Neutral Sodium Humate Modulates Growth, Slaughter Traits, Antioxidant Status, and Gut Health in Yellow-Feathered Broilers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design and Housing
2.3. Growth Performance
2.4. Serum Biochemical and Antioxidant Indices
2.5. Intestinal Tissue Morphology
2.6. cDNA Synthesis from Extracted RNA
2.7. Quantitative PCR
2.8. Slaughter Performance
2.9. Meat Quality
2.10. Intestinal Microbial Analysis
2.11. Data Analysis
3. Results
3.1. Growth Performance
3.2. Antioxidant Indices and Serum Biochemical Indices
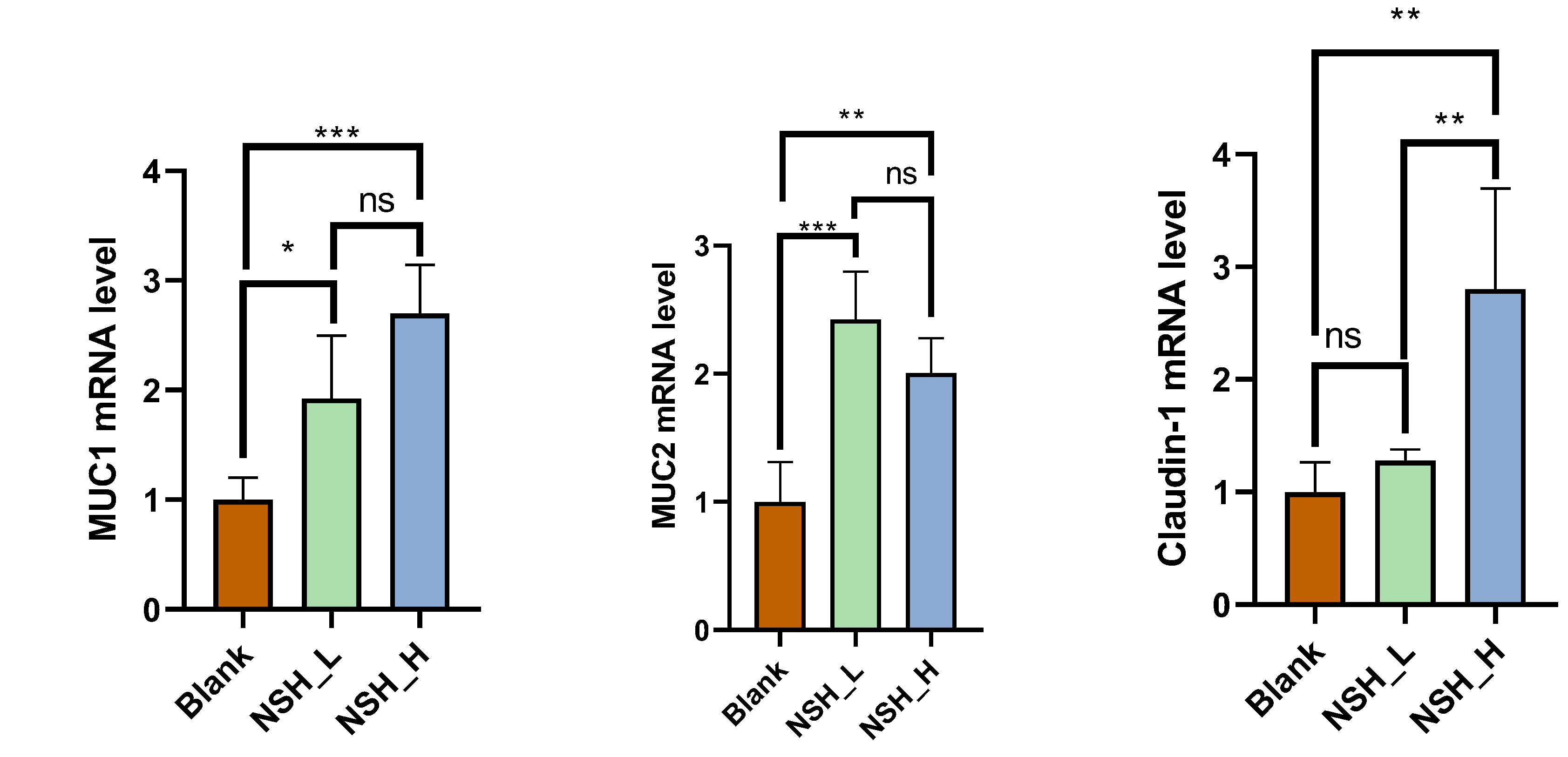
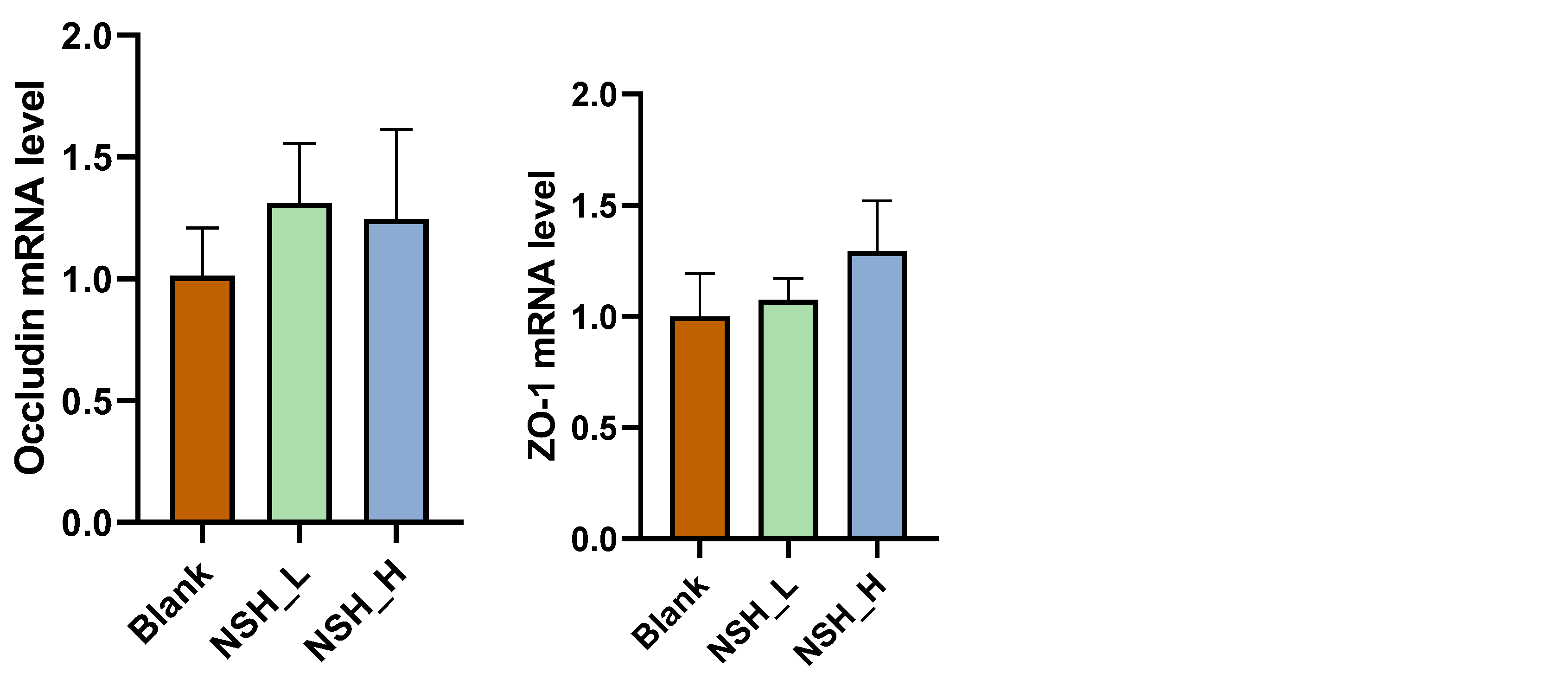
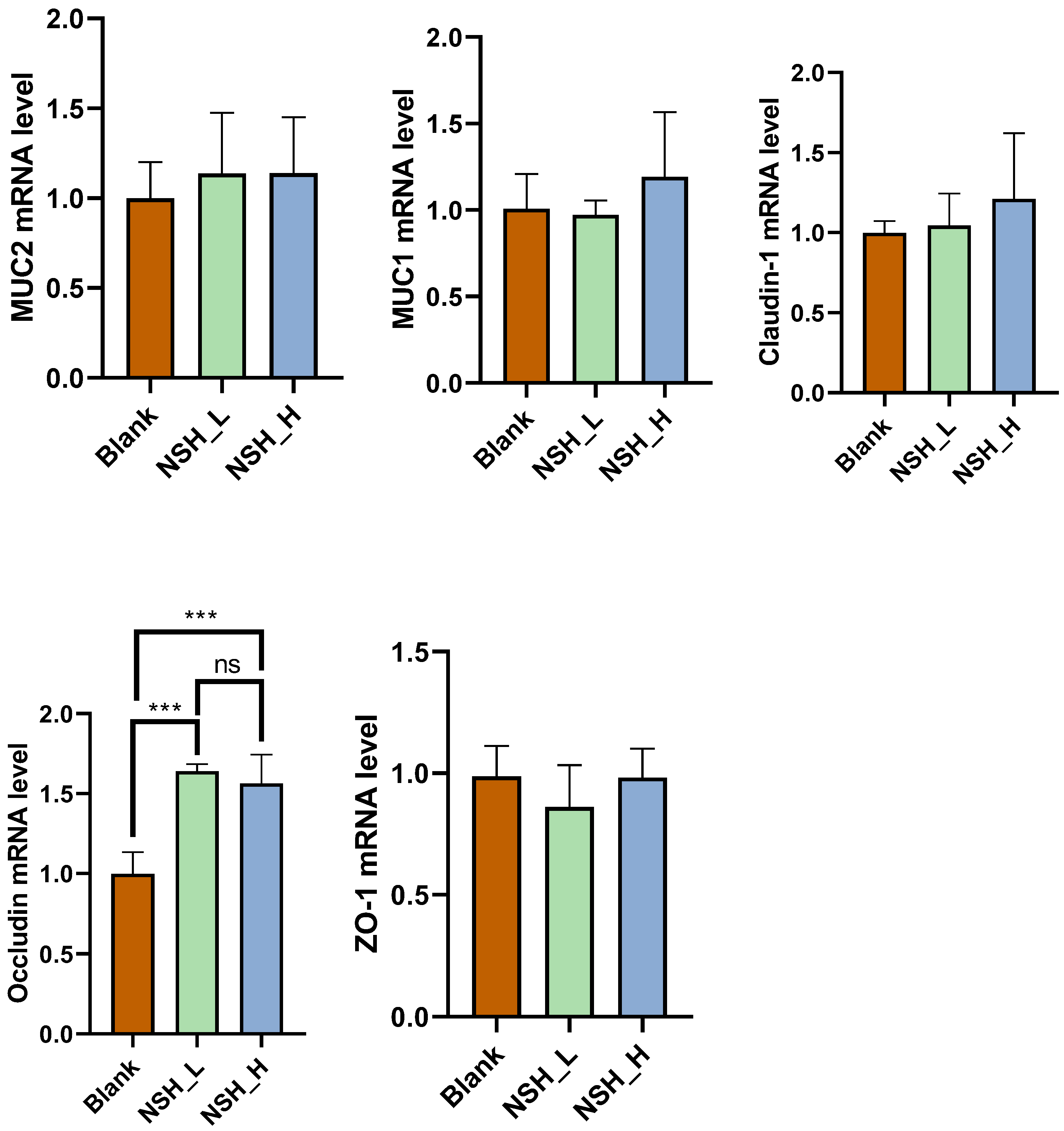
3.3. Intestinal Morphology and Intestinal Mucosal Barrier Function
3.4. Carcass Traits and Meat Quality
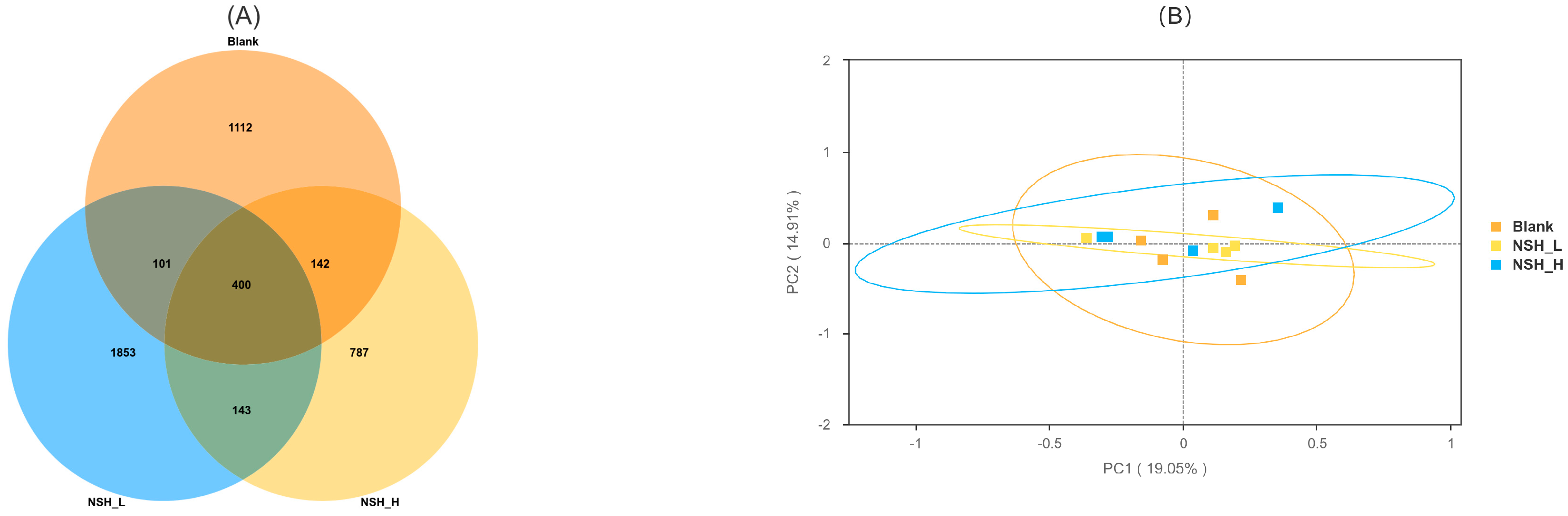
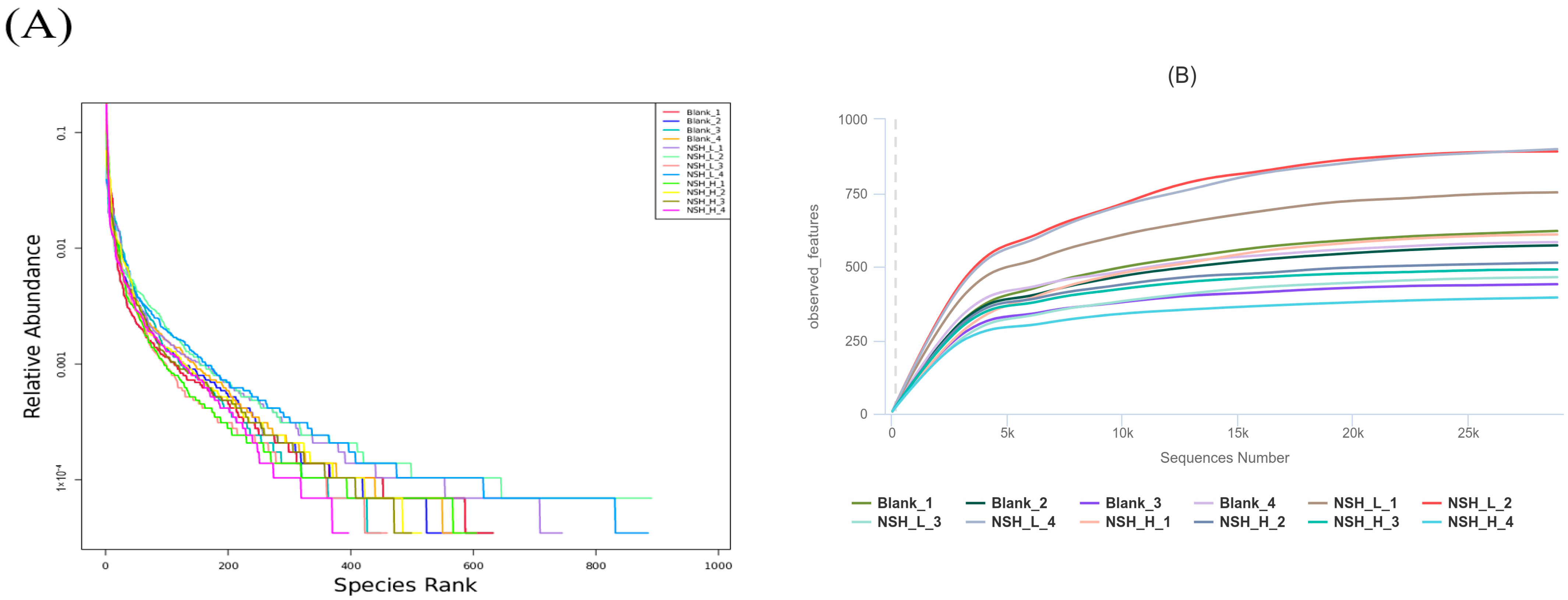
3.5. Analysis of Intestinal Microorganisms
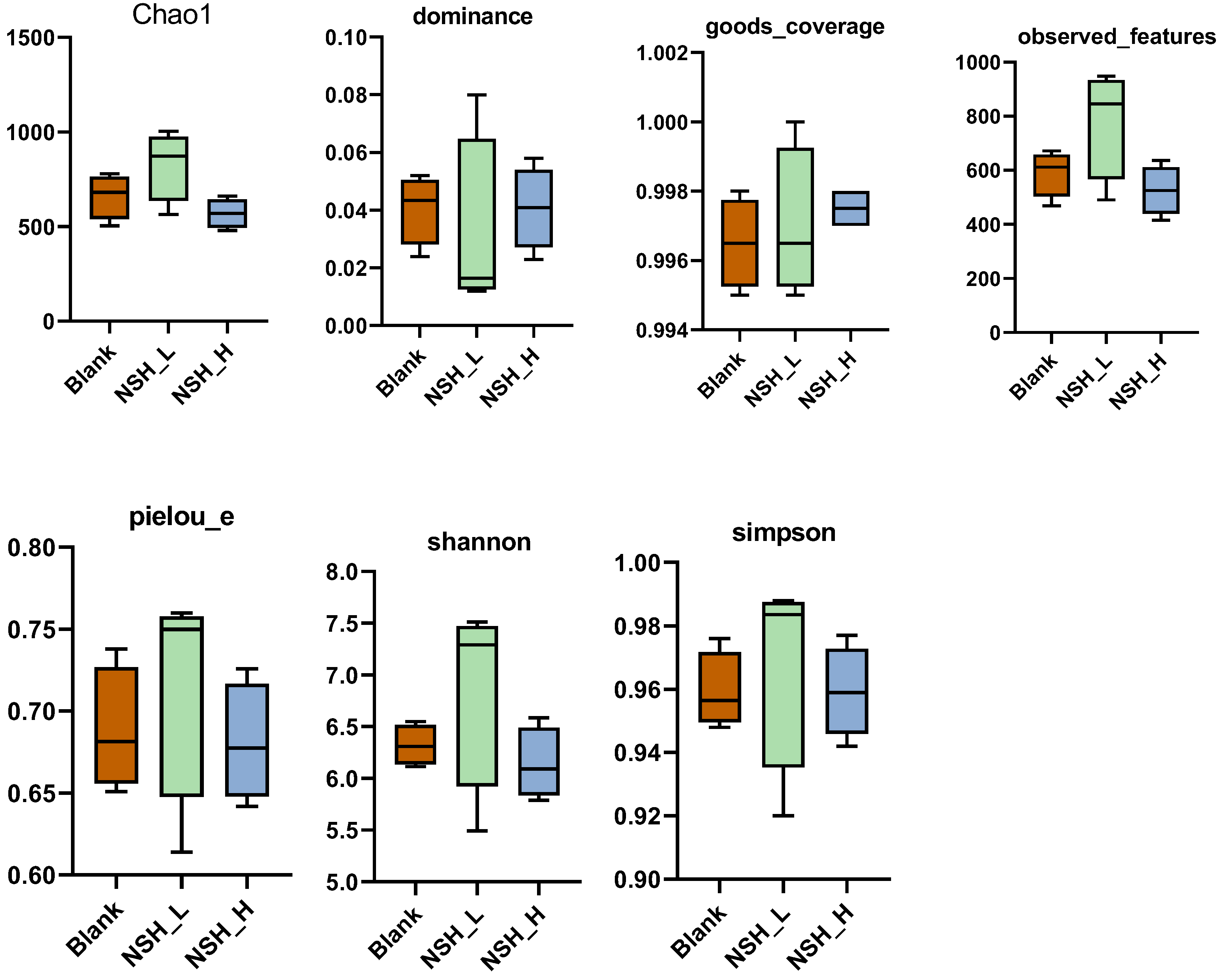
