Hematological, Enzymatic, and Endocrine Response to Intense Exercise in Lidia Breed Cattle During the Roping Bull Bullfighting Celebration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
2.2.1. Study Population
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Sample Size and Geographic Distribution
2.3. Experimental Design and Exercise Protocol
2.3.1. Standardized Exercise Protocol
2.3.2. Pre-Event Standardization Procedures
2.3.3. Blood Sampling Protocol
2.4. Sample Collection and Laboratory Processing
2.4.1. Blood Collection Procedures
2.4.2. Sample Processing and Storage Protocols
2.4.3. Quality Control Measures
2.5. Sample Analyses
2.5.1. Hematology
2.5.2. Biochemistry
2.6. Quality Assurance and Method Validation
2.7. Statistical Analysis
2.7.1. Software and Data Management
2.7.2. Descriptive Statistics and Normality Assessment
2.7.3. Comparative Analysis Methods
3. Results
3.1. Hematological Parameters
3.2. Biochemical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adenocorticotropic hormone or adenocorticotropin |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| CK | Creatine kinase |
| HCT | Hematocrit |
| HGB | Hemoglobin |
| LBBSB | Lidia Bovine Breed Stud Book |
| LDH | Lactate dehydrogenase |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| NAD | Nicotinamide adenine dinucleotide |
| NADH | Nicotinamide adenine dinucleotide hydrogenase |
| PLT | Platelets |
| POST | After |
| PRE | Before |
| RBC | Red blood cells |
| SD | Standard deviation |
| WBC | White blood cells |
References
- Rodríguez Montesinos, A. Prototipos Raciales del Vacuno de Lidia (Breed Prototypes of Fighting Cattle); MAPA: Madrid, Spain, 2002. [Google Scholar]
- Real Decreto 60/2001, de 26 de Enero, Sobre Prototipo Racial de la Raza Bovina de Lidia. Bol. Of. Estado 2001, 38, 5255–5261.
- Lomillos, J.M.; Alonso, M.E. The Lidia Breed: Management and Medicine. In Animal Reproduction in Veterinary Medicine; IntechOpen: London, UK, 2020; pp. 1–22. [Google Scholar]
- Lomillos, J.M.; Alonso, M.E.; Sánchez García, C.; Gaudioso, V.R. Evolución del sector de la producción del toro de Lidia en España. Censos y Ganaderías. ITEA-Inf. Tec. Econ. Agrar. 2012, 108, 207–221. [Google Scholar]
- Federación Española de Toro de Cuerda. Available online: https://torodecuerda.es/ (accessed on 15 June 2025).
- Aceña, M.C.; García-Belenguer, S.; Gascón, M.; Purroy, A. Modifications hématologiques et musculaires pendant la corrida chez le taureau de combat. Rev. Med. Vet. 1995, 146, 277–282. [Google Scholar]
