Ultrasound-Guided Radiofrequency Ablation and Pulsed Radiofrequency Treatment for Chronic Lameness Due to Distal Forelimb Disease in Horses: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
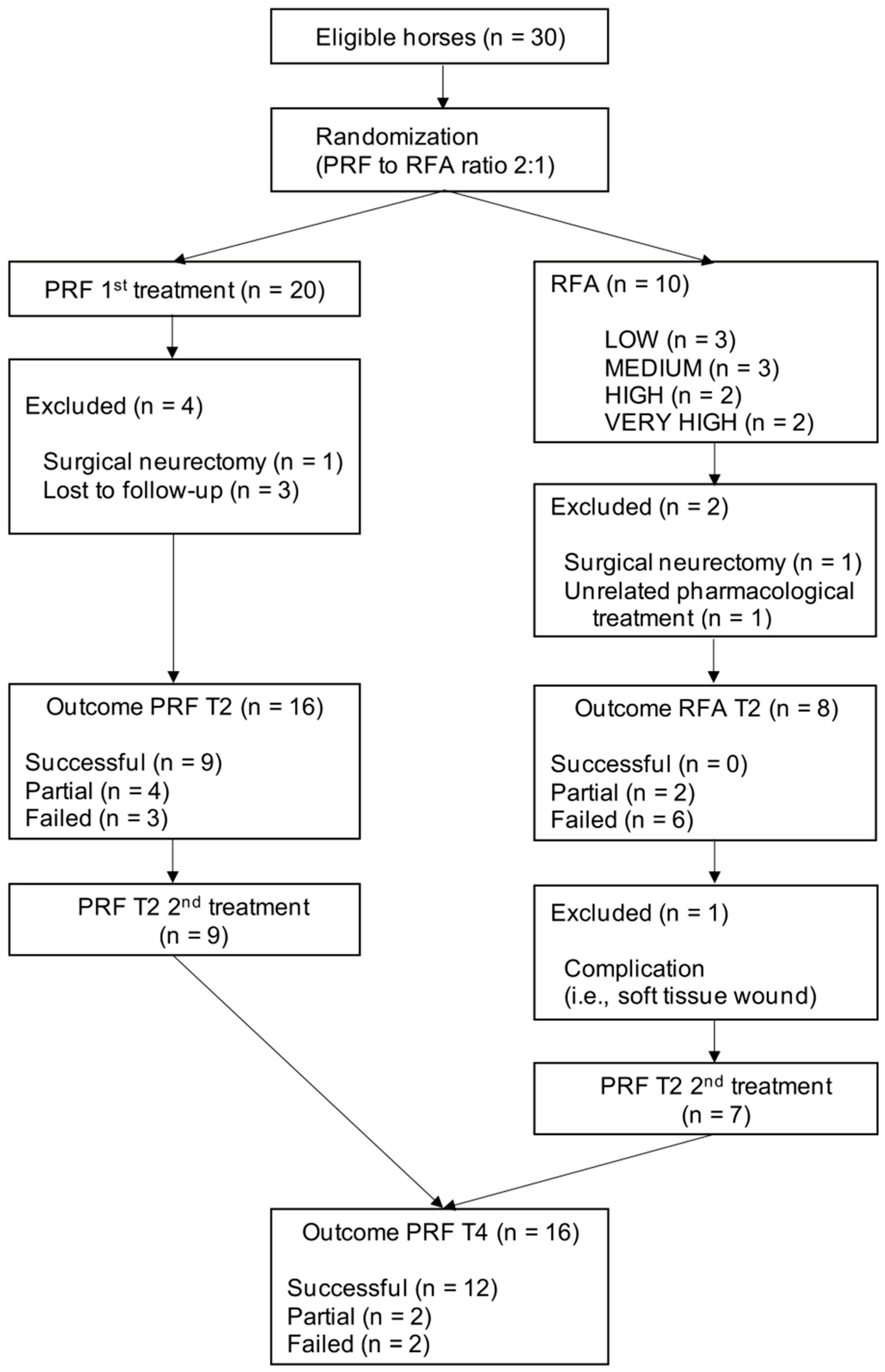
2.2. Study Design
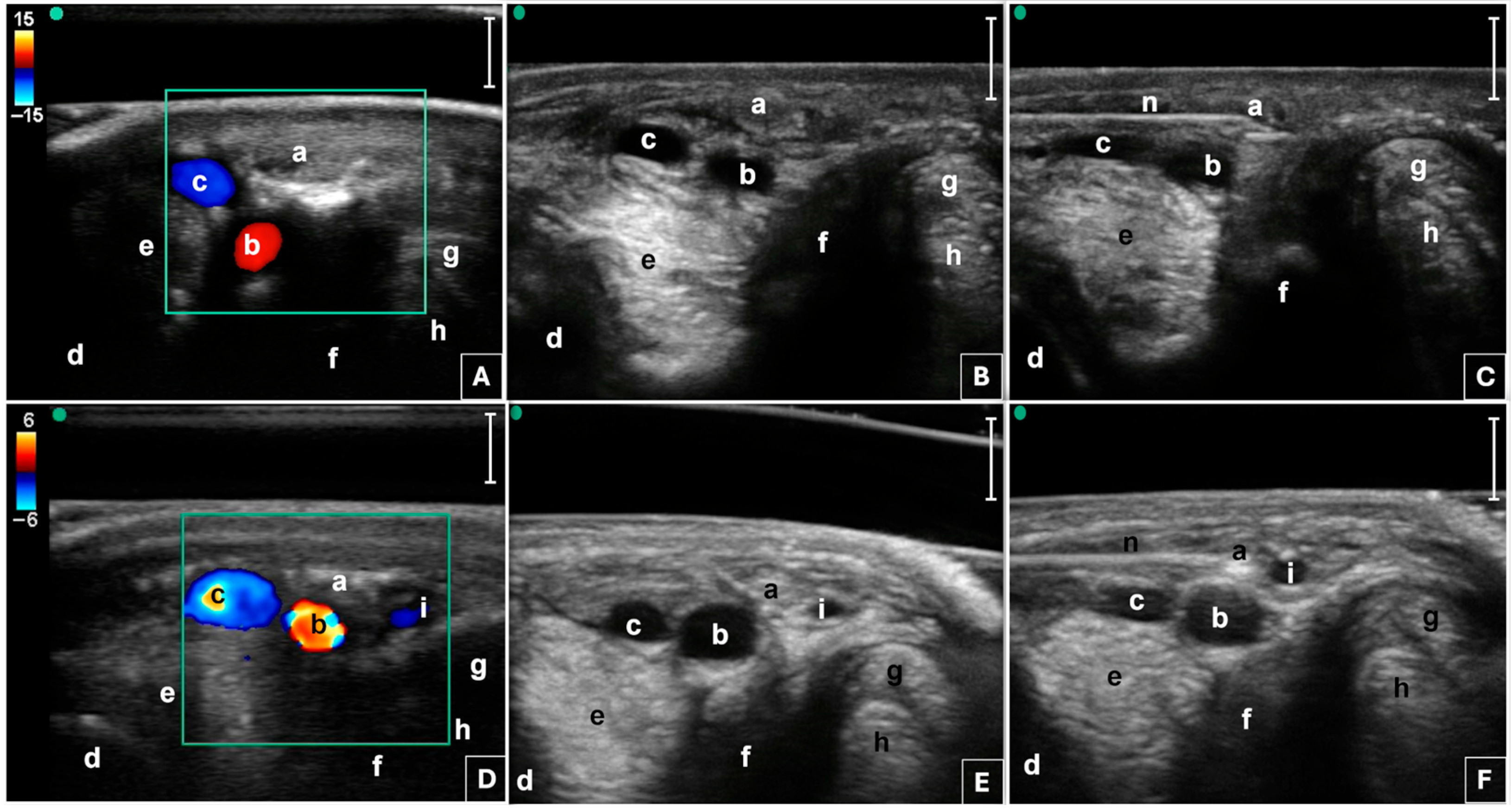
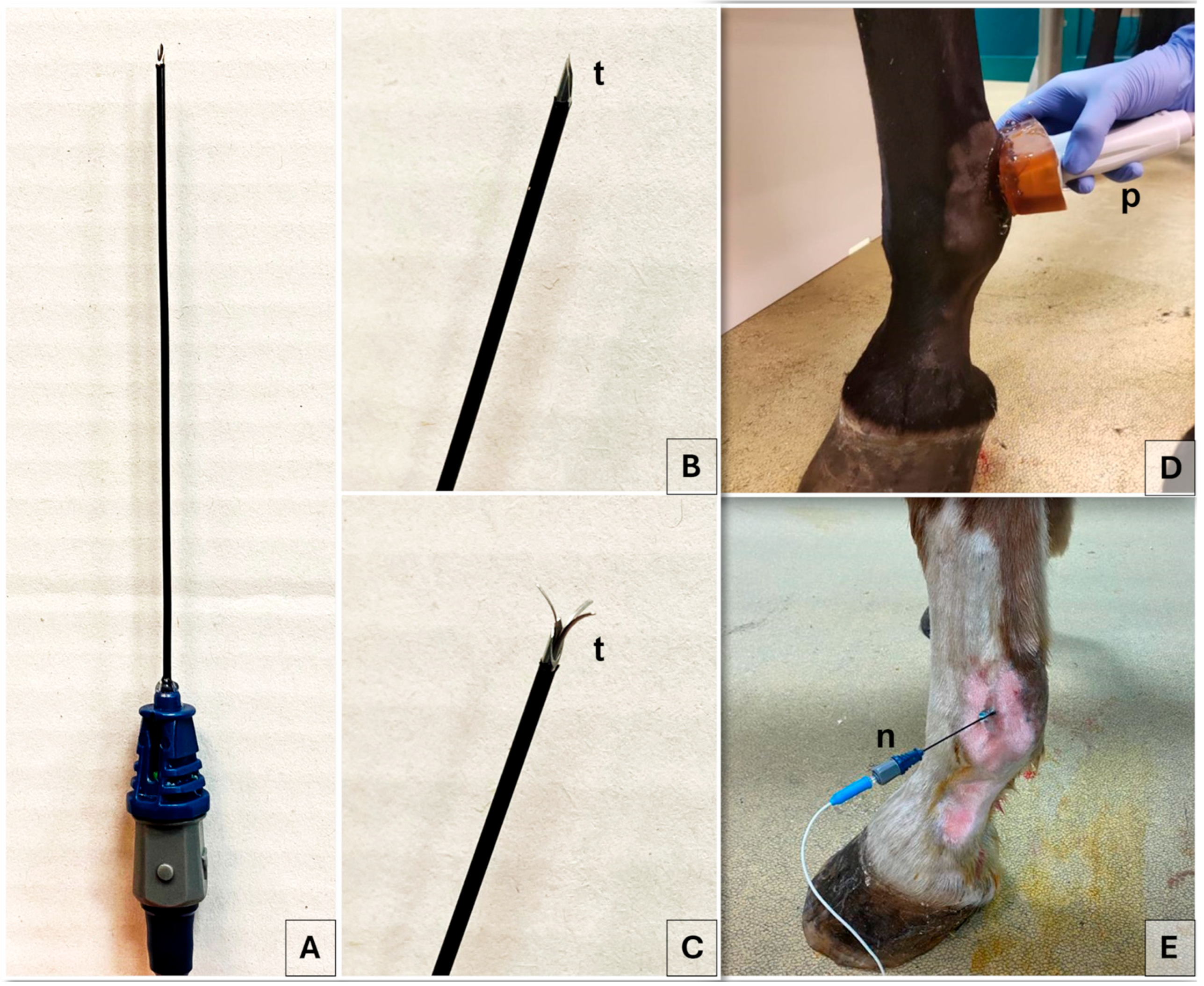
2.3. Radiofrequency Procedure
2.4. Outcome Measure
2.5. Statistical Analysis
3. Results
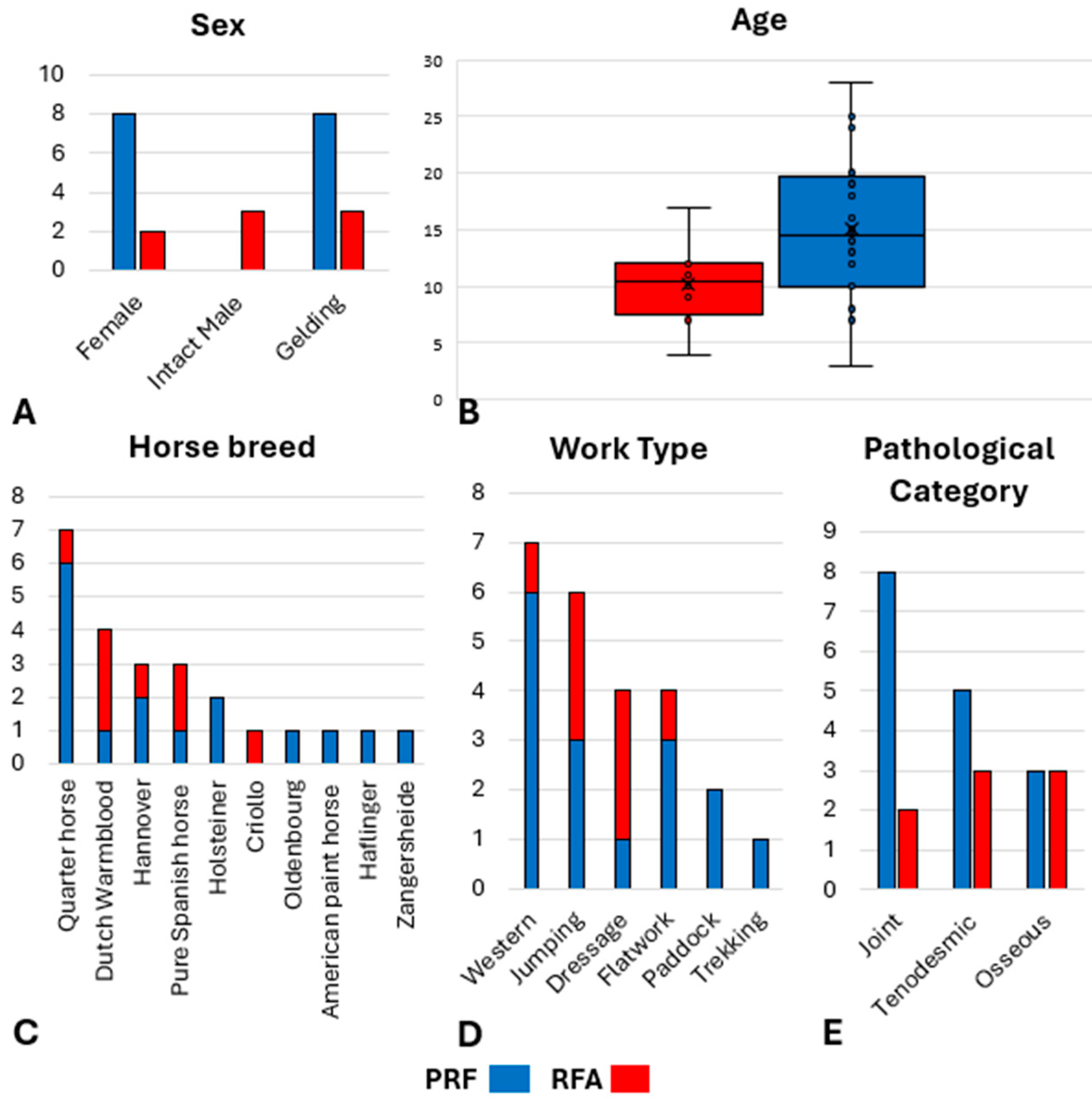
3.1. Animals
3.2. Outcome Measure
4. Discussion
4.1. RFA Clinical Outcomes and Neuroablation
4.2. PRF Clinical Outcomes and Neuromodulation
4.3. Complications: RFA vs. PRF
4.4. Potential Risks Related to RF
4.5. PRF Second Treatment Strategy
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAEP | American Association of Equine Practitioners |
| ASNB | Abaxial Sesamoid Nerve Block |
| ASNBs | Abaxial Sesamoid Nerve Blocks |
| CI | Confidence Interval |
| CT | Computed Tomography |
| DIPJ | Distal Interphalangeal Joint |
| MRI | Magnetic Resonance Imaging |
| NSAID | Nonsteroidal Anti-inflammatory Drug |
| OA | Osteoarthritis |
| PDA | Palmar Digital Artery |
| PDN | Palmar Digital Nerve |
| PDNs | Palmar Digital Nerves |
| PIPJ | Proximal Interphalangeal Joint |
| PRF | Pulsed Radiofrequency |
| RF | Radiofrequency |
| RFA | Radiofrequency Ablation |
| SD | Standard Deviation |
| US | Ultrasound |
Appendix A
References
- Pollard, D.; Wylie, C.E.; Newton, J.R.; Verheyen, K.L.P. Factors Associated with Euthanasia in Horses and Ponies Enrolled in a Laminitis Cohort Study in Great Britain. Prev. Vet. Med. 2020, 174, 104833. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Nibeyro, S.; Werpy, N.; White, N. Standing Low-Field Magnetic Resonance Imaging in Horses with Chronic Foot Pain. Aust. Vet. J. 2012, 90, 75–83. [Google Scholar] [CrossRef]
- Koch, D.W.; Barrett, M.F.; Jackman, B.R.; MacDonald, D.; Goodrich, L.R. Comparison of Lameness Outcomes in Horses with Acute or Chronic Digital Lameness That Underwent Magnetic Resonance Imaging. New Zealand Veter J. 2020, 68, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, F.; Hartmann, A.; Alawneh, J.; Wightman, P.; Saunders, J. Contrast Enhanced Computed Tomography Findings in 105 Horse Distal Extremities. J. Equine Vet. Sci. 2021, 104, 103704. [Google Scholar] [CrossRef] [PubMed]
- Himani, H.; Kumar, A.; Anand, A.; Singh, N.; Uppal, V.; Mohindroo, J. Clinical Occurrence and Radiographic Diagnosis of Distal Limb Lameness in Equine. Indian J. Anim. Sci. 2019, 89, 15–24. [Google Scholar] [CrossRef]
- Schaer, T.P.; Bramlage, L.R.; Embertson, R.M.; Hance, S. Proximal Interphalangeal Arthrodesis in 22 Horses. Equine Vet. J. 2001, 33, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Sampson, S.N.; Schneider, R.K.; Tucker, R.L.; Gavin, P.R.; Zubrod, C.J.; Ho, C.P. Magnetic Resonance Imaging Features of Oblique and Straight Distal Sesamoidean Desmitis in 27 Horses. Vet. Radiol. Ultrasound 2007, 48, 303–311. [Google Scholar] [CrossRef]
- Hawkins, A.; O’Leary, L.; Bolt, D.; Fiske-Jackson, A.; Berner, D.; Smith, R. Retrospective Analysis of Oblique and Straight Distal Sesamoidean Ligament Desmitis in 52 Horses. Equine Vet. J. 2022, 54, 312–322. [Google Scholar] [CrossRef]