OTU Diversity, Beta Diversity, and Alpha Diversity Analysis
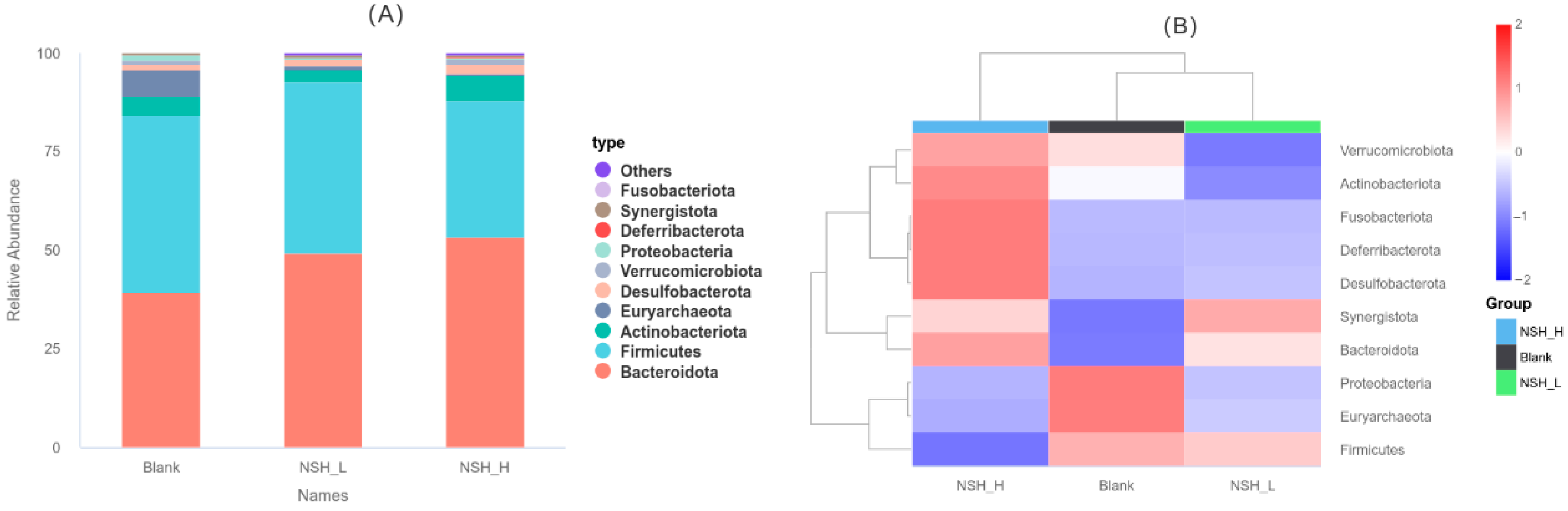
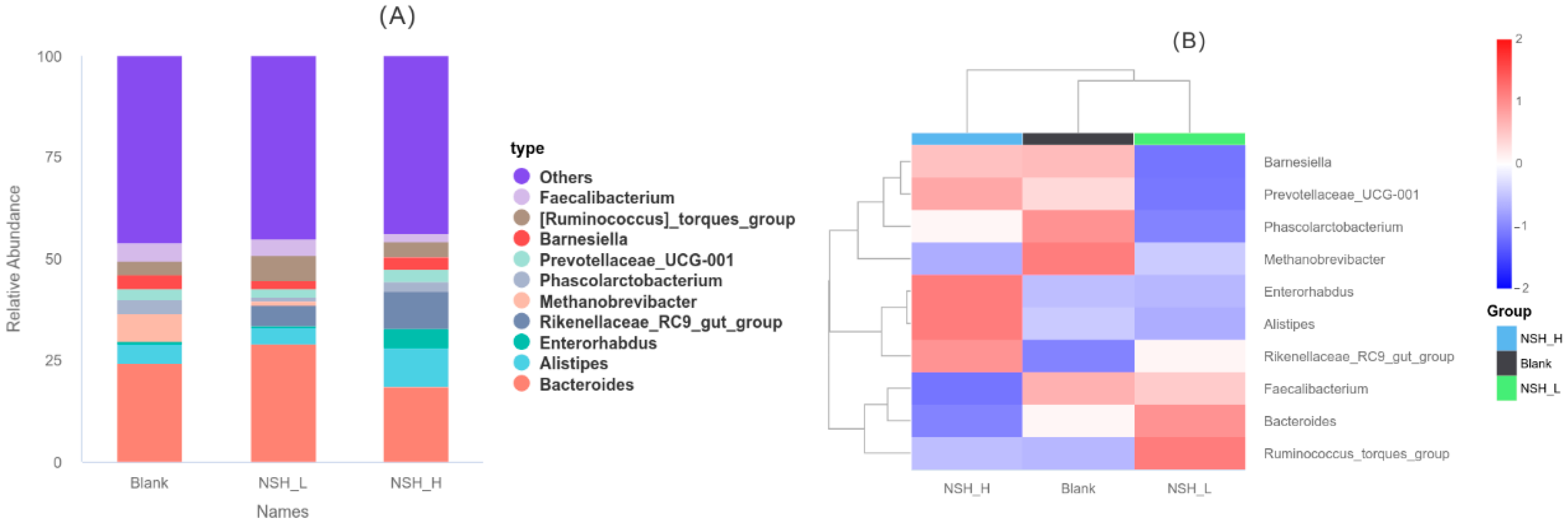
3.6. Taxonomic Composition of Cecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wirsenius, S.; Azar, C.; Berndes, G. How much land is needed for global food production under scenarios of dietary changes and livestock productivity increases in 2030? Agric. Syst. 2010, 103, 621–638. [Google Scholar] [CrossRef]
- Wang, J.; Deng, L.; Chen, M.; Che, Y.; Li, L.; Zhu, L.; Chen, G.; Feng, T. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: A review of the literature of the last decade. Anim. Nutr. 2024, 17, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.; Dunshea, F.R. Cinnamon: A natural feed additive for poultry health and production—A review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Alagawany, M.; Abd El-Hack, M.; Saeed, M.; Arain, M.; Elnesr, S. Humic acid as a feed additive in poultry diets: A review. Iran. J. Vet. Res. 2019, 20, 167. [Google Scholar] [PubMed]