- Alonso, M.E.; Sánchez, J.M.; Robles, R.; Zarza, A.M.; Gaudioso, V.R. Relation entre la fréquence des chutes et différents paramètres hématologiques chez le taureau de combat. Rev. Med. Vet. 1997, 148, 999–1004. [Google Scholar]
- Escalera-Valente, F.; González-Montaña, J.R.; Alonso de la Varga, M.E.; Lomillos-Perez, J.M.; Gaudioso-Lacasa, V.R. Influence of intense exercise on acid-base, blood gas and electrolyte status in bulls. Res. Vet. Sci. 2013, 95, 623–628. [Google Scholar] [CrossRef]
- Escribano, B.; Tunez, I.; Requena, F.; Rubio, M.D.; De Miguel, R.; Montilla, P.; Tovar, P.; Aguera, E. Effects of an aerobic training program on oxidative stress biomarkers in bulls. Vet. Med. 2010, 55, 422–428. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Castro, M.J.; Alonso, M.E.; Gaudioso, V.R. Adaptive metabolic responses in females of the fighting breed submitted to different sequences of stress stimuli. Physiol. Behav. 1996, 60, 1047–1052. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Napolitano, F.; Strappini, A.; Orihuela, A.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Mora-Medina, P.; Velarde, A. Quality of Death in Fighting Bulls during Bullfights: Neurobiology and Physiological Responses. Animals 2021, 11, 2820. [Google Scholar] [CrossRef] [PubMed]
- Roches, A.d.B.D.; Faure, M.; Lussert, A.; Herry, V.; Rainard, P.; Durand, D.; Foucras, G. Behavioral and patho-physiological response as possible signs of pain in dairy cows during Escherichia coli mastitis: A pilot study. J. Dairy Sci. 2017, 100, 8385–8397. [Google Scholar] [CrossRef]
- Edwards-Callaway, L.N.; Cramer, M.C.; Cadaret, C.N.; Bigler, E.J.; Engle, T.E.; Wagner, J.J.; Clark, D.L. Impacts of shade on cattle well-being in the beef supply chain. J. Anim. Sci. 2021, 99, skaa375. [Google Scholar] [CrossRef] [PubMed]
- García-Torres, S.; Cabeza de Vaca, M.; Tejerina, D.; Romero-Fernández, M.P.; Ortiz, A.; Franco, D.; Sentandreu, M.A.; Oliván, M. Assessment of stress by serum biomarkers in calves and their relationship to ultimate pH as an indicator of meat quality. Animals 2021, 11, 2291. [Google Scholar] [CrossRef]
- Kareklas, K.; Kunc, H.P.; Arnott, G. Extrinsic stressors modulate resource evaluations: Insights from territoriality under artificial noise. Front. Zool. 2021, 18, 12. [Google Scholar] [CrossRef]
- Gimsa, U.; Tuchscherer, M.; Kanitz, E. Psychosocial stress and immunity—What can we learn from pig studies? Front. Behav. Neurosci. 2018, 12, 64. [Google Scholar] [CrossRef]