- Van Weeren, P.R.; Back, W. Musculoskeletal Disease in Aged Horses and Its Management. Vet. Clin. N. Am. Equine Pract. 2016, 32, 229–247. [Google Scholar] [CrossRef]
- Daglish, J.; Mama, K.R. Pain: Its Diagnosis and Management in the Rehabilitation of Horses. Vet. Clin. N. Am. Equine Pract. 2016, 32, 13–29. [Google Scholar] [CrossRef]
- Flood, J.; Stewart, A.J. Non-Steroidal Anti-Inflammatory Drugs and Associated Toxicities in Horses. Animals 2022, 12, 2939. [Google Scholar] [CrossRef]
- Matthews, B.G.; Hurn, S.E.; Harding, M.P.; Henry, R.A.; Ware, R.S. The Effectiveness of Non-Surgical Interventions for Common Plantar Digital Compressive Neuropathy (Morton’s Neuroma): A Systematic Review and Meta-Analysis. J. Foot Ankle Res. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Nibeyro, S.D.; Werpy, N.M.; White, N.A.; Mitchell, M.A.; Edwards, R.B.; Mitchell, R.D.; Gold, S.J.; Allen, A.K. Outcome of Palmar/Plantar Digital Neurectomy in Horses with Foot Pain Evaluated with Magnetic Resonance Imaging: 50 Cases (2005–2011). Equine Vet. J. 2015, 47, 160–164. [Google Scholar] [CrossRef]
- Madison, J.B.; Dyson, S.J. Treatment and Prognosis of Horses with Navicular Disease. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Saunders: St. Louis, MO, USA, 2003; pp. 299–304. [Google Scholar]
- Albishi, W.; Abudujain, N.M.; Bin Dakhil, A.; Alzeer, M. The Utilization of Radiofrequency Techniques for Upper Extremity Pain Management. Pain Physician 2023, 26, 125–135. [Google Scholar] [CrossRef]
- Vanneste, T.; Van Lantschoot, A.; Van Boxem, K.; Van Zundert, J. Pulsed Radiofrequency in Chronic Pain. Curr. Opin. Anaesthesiol. 2017, 30, 577–582. [Google Scholar] [CrossRef]
- Senthelal, S.; Dydyk, A.M.; Mesfin, F.B. Ablative Nerve Block. In StatPearls; StatPearls Publishing, Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Michaud, K.; Cooper, P.; Abd-Elsayed, A.; Kohan, L. Review of Radiofrequency Ablation for Peripheral Nerves. Curr. Pain Headache Rep. 2021, 25, 63. [Google Scholar] [CrossRef]
- Iannaccone, F.; Dixon, S.; Kaufman, A. A Review of Long-Term Pain Relief after Genicular Nerve Radiofrequency Ablation in Chronic Knee Osteoarthritis. Pain Physician 2017, 20, E437–E444. [Google Scholar] [CrossRef]
- Koshi, E.; Meiling, J.B.; Conger, A.M.; McCormick, Z.L.; Burnham, T.R. Long-Term Clinical Outcomes of Genicular Nerve Radiofrequency Ablation for Chronic Knee Pain Using a Three-Tined Electrode for Expanded Nerve Capture. Interv. Pain Med. 2022, 1, 100003. [Google Scholar] [CrossRef] [PubMed]
- Seale, C.; Connolly, B.R.; Hulk, K.; Yu, G.G.; Nagpal, A.S. The Use of Radiofrequency in the Treatment of Pelvic Pain. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 683–701. [Google Scholar] [CrossRef]
- Russo, M.A.; Santarelli, D.M. Development and Description of a New Multifidus-Sparing Radiofrequency Neurotomy Technique for Facet Joint Pain. Pain Pract. 2021, 21, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Tekin, I.; Mirzai, H.; Ok, G.; Erbuyun, K.; Vatansever, D. A Comparison of Conventional and Pulsed Radiofrequency Denervation in the Treatment of Chronic Facet Joint Pain. Clin. J. Pain 2007, 23, 524–529. [Google Scholar] [CrossRef]