- Abd El-Hack, M.E.; Alagawany, M.; Arif, M.; Emam, M.; Saeed, M.; Arain, M.A.; Siyal, F.A.; Patra, A.; Elnesr, S.S.; Khan, R.U. The uses of microbial phytase as a feed additive in poultry nutrition—A review. Ann. Anim. Sci. 2018, 18, 639–658. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, A.; Chen, X.; Che, X.; Zhou, K.; Wang, Z. Sodium humate accelerates cutaneous wound healing by activating TGF-β/Smads signaling pathway in rats. Acta Pharm. Sin. B 2016, 6, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; You, Z.; Du, Y.; Zheng, D.; Jia, H.; Liu, Y. Influence of sodium humate on the growth performance, diarrhea incidence, blood parameters, and fecal microflora of pre-weaned dairy calves. Animals 2022, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Jooné, G.K.; Dekker, J.; van Rensburg, C.E.J. Investigation of the immunostimulatory properties of oxihumate. Z. Naturforsch. C 2003, 58, 263–267. [Google Scholar] [CrossRef] [PubMed]
- El-Ratel, I.T.; El Basuini, M.F.; Khattab, A.A.; Mekawy, A.I.; Fouda, S.F. Ameliorative impacts of sodium humate on heat-stressed laying Japanese quail (Coturnix coturnix Japonica). Anim. Physiol. Anim. Nutr. 2023, 107, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Pirzado, S.; Liu, G.; Chen, Z.; Chang, W.; Cai, H.; Bryden, W.; Zheng, A. Dietary supplementation with sodium humate improves egg quality and immune function of laying hens. J. Appl. Anim. Nutr. 2020, 8, 93–99. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, J.; Zou, P.; Zhou, Y.; Wang, B.; Yu, D.; Li, W.; Zhan, X. Effects of dietary supplementation of humic acid sodium and zinc oxide on growth performance, immune status and antioxidant capacity of weaned piglets. Animals 2020, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Skalická, M.; Koréneková, B.; Nad, P.; Makóová, Z. The role of natrium humate on cadmium elimination and copper level in poultry. Chem. Inż. Ekol. 2002, 9, 10. [Google Scholar]
- Proskina, L.; Barzdina, D.; Valdovska, A.; Pilvere, I.; Vircava, I.; Cerina, S.; Meskis, S. Assessment of the inclusion of a feed additive of sodium humate derived from freshwater sapropel in diets for broiler chickens. Vet. World 2023, 16, 2029. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Li, P.; Wei, B.; Zhang, C.; Zhu, X.; Jie, Z. Sodium humate alters the intestinal microbiome, short-chain fatty acids, eggshell ultrastructure, and egg performance of old laying hens. Front. Vet. Sci. 2022, 9, 986562. [Google Scholar] [CrossRef] [PubMed]
- NY/T 3645-2020; Yellow-Feathered Broiler Chicken Nutritional Requirements. China Agriculture Press: Beijing, China, 2020.