- Escalera-Valente, F.; Alonso, M.E.; Lomillos-Pérez, J.M.; Gaudioso-Lacasa, V.R.; Alonso, A.J.; González-Montaña, J.R. Blood Biochemical Variables Found in Lidia Cattle after Intense Exercise. Animals 2021, 11, 2866. [Google Scholar] [CrossRef]
- Mpakama, T.; Chulayo, A.Y.; Muchenje, V. Bruising in slaughter cattle and its relationship with creatine kinase levels and beef quality as affected by animal related factors. Asian-Australas. J. Anim. Sci. 2014, 27, 717–725. [Google Scholar] [CrossRef]
- Purroy, A.; García-Belenguer, S.; González, J.; Gascon, M.; Barberan, M. Muscular lesions and enzymatic activities in fighting bulls. Ann. Rech. Vet. 1992, 23, 59–62. [Google Scholar] [PubMed]
- Mbiydzenyuy, N.E.; Qulu, L.A. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab. Brain Dis. 2024, 39, 1613–1636. [Google Scholar] [CrossRef] [PubMed]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine. In A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Saunders Ltd.: New York, NY, USA, 2006. [Google Scholar]
- Constable, P.; Hinchcliff, K.W.; Done, S.; Gruenberg, W. Veterinary Medicine. In A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 11th ed.; Elsevier: St. Louis, MO, USA, 2017. [Google Scholar]
- Harris, R.; Marlin, D.; Dunnett, M.; Snow, D.; Hultman, E. Muscle buffering capacity and dipeptide content in the thoroughbred horse, greyhound dog and man. Comp. Biochem. Physiol. 1990, 97, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Pösö, A.R.; Soveri, T.; Oksanen, H.E. The effect of exercise on blood parameters in standardbred and Finnish-bred horses. Acta Vet. Scand. 1983, 24, 170–184. [Google Scholar] [CrossRef]
- Tadich, N.; Gallo, C.; Bustamante, H.; Schwerter, M.; van Schaik, G. Effects of transport and lairage time on some blood constituents of Friesian-cross steers in Chile. Livest. Prod. Sci. 2005, 93, 223–233. [Google Scholar] [CrossRef]
- Grandin, T. Assessment of stress during handling and transport. J. Anim. Sci. 1997, 75, 249–257. [Google Scholar] [CrossRef]
- Kramer, J.W.; Hoffmann, W.E. Clinical enzymology. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 303–325. [Google Scholar]
- Brooks, G.A. Lactate production under fully aerobic conditions: The lactate shuttle during rest and exercise. Fed. Proc. 1986, 45, 2924–2929. [Google Scholar]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81, 209–230. [Google Scholar] [CrossRef]
- Valberg, S.J. Muscle conditions affecting sport horses. Vet. Clin. N. Am. Equine Pract. 2018, 34, 253–276. [Google Scholar] [CrossRef]