- Choi, G.; Ahn, S.-H.; Cho, Y.-W.; Lee, D.-G. Long-Term Effect of Pulsed Radiofrequency on Chronic Cervical Radicular Pain Refractory to Repeated Transforaminal Epidural Steroid Injections. Pain Med. 2012, 13, 368–375. [Google Scholar] [CrossRef]
- Van Zundert, J.; Patijn, J.; Kessels, A.; Lamé, I.; van Suijlekom, H.; van Kleef, M. Pulsed Radiofrequency Adjacent to the Cervical Dorsal Root Ganglion in Chronic Cervical Radicular Pain: A Double Blind Sham Controlled Randomized Clinical Trial. Pain 2007, 127, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Sujith, J.; Singla, N.; Panda, N.B.; Bala, I. Radiofrequency Thermoablation of the Gasserian Ganglion versus the Peripheral Branches of the Trigeminal Nerve for Treatment of Trigeminal Neuralgia: A Randomized, Control Trial. Pain Physician 2019, 22, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Masala, S.; Cuzzolino, A.; Morini, M.; Raguso, M.; Fiori, R. Ultrasound-Guided Percutaneous Radiofrequency for the Treatment of Morton’s Neuroma. Cardiovasc. Interv. Radiol. 2018, 41, 137–144. [Google Scholar] [CrossRef]
- Alférez, M.D.; Corda, A.; de Blas, I.; Gago, L.; Fernandes, T.; Rodríguez-Piza, I.; Balañá, B.; Corda, F.; Gómez Ochoa, P. Percutaneous Ultrasound-Guided Radiofrequency Ablation as a Therapeutic Approach for the Management of Insulinomas and Associated Metastases in Dogs. Animals 2024, 14, 3301. [Google Scholar] [CrossRef]
- Wright, K.N.; Connor, C.E.; Irvin, H.M.; Knilans, T.K.; Webber, D.; Kass, P.H. Atrioventricular Accessory Pathways in 89 Dogs: Clinical Features and Outcome after Radiofrequency Catheter Ablation. J. Vet. Intern. Med. 2018, 32, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Choi, Y.M.; Jang, E.J.; Kim, J.Y.; Kim, T.K.; Kim, K.H. Neural Ablation and Regeneration in Pain Practice. Korean J. Pain 2016, 29, 3–11. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal Ablation of Tumours: Biological Mechanisms and Advances in Therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Yao, B.; Song, P.; Xu, R.; Li, R.; Liu, P.; Zhang, Y.; Mu, L.; Tong, X.; et al. Neuropathologic Damage Induced by Radiofrequency Ablation at Different Temperatures. Clinics 2022, 77, 100033. [Google Scholar] [CrossRef]
- Zachariah, C.; Mayeux, J.; Alas, G.; Adesina, S.; Mistretta, O.C.; Ward, P.J.; Chen, A.; English, A.W.; Washington, A.V. Physiological and Functional Responses of Water-Cooled versus Traditional Radiofrequency Ablation of Peripheral Nerves in Rats. Reg. Anesth. Pain Med. 2020, 45, 792–798. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, C.H.; Lee, S.H.; Derby, R.; Yang, S.N.; Lee, J.E.; Kim, J.H.; Kim, S.S.; Lee, J.H. Ultrasound-Guided Radiofrequency Neurotomy in Cervical Spine: Sonoanatomic Study of a New Technique in Cadavers. Clin. Radiol. 2008, 63, 1205–1212. [Google Scholar] [CrossRef]
- Amari, M.; Rabbogliatti, V.; Ravasio, G.; Auletta, L.; Brioschi, F.A.; Riccaboni, P.; Aere, S.D.; Roccabianca, P. Development of an Ultrasound-Guided Radiofrequency Ablation Technique in the Equine Cadaveric Distal Limb: Histological Findings and Potential for Treating Chronic Lameness. Front. Vet. Sci. 2024, 11, 1437989. [Google Scholar] [CrossRef]
