diabetes Sci. Technol. 2008, 2, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Xu, Y.B.; Wu, J.L.; Li, N.; Guo, M.Q. Hypoglycemic and hypolipidemic effects of Moringa oleifera leaves and their functional chemical constituents. Food Chem. 2020, 333, 127478. [Google Scholar] [CrossRef]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Liyanage, R. A simple microplate-based method for the determination of alpha-amylase activity using the glucose assay kit (GOD method). Food Chem. 2016, 211, 853–859. [Google Scholar] [CrossRef]
- Qaisar, M.N.; Chaudhary, B.A.; Sajid, M.U.; Hussain, N. α-glucosidase inhibitory activity of dichoromethane and methanol extracts of croton bonpladianum baill. Trop. J. Pharm. Res. 2014, 13, 1833–1836. [Google Scholar] [CrossRef]
- Azad, S.B.; Ansari, P.; Azam, S.; Hossain, S.M.; Shahid, M.I.; Hasan, M.; Hannan, J.M.A. Anti-hyperglycaemic activity of Moringa oleifera is partly mediated by carbohydrase inhibition and glucose-fibre binding. Biosci. Rep. 2017, 37, BSR20170059. [Google Scholar] [CrossRef]
- Gomes, S.M.; Miranda, R.; Santos, L. Enhancing the biological properties of white chocolate: Moringa oleifera leaf extract as a natural functional ingredient. Foods 2025, 14, 359. [Google Scholar] [CrossRef]
- Ferreira, T.; Gomes, S.M.; Santos, L. Elevating cereal-based nutrition: Moringa oleifera supplemented bread and biscuits. Antioxidants 2023, 12, 2069. [Google Scholar] [CrossRef]
- Magaji, U.F.; Sacan, O.; Yanardag, R. Alpha amylase, alpha glucosidase and glycation inhibitory activity of Moringa oleifera extracts. S. Afr. J. Bot. 2020, 128, 225–230. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Aladeselu, O.H.; Oboh, G.; Boligon, A.A. Drying alters the phenolic constituents, antioxidant properties, alpha-amylase, and alpha-glucosidase inhibitory properties of Moringa (Moringa oleifera) leaf. Food Sci. Nutr. 2018, 6, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.M.; Sreerama, Y.N. Soluble and bound phenolics of two different millet genera and their milled fractions: Comparative evaluation of antioxidant properties and inhibitory effects on starch hydrolysing enzyme activities. J. Funct. Foods. 2017, 35, 682–693. [Google Scholar] [CrossRef]
- Pradeep, P.M.; Sreerama, Y.N. Phenolic antioxidants of foxtail and little millet cultivars and their inhibitory effects on alpha-amylase and alpha-glucosidase activities. Food Chem. 2018, 247, 46–55. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.J.; Ren, G.X. Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef]
- Xiao, J.B.; Ni, X.L.; Kai, G.Y.; Chen, X.Q. A review on structure-activity relationship of dietary polyphenols inhibiting alpha-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef]
- Di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional significance and structure-activity relationship of food-derived α-glucosidase inhibitors. Curr. Opin. Food Sci. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Mousavi, B.; Azizi, M.H.; Abbasi, S. Antidiabetic bio-peptides of soft and hard wheat glutens. Food Chem. 2022, 4, 100104. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef]
- William, F.; Lakshminarayanan, S.; Chegu, H. Effect of some indian vegetables on the glucose and insulin response in diabetic subjects. Int. J. Food Sci. Nutr. 1993, 44, 191–195. [Google Scholar] [CrossRef]
- Leone, A.; Bertoli, S.; Di Lello, S.; Bassoli, A.; Ravasenghi, S.; Borgonovo, G.; Forlani, F.; Battezzati, A. Effect of Moringa oleifera leaf powder on postprandial blood glucose response: In vivo study on Saharawi people living in refugee camps. Nutrients 2018, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Behrend, E.; Holford, A.; Lathan, P.; Rucinsky, R.; Schulman, R. 2018 AAHA diabetes management guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2018, 54, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.W.; Robertson, J.; Feldman, E.C.; Briggs, C. Effect of the alpha-glucosidase inhibitor acarbose on control of glycemia in dogs with naturally acquired diabetes mellitus. J. Am. Vet. Med. Assoc. 