- McKenzie, E.C.; Hinchcliff, K.W.; Valberg, S.J.; Williamson, K.K.; Payton, M.E.; Davis, M.S. Assessment of alterations in triglyceride and glycogen concentrations in muscle tissue of Alaskan sled dogs during repetitive prolonged exercise. Am. J. Vet. Res. 2008, 69, 1097–1103. [Google Scholar] [CrossRef]
- Arias, C.; Hernández, A.; Hernández, J. Plasma biochemistry in Spanish purebred horses: Reference values and evaluation of the effects of training. Comp. Clin. Pathol. 2013, 22, 283–289. [Google Scholar]
- Salamanca Llorente, F. Influencia del Encierro en la Respuesta Fisiológica del toro Durante la Lidia. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2012; pp. 1–244. [Google Scholar]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Ballou, M.A.; Randel, R.D.; Willard, S.T.; Vann, R.C.; Welsh, T.H. Temperament influences endothelial function and inflammation in lactating dairy cows. J. Dairy Sci. 2011, 94, 4704–4714. [Google Scholar]
- Sporer, K.R.; Weber, P.S.; Burton, J.L.; Earley, B.; Crowe, M.A. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress. J. Anim. Sci. 2008, 86, 1325–1334. [Google Scholar] [CrossRef]
- Café, L.M.; Robinson, D.L.; Ferguson, D.M.; McIntyre, B.L.; Geesink, G.H.; Greenwood, P.L. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef]
- Curley, K.O.; Paschal, J.C.; Welsh, T.H.; Randel, R.D. Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 1–21. [Google Scholar]
- Earley, B.; Murray, M.; Prendiville, D.J.; Pintado, B.; Borque, C.; Canali, E. The effect of transport by road and sea on physiology, immunity and behaviour of beef cattle. Res. Vet. Sci. 2012, 92, 531–541. [Google Scholar] [CrossRef] [PubMed]
- McKeever, K.H. The endocrine system and the challenge of exercise. Vet. Clin. N. Am. Equine Pract. 2002, 18, 321–353. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Fazio, E.; Cravana, C.; Medica, P. Circulating β-endorphin, adrenocorticotrophic hormone and cortisol levels of horses before and after competitive show jumping with different fence heights. Ann. Anim. Sci. 2012, 12, 645–652. [Google Scholar]
- Cayado, P.; Muñoz-Escassi, B.; Domínguez, C.; Manley, W.; Olabarri, B.; Sanchez de la Muela, M.; Castejon, F.; Marañón, G.; Vara, E. Hormone response to training and competition in athletic horses. Equine Vet. J. 2006, 38, 274–278. [Google Scholar] [CrossRef]
- Noakes, T.D. Effect of exercise on serum enzyme activities in humans. Sports Med. 1987, 4, 245–267. [Google Scholar] [CrossRef]


| Variable | Unit | PRE | POST | Normality | p-Value | Reference Values [21] |
|---|---|---|---|---|---|---|