- Boesch, J.M.; Campoy, L.; Southard, T.; Dewey, C.; Erb, H.N.; Gleed, R.D.; Martin-Flores, M.; Sakai, D.M.; Sutton, J.; Williamson, B.; et al. Histological, Electrophysiological and Clinical Effects of Thermal Radiofrequency Therapy of the Saphenous Nerve and Pulsed Radiofrequency Therapy of the Sciatic Nerve in Dogs. Vet. Anaesth. Analg. 2019, 46, 689–698. [Google Scholar] [CrossRef]
- Vatansever, D.; Tekin, I.; Tuglu, I.; Erbuyun, K.; Ok, G. A Comparison of the Neuroablative Effects of Conventional and Pulsed Radiofrequency Techniques. Clin. J. Pain 2008, 24, 717–724. [Google Scholar] [CrossRef]
- Sam, J.; Catapano, M.; Sahni, S.; Ma, F.; Abd-Elsayed, A.; Visnjevac, O. Pulsed Radiofrequency in Interventional Pain Management: Cellular and Molecular Mechanisms of Action—An Update and Review. Pain Physician 2021, 24, 525–532. [Google Scholar] [PubMed]
- Ren, H.; Jin, H.; Jia, Z.; Ji, N.; Luo, F. Pulsed Radiofrequency Applied to the Sciatic Nerve Improves Neuropathic Pain by Down-Regulating The Expression of Calcitonin Gene-Related Peptide in the Dorsal Root Ganglion. Int. J. Med. Sci. 2018, 15, 153–160. [Google Scholar] [CrossRef]
- Erdine, S.; Bilir, A.; Cosman, E.R.; Cosman, E.R. Ultrastructural Changes in Axons Following Exposure to Pulsed Radiofrequency Fields. Pain Pract. 2009, 9, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, W. Treatment of Neuropathic Pain Using Pulsed Radiofrequency: A Meta-Analysis. Pain Physician 2016, 19, 429–444. [Google Scholar] [CrossRef]
- Almuhanna, A.H.; Cahalan, S.D.; Lane, A.; Goodwin, D.; Perkins, J.; Piercy, R.J. Optimisation and Validation of Immunohistochemical Axonal Markers for Morphological and Functional Characterisation of Equine Peripheral Nerves. Equine Vet. J. 2021, 53, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Bassage, L.H.; Ross, M.W. Diagnostic Analgesia. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Saunders: South Linn Township, MO, USA, 2003; p. 1206. ISBN 0721683428. [Google Scholar]
- Baxter, G.M. Examination for Lameness—Perineural and Intrasynovial Anesthesia. In Adams and Stashak’s Lameness in Horses; Baxter, G.M., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 157–188. ISBN 9781119276685. [Google Scholar]
- Moyer, W. Equine Joint Injection and Regional Anesthesia, 5th ed.; Academic Veterinary Solutions, LLC: Chadds Ford, PA, USA, 2011; ISBN 9780615420332. [Google Scholar]
- Denoix, J.M. Diagnostic Techniques for Identification and Documentation of Tendon and Ligament Injuries. Vet. Clin. N. Am. Equine Pract. 1994, 10, 365–407. [Google Scholar] [CrossRef]
- Keegan, K.G. Examination for Lameness—Subjective Assessment of Lameness. In Adams and Stashak’s Lameness in Horses; Baxter, G.M., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 123–138. ISBN 9781119276685. [Google Scholar]
- Young, J.M.; Schoonover, M.J.; Kembel, S.L.; Taylor, J.D.; Bauck, A.G.; Gilliam, L.L. Efficacy of Orally Administered Gabapentin in Horses with Chronic Thoracic Limb Lameness. Vet. Anaesth. Analg. 2020, 47, 259–266. [Google Scholar] [CrossRef]