2000, 216, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liang, K.; Jin, Y.Q.; Zhang, M.M.; Chen, Y.; Wu, H.; Lai, F.R. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and alpha-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food. Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Yamada, Y.; Muraki, A.; Oie, M.; Kanegawa, N.; Oda, A.; Sawashi, Y.; Kaneko, K.; Yoshikawa, M.; Goto, T.; Takahashi, N.; et al. Soymorphin-5, a soy-derived mu-opioid peptide, decreases glucose and triglyceride levels through activating adiponectin and PPARalpha systems in diabetic KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E433–E440. [Google Scholar] [CrossRef]

| Ingredients | Content (%) |
|---|---|
| Peptide molecular weight analysis (Dalton, Da) | |
| <180 | 23.84 |
| 180–500 | 53.10 |
| 500–1000 | 15.11 |
| 1000–3000 | 7.28 |
| 3000–5000 | 0.53 |
| 5000–10,000 | 0.11 |
| >10,000 | 0.04 |
| Proximate analysis (DM basis, %) | |
| Crude protein | 47.73 |
| Crude fat | 10.01 |
| Crude fiber | 5.90 |
| Ash | 5.94 |
| Moisture | 7.47 |
| Ingredients | Proportion (%) | |
|---|---|---|
| Control Snacks | Experimental Snacks | |
| Water | 12.10 | 11.74 |
| Corn flour | 9.31 | 9.03 |
| Potato starch | 8.38 | 8.13 |
| Rice flour | 27.93 | 27.09 |
| Peptone | 18.62 | 18.06 |
| Chicken liver powder | 1.86 | 1.81 |
| Chicken paste | 4.65 | 4.51 |
| Potassium sorbate | 0.37 | 0.36 |
| Chicken meal flour | 2.79 | 2.71 |
| Sodium pyrophosphate | 0.47 | 0.45 |
| Sodium hexametaphosphate | 0.47 | 0.45 |
| Calcium carbonate | 1.86 | 1.81 |
| Glycerol | 11.17 | 10.83 |
| Vitamin E | 0.03 | 0.03 |
| MOLP | - | 3.00 |
| Proximate analysis (DM basis, %) | ||
| Crude protein | 19.84 | 19.97 |
| Crude fat | 2.84 | 2.71 |
| Crude fiber | 0.67 | 0.74 |
| Ash | 7.86 | 7.63 |
| Moisture | 9.05 | 9.65 |
| Gross energy (kcal/kg) | 3874.65 | 3854.67 |
| Sample | Sample Blank | Positive | Control | Blank | |
|---|---|---|---|---|---|
| MOLP | 50 μL | 50 μL | - | - | - |
| α-amylase (1 U/mL) | 50 μL | - | 50 μL | 50 μL | - |
| PBS | - | 50 μL | - | 50 μL | 100 μL |
| Acarbose | - | - | 50 μL | - | - |
| Sample | Sample Blank | Positive | Control | Blank | |
|---|---|---|---|---|---|
| MOLP | 50 μL | 50 μL | - | - | - |
| α-glucosidase (0.25 U/mL) | 100 μL | - | 100 μL | 100 μL | - |
| PBS | - | 100 μL | - | 50 μL | 150 μL |
| Acarbose | - | - | 50 μL | - | - |
| Samples | α-Amylase (IC50, mg/mL) | α-Glucosidase (IC50, mg/mL) |
|---|---|---|
| MOLP | 2.29 ± 0.10 | 2.80 ± 0.04 |
| Acarbose | 0.06 ± 0.00 | 1.02 × 10−3 ± 0.00 |
| Items | Glucose | CONT | MOLP |
|---|---|---|---|
| Fasted blood glucose (mmol/L) | 4.40 ± 0.06 | 4.38 ± 0.08 | 4.40 ± 0.05 |
| Peak glucose (mmol/L) | 7.83 ± 0.05 | 7.10 ± 0.06 A | 6.24 ± 0.04 B |
| Time to peak (min) | 43.13 ± 1.88 | 48.75 ± 2.45 B | 71.25 ± 5.49 A |
| AUC (0–180 min) | 1002.31 ± 9.87 | 969.34 ± 7.40 A | 924.14 ± 7.46 B |
| IAUC (0–180 min) | 211.70 ± 7.99 | 183.39 ± 9.85 A | 129.36 ± 6.28 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Xu, L.; Cao, Y.; Liu, G.; Zhang, Y.; Mao, X. Effects of Moringa oleifera Leaf Peptide on Hypoglycemic Activity In Vitro and Postprandial Glycemic Response in Beagle Dogs. Animals 2025, 15, 2361. https://doi.org/10.3390/ani15162361
Wang W, Xu L, Cao Y, Liu G, Zhang Y, Mao X. Effects of Moringa oleifera Leaf Peptide on Hypoglycemic Activity In Vitro and Postprandial Glycemic Response in Beagle Dogs. Animals. 2025; 15(16):2361. https://doi.org/10.3390/ani15162361
Chicago/Turabian StyleWang, Wencan, Ling Xu, Yong Cao, Guo Liu, Yan Zhang, and Xin Mao. 2025. "Effects of Moringa oleifera Leaf Peptide on Hypoglycemic Activity In Vitro and Postprandial Glycemic Response in Beagle Dogs" Animals 15, no. 16: 2361. https://doi.org/10.3390/ani15162361
APA StyleWang, W., Xu, L., Cao, Y., Liu, G., Zhang, Y., & Mao, X. (2025). Effects of Moringa oleifera Leaf Peptide on Hypoglycemic Activity In Vitro and Postprandial Glycemic Response in Beagle Dogs. Animals, 15(16), 2361. https://doi.org/10.3390/ani15162361