- Hou, J.; Lu, L.; Lian, L.; Tian, Y.; Zeng, T.; Ma, Y.; Li, S.; Chen, L.; Xu, W.; Gu, T. Effects of coated sodium butyrate on the growth performance, serum biochemistry, antioxidant capacity, intestinal morphology, and intestinal microbiota of broiler chickens. Front. Microbiol. 2024, 15, 1368736. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, M.; Wu, Z.; Huang, P.; Zeng, J. Dihydrosanguinarine enhances tryptophan metabolism and intestinal immune function via AhR pathway activation in broilers. J. Anim. Sci. Biotechnol. 2025, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhan, X.; Wang, B.; Wang, F.; Zhou, Y.; Xu, S.; Li, X.; Tang, L.; Jin, Q.; Li, W. Modified montmorillonite improved growth performance of broilers by modulating intestinal microbiota and enhancing intestinal barriers, anti-inflammatory response, and antioxidative capacity. Antioxidants 2022, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, S.; Li, X.; Yang, X.; Long, F.; Yang, X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poult. Sci. 2019, 98, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Luo, Z.; Liu, D. Supplemental Bacillus subtilis PB6 improves growth performance and gut health in broilers challenged with Clostridium perfringens. J. Immunol. Res. 2021, 2021, 2549541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xiao, G.; Yan, X.; Qiu, T.; Liu, S.; Ou, J.; Cen, M.; Gong, L.; Shi, J.; Zhang, H. Complex of lauric acid monoglyceride and cinnamaldehyde ameliorated subclinical necrotic enteritis in yellow-feathered broilers by regulating gut morphology, barrier, inflammation and serum biochemistry. Animals 2023, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- NY/T 823-2004; Nomenclature of Poultry Production Performance Terms and Statistical Methods. China Agriculture Press: Beijing, China, 2004.
- Chen, K.; Lv, J.; Luo, Z.; Liu, Z.; Cen, M.; Li, B.; Ou, J.; Zhang, H. The effect of amylase, chromium propionate and their combination supplementation on growth performance, carcass traits, serum parameters, antioxidant capacity and intestinal health in yellow feathered broilers. Poult. Sci. 2025, 104, 105229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xing, T.; Zhang, L.; Zhao, L.; Gao, F. Effects of substituting soybean meal with fermented rapeseed meal mixture on the growth performance, slaughter performance, meat quality, blood biochemical indices and intestinal barrier function in Langshan Chickens. Poult. Sci. 2024, 103, 104478. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, H.; Gao, X.; Wang, J. The intratumor microbiota signatures associate with subtype, tumor stage, and survival status of esophageal carcinoma. Front. Oncol. 2021, 11, 754788. [Google Scholar] [CrossRef] [PubMed]
- Kocabağli, N.; Alp, M.; Acar, N.; Kahraman, R. The effects of dietary humate supplementation on broiler growth and carcass yield. Poult. Sci. 2002, 81, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S. Effect of adding dietary humate on productive performance of broiler chicks. CABI Digit. Libr. 2014, 2014, 23–31. [Google Scholar] [CrossRef]
- Surai, P.; Fisinin, V. Vitagenes in poultry production: Part 1. Technological and environmental stresses. World’s Poult. Sci. J. 2016, 72, 721–734. [Google Scholar] [CrossRef]
- Surai, P.; Fisinin, V. Vitagenes in poultry production: Part 2. Nutritional and internal stresses. World’s Poult. Sci. J. 2016, 72, 761–772. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.M.; Elhady, M.; El Iraqi, K.; Wahba, F. Biological immune stimulants effects on immune response, behavioural and productive performance of broilers. Egypt. Poult. Sci. 2015, 35, 691–702. Available online: https://www.researchgate.net/publication/281614793 (accessed on 20 December 2024).