| WBC | 103/µL | 7.7 ± 1.8 | 8.8 ± 2.0 | 0.085 | <0.001 | 4 to 12 |
| Lymphocytes | % | 50.1 ± 6.4 | 44.7 ± 6.9 | 0.011 | <0.001 | 45 to 75 |
| Neutrophils | % | 44.8 ± 6.5 | 50.5 ± 6.4 | 0.066 | <0.001 | 15 to 45 |
| Eosinophils | % | 2.4 ± 1.4 | 2.1 ± 1.6 | <0.001 | 0.019 | 0 to 20 |
| Monocytes | % | 2.5 ± 1.5 | 2.2 ± 1.3 | <0.001 | 0.039 | 2 to 7 |
| Basophils | % | 0.0 ± 0.0 | 0.0 ± 0.0 | - | - | 0 to 2 |
| RBC | 106/µL | 7.5 ± 1.0 | 8.0 ± 1.0 | 0.014 | <0.001 | 5 to 10 |
| HCT | % | 37.6 ± 5.5 | 39.5 ± 5.2 | 0.012 | <0.001 | 24 to 46 |
| HGB | g/dL | 12.1 ± 1.7 | 12.9 ± 1.7 | 0.088 | <0.001 | 8 to 15 |
| VCM | fL | 49.6 ± 3.9 | 49.4 ± 3.8 | 0.020 | 0.683 | 40 to 60 |
| MCH | pg | 16.1 ± 1.3 | 16.1 ± 1.2 | <0.0001 | 0.957 | 11 to 17 |
| MCHC | g/dL | 32.4 ± 1.0 | 32.7 ± 1.5 | <0.0001 | 0.121 | 30 to 36 |
| PL | 103/µL | 289.3 ± 113.3 | 321.6 ± 120.0 | 0.029 | <0.001 | 100–800 |
| Variable | Unit | PRE | POST | Normality | p-Value | Reference Values [21,22] |
|---|---|---|---|---|---|---|
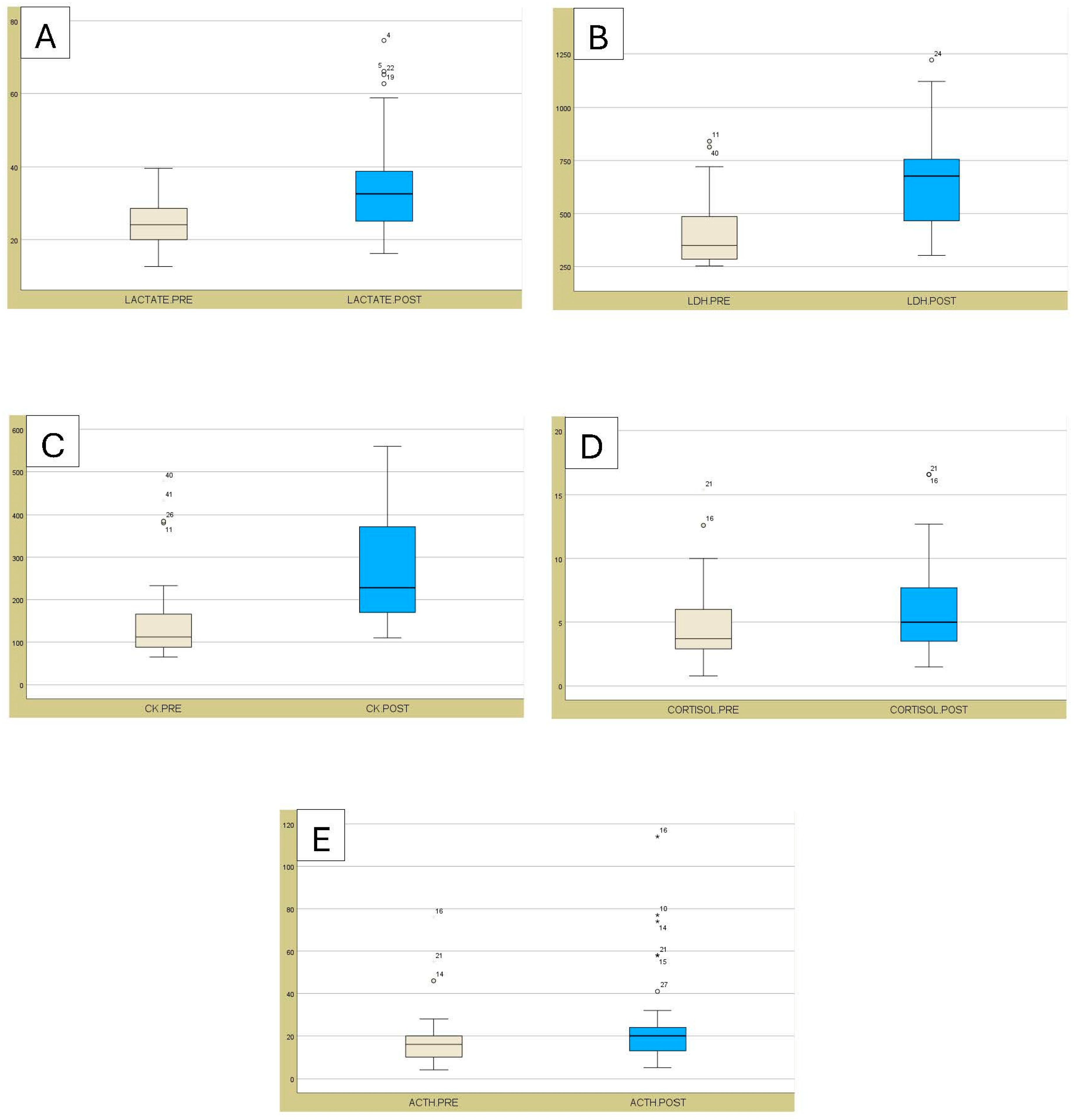
| Lactate | mg/dL | 24.4 ± 6.1 | 35.2 ± 13.7 | 0.004 | <0.001 | 18 to 40 |
| LDH | IU/L | 403.6 ± 152.5 | 661.5 ± 210.8 | <0.001 | <0.001 | 250 to 750 |
| CK | IU/L | 145.0 ± 89.6 | 270.2 ± 125.7 | <0.001 | <0.001 | 35 to 280 |
| Cortisol | µg/dL | 4.6 ± 2.7 | 6.1 ± 3.4 | 0.004 | <0.001 | 0.5 to 0.76 |
| ACTH | pg/mL | 17.1 ± 12.1 | 23.9 ± 19.5 | <0.001 | <0.001 | 10 to 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedeño, J.; Ruiz, S.; Martín, G.; Gardón, J.C. Hematological, Enzymatic, and Endocrine Response to Intense Exercise in Lidia Breed Cattle During the Roping Bull Bullfighting Celebration. Animals 2025, 15, 2303. https://doi.org/10.3390/ani15152303
Sedeño J, Ruiz S, Martín G, Gardón JC. Hematological, Enzymatic, and Endocrine Response to Intense Exercise in Lidia Breed Cattle During the Roping Bull Bullfighting Celebration. Animals. 2025; 15(15):2303. https://doi.org/10.3390/ani15152303
Chicago/Turabian StyleSedeño, Julio, Salvador Ruiz, Germán Martín, and Juan Carlos Gardón. 2025. "Hematological, Enzymatic, and Endocrine Response to Intense Exercise in Lidia Breed Cattle During the Roping Bull Bullfighting Celebration" Animals 15, no. 15: 2303. https://doi.org/10.3390/ani15152303
APA StyleSedeño, J., Ruiz, S., Martín, G., & Gardón, J. C. (2025). Hematological, Enzymatic, and Endocrine Response to Intense Exercise in Lidia Breed Cattle During the Roping Bull Bullfighting Celebration. Animals, 15(15), 2303. https://doi.org/10.3390/ani15152303