- Wray, J.K.; Dixon, B.; Przkora, R. Radiofrequency Ablation. In StatPearls; StatPearls Publishing, Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Alvites, R.; Rita Caseiro, A.; Santos Pedrosa, S.; Vieira Branquinho, M.; Ronchi, G.; Geuna, S.; Varejão, A.S.P.; Colette Maurício, A. Peripheral Nerve Injury and Axonotmesis: State of the Art and Recent Advances. Cogent Med. 2018, 5, 1466404. [Google Scholar] [CrossRef]
- Konig, H.E.; Liebich, H.-G. Nervous System (Systema Nervosum). In Veterinary Anatomy of Domestic Animals; Konig, H.E., Liebich, H.-G., Eds.; Schattauer GmbH: Stuttgart, Germany, 2007; pp. 489–560. [Google Scholar]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) Following Investigational Therapy in Dogs and Cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Lu, K.; Liliang, P.C.; Liang, C.L.; Wang, K.W.; Tsai, Y.D.; Chen, H.J. Efficacy of Conventional and Pulsed Radiofrequency for Treating Chronic Lumbar Facet Joint Pain. Formos. J. Surg. 2012, 45, 107–112. [Google Scholar] [CrossRef][Green Version]
- Agarwal, A.; Rastogi, S.; Bansal, M.; Kumar, S.; Malviya, D.; Thacker, A.K. Radiofrequency Treatment of Idiopathic Trigeminal Neuralgia (Conventional vs. Pulsed): A Prospective Randomized Control Study. Anesth. Essays Res. 2021, 15, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Hwang, S.J.; Song, J.G.; Leem, J.G.; Kang, Y.U.; Park, P.H.; Shin, J.W. Radiofrequency Treatment Relieves Chronic Knee Osteoarthritis Pain: A Double-Blind Randomized Controlled Trial. Pain 2011, 152, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Gazelka, H.M.; Knievel, S.; Mauck, W.D.; Moeschler, S.M.; Pingree, M.J.; Rho, R.H.; Lamer, T.J. Incidence of Neuropathic Pain after Radiofrequency Denervation of the Third Occipital Nerve. J. Pain Res. 2014, 7, 195–198. [Google Scholar] [CrossRef]
- Boesch, J.M.; Campoy, L.; Martin-Flores, M.; Gleed, R.D. Thermal Radiofrequency Ablation of the Saphenous Nerve in Dogs with Pain from Naturally-Occurring Stifle Osteoarthritis. Vet. Anaesth. Analg. 2020, 47, 417–418. [Google Scholar] [CrossRef]
- Cosman, E.R.; Dolensky, J.R.; Hoffman, R.A. Factors That Affect Radiofrequency Heat Lesion Size. Pain Med. 2014, 15, 2020–2036. [Google Scholar] [CrossRef] [PubMed]
- Künzle, A.; van Kuijk, S.M.J.; Koetsier, E. Efficacy of Cervical Facet Joint Radiofrequency Ablation Using a Multitined Cannula, a Technical Note, and Observational Study. Pain Physician 2023, 26, E353–E361. [Google Scholar] [CrossRef]
- Kidd, V.D.; Strum, S.R.; Strum, D.S.; Shah, J. Genicular Nerve Radiofrequency Ablation for Painful Knee Arthritis: The Why and the How. JBJS Essent. Surg. Tech. 2019, 9, e10. [Google Scholar] [CrossRef]
- Provenzano, D.A.; Lassila, H.C.; Somers, D. The Effect of Fluid Injection on Lesion Size during Radiofrequency Treatment. Reg. Anesth. Pain Med. 2010, 35, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Sheiman, R.G.; Mullan, C.; Ahmed, M. In Vivo Determination of a Modified Heat Capacity of Small Hepatocellular Carcinomas Prior to Radiofrequency Ablation: Correlation with Adjacent Vasculature and Tumour Recurrence. Int. J. Hyperth. 2012, 28, 122–131. [Google Scholar] [CrossRef]