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I. Cholesterol in health and disease. J. Clin. Investig. 2002, 110, 583–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schneeman, B.O. Gastrointestinal physiology and functions. Br. J. Nutr. 2002, 88, S159–S163. [Google Scholar] [CrossRef] [PubMed]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Eivollahi, L.; Ahady, M.T.; Sahraei, M. The Effect of Sodium Humate and Probiotic on Performance, Carcass traits, Immunological Indices and Gut Morphology in Broiler Chickens. Vet. Res. 2019, 74, 4. [Google Scholar] [CrossRef]
- Kogut, M.H.; Genovese, K.J.; Swaggerty, C.L.; He, H.; Broom, L. Inflammatory phenotypes in the intestine of poultry: Not all inflammation is created equal. Poult. Sci. 2018, 97, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, D.; Liu, K.; Deng, S.; Liu, Y. Sodium humate alleviates LPS-induced intestinal barrier injury by improving intestinal immune function and regulating gut microbiota. Mol. Immunol. 2023, 161, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Słowiñski, M.; Janakowski, S. The quality evaluation of RFN and PSE pork longissimus lumborum muscle considering its microstructure. Ann. Anim. Sci. 2014, 14, 737–747. [Google Scholar] [CrossRef]
- Jankowiak, H.; Cebulska, A.; Bocian, M. The relationship between acidification (pH) and meat quality traits of polish white breed pigs. Eur. Food Res. Technol. 2021, 247, 2813–2820. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Disetlhe, A.R.; Marume, U.; Mlambo, V.; Hugo, A. Effects of dietary humic acid and enzymes on meat quality and fatty acid profiles of broiler chickens fed canola-based diets. Asian-Australas. J. Anim. Sci. 2018, 32, 711. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.A.; Hugenholtz, F.; Lahti, L.; Smidt, H.; de Vos, W.M. Intestinal microbiome landscaping: Insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol. Rev. 2017, 41, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Wexler, A.; Goodman, A. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [PubMed]
- Dehority, B. Pectin-fermenting bacteria isolated from the bovine rumen. J. Bacteriol. 1969, 99, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M. Nutritional features and ecology of predominant anaerobic bacteria of the intestinal tract. Am. J. Clin. Nutr. 1974, 27, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Salyers, A.; Vercellotti, J.; West, S.; Wilkins, T. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 1977, 33, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.D.; Ihekweazu, F.D.; Haidacher, S.J.; Ruan, W.; Engevik, K.A.; Fultz, R.; Hoch, K.M.; Luna, R.A.; Oezguen, N.; Spinler, J.K.; et al. Bacteroides ovatus colonization influences the abundance of intestinal short chain fatty acids and neurotransmitters. iScience 2022, 25, 104158. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Arboleya, S.; Hernandez-Barranco, A.M.; Alvarez-Buylla, J.R.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Interactions between Bifidobacterium and Bacteroides species in cofermentations are affected by carbon sources, including exopolysaccharides produced by bifidobacteria. Appl. Environ. Microbiol. 2013, 79, 7518–7524. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Sánchez, B.; Salazar, N.; Martínez, N.; Redruello, B.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. Different metabolic features of Bacteroides fragilis growing in the presence of glucose and exopolysaccharides of bifidobacteria. Front. Microbiol. 2015, 6, 825. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.W.; Pinchak, W.E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D.; et al. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.G. Carbohydrate metabolism: Structural carbohydrates. In Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 205–236. [Google Scholar]
- Mohammadzadeh, R.; Mahnert, A.; Duller, S.; Moissl-Eichinger, C. Archaeal key-residents within the human microbiome: Characteristics, interactions and involvement in health and disease. Curr. Opin. Microbiol. 2022, 67, 102146. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (100%) | 1–21 d | 22–42 d | 43–56 d |
|---|---|---|---|
| Corn | 44.50 | 39.50 | 42.20 |
| Soybean meal (CP = 46%) | 33.50 | 33.00 | 27.70 |
| Extruded soybean | 5.00 | 0.00 | 0.00 |
| Sorghum | 10.00 | 20.00 | 20.00 |
| Lard | 1.50 | 3.50 | 6.40 |