- Carpenedo, R.; Al-Wardat, M.; Vizzolo, L.; Germani, G.; Chinè, E.; Ridolfo, S.; Dauri, M.; Natoli, S. Ultrasound-Guided Pulsed Radiofrequency of the Saphenous Nerve for Knee Osteoarthritis Pain: A Pilot Randomized Trial. Pain Manag. 2022, 12, 181–193. [Google Scholar] [CrossRef]
- Fang, L.; Tao, W.; Jingjing, L.; Nan, J. Comparison of High-Voltage- with Standard-Voltage Pulsed Radiofrequency of Gasserian Ganglion in the Treatment of Idiopathic Trigeminal Neuralgia. Pain Pract. 2015, 15, 595–603. [Google Scholar] [CrossRef]
- Jia, Y.; Cheng, H.; Shrestha, N.; Ren, H.; Zhao, C.; Feng, K.; Luo, F. Effectiveness and Safety of High-Voltage Pulsed Radiofrequency to Treat Patients with Primary Trigeminal Neuralgia: A Multicenter, Randomized, Double-Blind, Controlled Study. J. Headache Pain 2023, 24, 91. [Google Scholar] [CrossRef]
- Choi, S.; Choi, H.J.; Cheong, Y.; Lim, Y.J.; Park, H.K. Internal-Specific Morphological Analysis of Sciatic Nerve Fibers in a Radiofrequency-Induced Animal Neuropathic Pain Model. PLoS ONE 2013, 8, e73913. [Google Scholar] [CrossRef] [PubMed]
- Bowker, R.M.; Linder, K.; Sonea, I.M.; Guida, L.A. Sensory Nerve Fibres and Receptors in Equine Distal Forelimbs and Their Potential Roles in Locomotion. Equine Vet. J. 1995, 27, 141–146. [Google Scholar] [CrossRef]
- Facchini, G.; Spinnato, P.; Guglielmi, G.; Albisinni, U.; Bazzocchi, A. A Comprehensive Review of Pulsed Radiofrequency in the Treatment of Pain Associated with Different Spinal Conditions. Br. J. Radiol. 2017, 90, 20150406. [Google Scholar] [CrossRef] [PubMed]
- Roca, G.; de Andrés Ares, J.; Luisa Franco Gay, M.; Nieto, C.; Teresa Bovaira, M. Radiofrequency Techniques: Complications and Troubleshooting. Tech. Reg. Anesth. Pain Manag. 2014, 18, 25–34. [Google Scholar] [CrossRef][Green Version]
- Tzaan, W.C.; Tasker, R.R. Percutaneous Radiofrequency Facet Rhizotomy—Experience with 118 Procedures and Reappraisal of Its Value. Can. J. Neurol. Sci. 2000, 27, 125–130. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C.; Delgado-Martínez, A.D.; De Andrés-Ares, J. Upper Limb and Lower Limb Radiofrequency Treatments in Orthopaedics. EFORT Open Rev. 2023, 8, 424–435. [Google Scholar] [CrossRef]
- Reddy, A.T.; Goyal, N.; Cascio, M.; Leal, J.; Singh, K. Abnormal Paresthesias Associated with Radiofrequency Ablation of Lumbar Medial Branch Nerves: A Case Report. Cureus 2023, 15, e35176. [Google Scholar] [CrossRef] [PubMed]
- Boswell, M.V.; Colson, J.D.; Sehgal, N.; Dunbar, E.E.; Epter, R. A Systematic Review of Therapeutic Facet Joint Interventions in Chronic Spinal Pain. Pain Physician 2007, 10, 229–253. [Google Scholar] [CrossRef]
- Stolzenberg, D.; Gordin, V.; Vorobeychik, Y. Incidence of Neuropathic Pain after Cooled Radiofrequency Ablation of Sacral Lateral Branch Nerves. Pain Med. 2014, 15, 1857–1860. [Google Scholar] [CrossRef]
- Kornick, C.; Kramarich, S.S.; Lamer, T.J.; Sitzman, B.T. Complications of Lumbar Facet Radiofrequency Denervation. Spine 2004, 29, 1352–1354. [Google Scholar] [CrossRef]
- Erken, H.Y.; Ayanoglu, S.; Akmaz, I.; Erler, K.; Kiral, A. Prospective Study of Percutaneous Radiofrequency Nerve Ablation for Chronic Plantar Fasciitis. Foot Ankle Int. 2014, 35, 95–103. [Google Scholar] [CrossRef]
- Huang, Y.H.; Hou, S.Y.; Cheng, J.K.; Wu, C.H.; Lin, C.R. Pulsed Radiofrequency Attenuates Diabetic Neuropathic Pain and Suppresses Formalin-Evoked Spinal Glutamate Release in Rats. Int. J. Med. Sci. 2016, 13, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yamaga, M.; Tateyama, S.; Uno, T.; Tsuneyoshi, I.; Takasaki, M. The Effect of Pulsed Radiofrequency Current on Mechanical Allodynia Induced with Resiniferatoxin in Rats. Anesth. Analg. 2010, 111, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M. Significance of Clinical Treatments on Peripheral Nerve and Its Effect on Nerve Regeneration. J. Neurol. Disord. 2014, 02, 1000168. [Google Scholar] [CrossRef]
- Schmidt, S.; Karri, J.; Singh, M.; Abd-Elsayed, A. Incidence, Diagnosis, and Management of Neuromas Following Radiofrequency Ablation Treatment: A Narrative Review. Curr. Pain Headache Rep. 2021, 25, 45. [Google Scholar] [CrossRef]
- Gekht, G.; Nottmeier, E.W.; Lamer, T.J. Painful Medial Branch Neuroma Treated with Minimally Invasive Medial Branch Neurectomy. Pain Med. 2010, 11, 1179–1182. [Google Scholar] [CrossRef]
- Matthews, S.; Dart, A.J.; Dowling, B.A. Palmar Digital Neurectomy in 24 Horses Using the Guillotine Technique. Aust. Vet. J. 2003, 81, 402–405. [Google Scholar] [CrossRef]
- Jackman, B.R.; Baxter, G.M.; Doran, R.E.; Allen, D.; Parks, A.H. Palmar Digital Neurectomy in Horses 57 Cases (1984–1990). Vet. Surg. 1993, 22, 285–288. [Google Scholar] [CrossRef]
- Dabareiner, R.M.; White, N.A.; Sullins, K.E. Comparison of Current Techniques for Palmar Digital Neurectomy in Horses. In Proceedings of the Annual Convention of the AAEP 1997, Phoenix, AZ, USA, 10 December 1997; pp. 231–232. [Google Scholar]
- Maher, O.; Davis, D.M.; Drake, C.; Myhre, G.D.; Labbe, K.M.; Han, J.H.; Lejeune, S.S. Pull-through Technique for Palmar Digital Neurectomy: Forty-One Horses (1998–2004). Vet. Surg. 2008, 37, 87–93. [Google Scholar] [CrossRef]
- Belba, A.; Vanneste, T.; Jerjir, A.; Smeets, K.; Van Buyten, J.P.; Bellemans, J.; Van Zundert, J. Complex Regional Pain Syndrome of the Knee after Conventional Radiofrequency Ablation of the Genicular Nerves Treated Successfully with Dorsal Root Ganglion Stimulation: A Case Report. Pain Pract. 2022, 22, 541–546. [Google Scholar] [CrossRef]
- Guerra, J.A.; Schumacher, J.; Salcedo-Jiménez, R.; Rohrbach, B.W.; Monterde, A.R.; Romero, L.R.; Donnell, R. Denervating the Pelvic Suspensory Ligaments of Horses Causes Morphological and Histological Changes in the Ligaments. Am. J. Vet. Res. 2022, 83, 399–404. [Google Scholar] [CrossRef]
- Eggleston, R.B.; Baxter, G.M. Lameness of the Distal Limb—Navicular Region/Palmar Foot. In Adams and Stashak’s Lameness in Horses; Baxter, G.M., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 439–462. ISBN 9781119276685. [Google Scholar]
- Cohen, S.P.; Mao, J. Neuropathic Pain: Mechanisms and Their Clinical Implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.M. Practical Review of Self-Mutilation in Horses. Anim. Reprod. Sci. 2008, 107, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zipu, J.; Hao, R.; Chunmei, Z.; Lan, M.; Ying, S.; Fang, L. Long-Term Follow-up of Pulsed Radiofrequency Treatment for Trigeminal Neuralgia: Kaplan-Meier Analysis in a Consecutive Series of 149 Patients. Pain Physician 2021, 24, E1263–E1271. [Google Scholar]
- Byrd, D.; Mackey, S. Pulsed Radiofrequency for Chronic Pain. Curr. Pain Headache Rep. 2008, 12, 37–41. [Google Scholar] [CrossRef]
- Malaithong, W.; Munjupong, S. Combined Continuous Radiofrequency Ablation and Pulsed Neuromodulation to Treat Cervical Facet Joint Pain and Alleviate Postcervical Radiofrequency Side Effects. Anesth. Pain Med. 2022, 12, e129747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.X.; Wang, Q.; He, M.W.; Yang, L.Q.; Wu, B.S.; Ni, J.X. Radiofrequency Thermocoagulation Combined with Pulsed Radiofrequency Helps Relieve Postoperative Complications of Trigeminal Neuralgia. Genet. Mol. Res. 2015, 14, 7616–7623. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.; Dobson, H. Ultrasonography of Peripheral Nerves in the Normal Adult Horse. Vet. Radiol. Ultrasound 2003, 44, 456–464. [Google Scholar] [CrossRef] [PubMed]
| Sample | Lameness Degree | Affected Forelimbs | |||||
|---|---|---|---|---|---|---|---|
| Group | Number of Horses | AAEP Score 2 | AAEP Score 3 | AAEP Score 4 | Right | Left | Bilateral |
| PRF | 16 | 6 | 7 | 3 | 5 | 3 | 8 |
| RFA | 8 | 2 | 6 | 0 | 4 | 3 | 1 |
| Total | 24 | 8 (33%) | 13 (54%) | 3 (13%) | 9 (37.5%) | 6 (25%) | 9 (37.5%) |
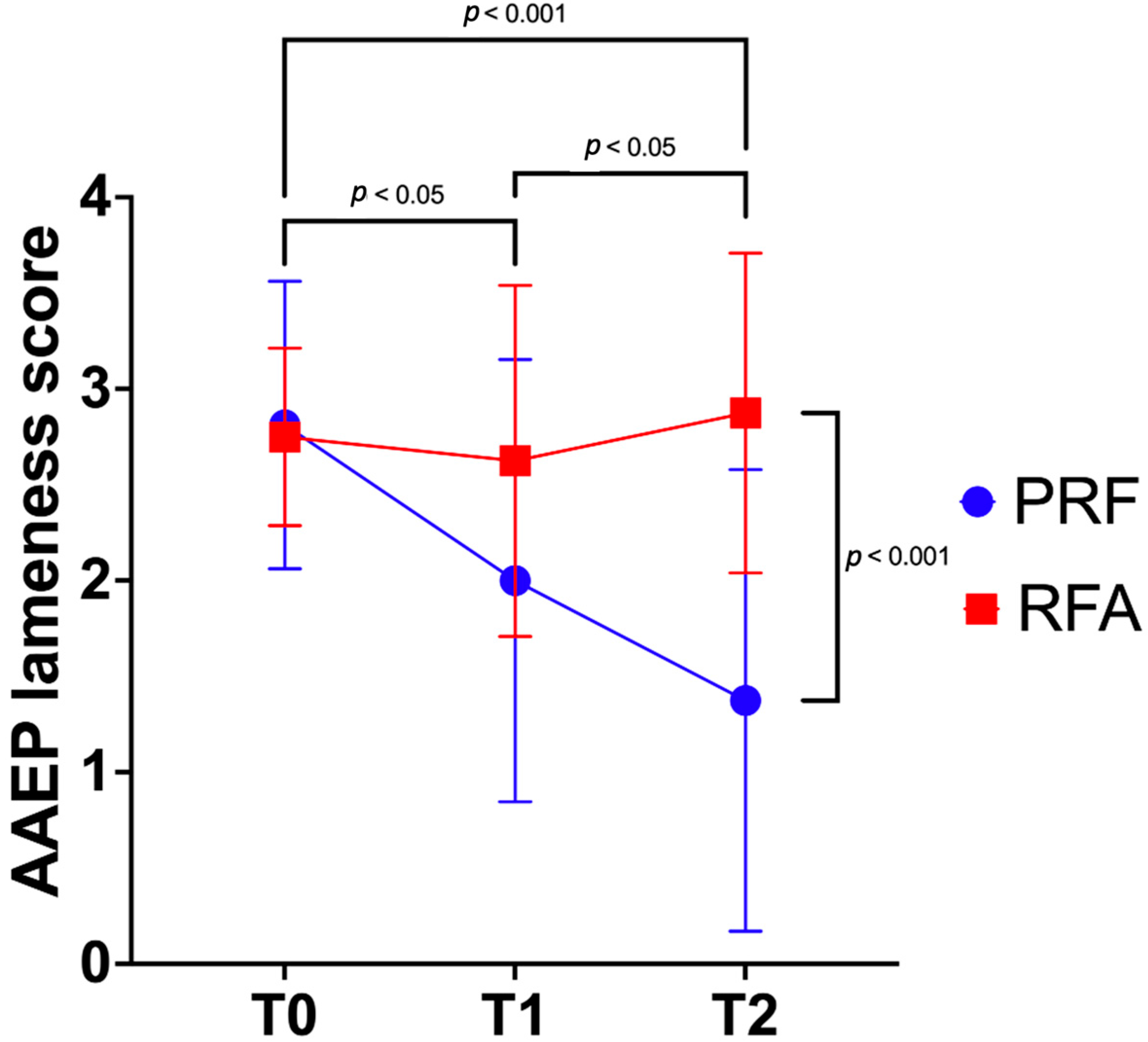
| Group | T0 | T1 | T2 | Within Groups Mean Diff. (95% CI) |
|---|---|---|---|---|
| PRF | 3 (2–4) a,A | 2 (0–3) b | 1 (0–3) *,c,B | T1–T0 −0.8 (−1.5–−0.1) T2–T1 −0.6 (−1.5–−0.2) |
| RFA | 3 (2–3) | 3 (1–4) | 3 (2–4) * | T0–T1 −0.1 (−0.9–0.6) T1–T2 0.2 (−0.7–1.2) |
| Between groups mean diff. (95% CI) | 0.1 (−0.5–0.7) | 0.6 (−0.3–1.6) | 1.5 (0.5–2.5) |
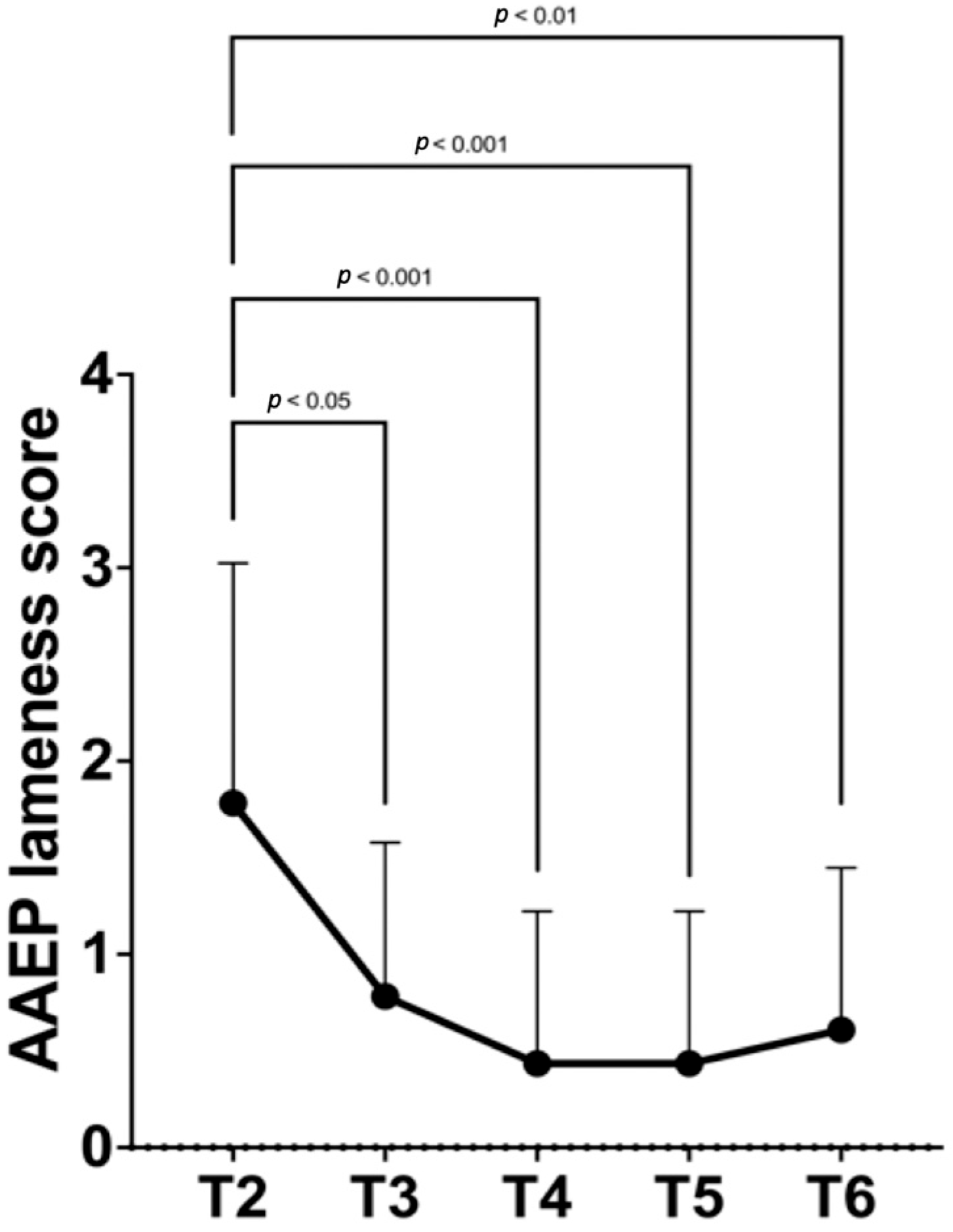
| T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|
| 2 (0–4) a,A | 1 (0–3) b | 0 (0–3) B | 0 (0–3) B | 0 (0–3) B |
| Between groups mean diff. (95% CI) | T3–T2 −1.0 (−1.6–−0.4) | T4–T2 −1.35 (−2.0–−0.7) | T5–T2 −1.3 (−2.0–−0.7) | T6–T2 −1.2 (−1.8–−0.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amari, M.; Brioschi, F.A.; Auletta, L.; Ravasio, G. Ultrasound-Guided Radiofrequency Ablation and Pulsed Radiofrequency Treatment for Chronic Lameness Due to Distal Forelimb Disease in Horses: A Pilot Study. Animals 2025, 15, 2341. https://doi.org/10.3390/ani15162341
Amari M, Brioschi FA, Auletta L, Ravasio G. Ultrasound-Guided Radiofrequency Ablation and Pulsed Radiofrequency Treatment for Chronic Lameness Due to Distal Forelimb Disease in Horses: A Pilot Study. Animals. 2025; 15(16):2341. https://doi.org/10.3390/ani15162341
Chicago/Turabian StyleAmari, Martina, Federica Alessandra Brioschi, Luigi Auletta, and Giuliano Ravasio. 2025. "Ultrasound-Guided Radiofrequency Ablation and Pulsed Radiofrequency Treatment for Chronic Lameness Due to Distal Forelimb Disease in Horses: A Pilot Study" Animals 15, no. 16: 2341. https://doi.org/10.3390/ani15162341
APA StyleAmari, M., Brioschi, F. A., Auletta, L., & Ravasio, G. (2025). Ultrasound-Guided Radiofrequency Ablation and Pulsed Radiofrequency Treatment for Chronic Lameness Due to Distal Forelimb Disease in Horses: A Pilot Study. Animals, 15(16), 2341. https://doi.org/10.3390/ani15162341