| Fish meal | 1.30 | 0.00 | 0.00 |
| Dicalcium phosphate | 1.30 | 1.30 | 1.20 |
| Limestone | 1.40 | 1.40 | 1.00 |
| L-lysine hydrochloride | 0.25 | 0.19 | 0.24 |
| DL-methionine | 0.23 | 0.21 | 0.19 |
| L-threonine | 0.05 | 0.06 | 0.05 |
| 1 Premix compound | 0.71 | 0.57 | 0.75 |
| Sodium chloride | 0.12 | 0.15 | 0.15 |
| Choline chloride | 0.12 | 0.10 | 0.10 |
| 2 Antioxidant | 0.01 | 0.01 | 0.01 |
| Phytase | 0.01 | 0.01 | 0.01 |
| Calculated nutrient composition | \ | ||
| Crude protein | 21.45 | 19.09 | 17.11 |
| Metabolizable Energy (kcal/kg) | 2877.00 | 2956.05 | 3173.48 |
| Crude fat | 4.87 | 6.02 | 8.85 |
| Calcium | 1.00 | 0.94 | 0.75 |
| Total Phosphorus | 0.64 | 0.60 | 0.56 |
| Lysine | 1.39 | 1.16 | 1.06 |
| Methionine | 0.56 | 0.49 | 0.45 |
| Threonine | 0.88 | 0.78 | 0.69 |
| Genes | Primer Sequence | Temperature (℃) | Number | Reference |
|---|---|---|---|---|
| β-actin | F: CATTGTCCACCGCAAATGCT R: AAGCCATGCCAATCTCGTCT | 57.2 | NM_205518.1 | [18] |
| Occludin | F: CGGAGCCCAGACTACCAAAG R: TTACACAGCTTCAGCCTTACA | 55.8 | XM_025144247.1 | [18] |
| Claudin-1 | F: GGTATGGCAACAGAGTGGCT R: CAGCCAATGAAGAGGGCTGA | 57.0 | NM_001013611.2 | [18] |
| MUC-2 | F: TTCATGATGCCTGCTCTTGTG R: CCTGAGCCTTGGTACATTCTTGT | 58.0 | XM_421035 | [19] |
| ZO-1 | F: CTTCAGGTGTTTCTCTTCCTCCTC R: CTGTGGTTTCATGGCTGGATC | 56.6 | XM_413773 | [20] |
| ZO-2 | F: AGTGGCCACCATTGTTGTGA R:ACTGTAGCCACTTCGAGCAC | 56.2 | XM_046934790 | [21] |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Blank | NSH-L | NSH-L | |||
| Initial Body Weight (g) | 32.26 ± 0.05 | 32.26 ± 0.06 | 32.23 ± 0.05 | 0.015 | 0.548 |
| 22 d FBW (g) | 372.96 ± 4.85 | 372.35 ± 9.48 | 384.39 ± 5.17 | 2.443 | 0.059 |
| 1–22 d ADG (g/d) | 16.22 ± 0.23 | 16.19 ± 0.45 | 16.76 ± 0.24 | 0.116 | 0.062 |
| 1–22 d Average Daily Feed Intake (g/d) | 27.53 ± 0.94 | 26.3 ± 0.94 | 28.06 ± 1.59 | 0.383 | 0.159 |
| 1–22 d FCR | 1.7 ± 0.05 | 1.61 ± 0.04 | 1.68 ± 0.08 | 0.019 | 0.122 |
| 43 d FBW (g) | 1272.75 ± 14.02 a | 1302.14 ± 15.31 ab | 1310.64 ± 18.38 b | 6.436 | 0.021 |
| 23–43 d ADG (g/d) | 42.84 ± 0.77 | 44.27 ± 1.14 | 44.1 ± 0.65 | 0.298 | 0.091 |
| 23–43 d Average Daily Feed Intake (g/d) | 71.28 ± 1.89 | 71.76 ± 0.99 | 72.13 ± 0.53 | 0.348 | 0.649 |
| 23–43 d FCR | 1.64 ± 0.05 | 1.62 ± 0.02 | 1.63 ± 0.02 | 0.009 | 0.679 |
| 57 d FBW (g) | 1949.77 ± 13.29 a | 1995.96 ± 10.64 b | 2006.07 ± 6.31 b | 7.879 | <0.001 |
| 44–57 d ADG (g/d) | 48.35 ± 0.99 | 49.55 ± 1.07 | 49.67 ± 1.06 | 0.326 | 0.197 |
| 44–57 d Average Daily Feed Intake (g/d) | 133.38 ± 3.01 | 130.61 ± 1.19 | 131.97 ± 1.42 | 0.632 | 0.214 |
| 44–57 d FCR | 2.71 ± 0.06 | 2.64 ± 0.08 | 2.72 ± 0.17 | 0.031 | 0.607 |
| 1–57 d ADG (g/d) | 34.24 ± 0.24 a | 35.07 ± 0.19 b | 35.25 ± 0.11 b | 0.141 | <0.001 |
| 1–57 d Average Daily Feed Intake (g/d) | 70.4 ± 1.18 | 69.43 ± 0.45 | 70.56 ± 0.48 | 0.254 | 0.14 |
| 1–57 d FCR | 2.03 ± 0.04 | 1.98 ± 0.02 | 2.01 ± 0.02 | 0.01 | 0.113 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Blank | NSH-L | NSH-H | |||
| 22 days | |||||
| GSH-Px (U/mgprot) | 2009.51 ± 119.56 a | 2697.11 ± 184.1 c | 2387.31 ± 123.46 b | 92.89 | <0.001 |
| T-SOD (U/mL) | 366.31 ± 6.68 a | 379.21 ± 15.16 a | 427.7 ± 34.06 b | 9.8 | 0.008 |
| T-AOC (mM) | 0.23 ± 0.02 a | 0.37 ± 0.06 b | 0.29 ± 0.03 ab | 0.02 | 0.003 |
| MDA (nmol/mL) | 4.92 ± 0.24 a | 3.06 ± 0.49 b | 4.31 ± 0.63 a | 0.264 | 0.001 |
| 43 days | |||||
| GSH-Px (U/mgprot) | 392.88 ± 13.41 a | 590.79 ± 29.28 b | 561.8 ± 11.84 b | 26.82 | <0.001 |
| T-SOD (U/mL) | 171.09 ± 8.81 | 182.17 ± 7.73 | 168.81 ± 14.13 | 3.28 | 0.217 |
| T-AOC (mM) | 0.44 ± 0.02 a | 0.48 ± 0.05 a | 0.78 ± 0.08 b | 0.048 | <0.001 |
| MDA (nmol/mL) | 5.06 ± 0.46 a | 2.81 ± 0.33 b | 2.78 ± 0.26 b | 0.335 | <0.001 |
| 57 days | |||||
| GSH-Px (U/mgprot) | 852.14 ± 16.65 a | 921.65 ± 4.31 b | 868.72 ± 6.52 a | 9.36 | <0.001 |
| T-SOD (U/mL) | 129.35 ± 3.48 a | 167.39 ± 7.45 b | 167.41 ± 6.28 b | 5.63 | <0.001 |
| T-AOC (mM) | 0.39 ± 0.02 | 0.45 ± 0.04 | 0.46 ± 0.02 | 0.015 | 0.102 |
| MDA (nmol/mL) | 3.94 ± 0.33 a | 2.83 ± 0.5 b | 2.93 ± 0.33 b | 0.183 | 0.006 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Blank | NSH-L | NSH-H | |||
| 22 days | |||||
| TCH (mmol/L) | 5.83 ± 0.99 | 5.83 ± 0.39 | 5.92 ± 0.7 | 0.193 | 0.982 |
| ALB (g/L) | 17.27 ± 0.42 | 18.36 ± 1.97 | 19.67 ± 1.66 | 0.493 | 0.133 |
| TP (g/L) | 28.73 ± 0.76 a | 32.85 ± 2.02 b | 32.39 ± 1.84 b | 0.7 | 0.012 |
| AKP (Kinyoun Units/100 mL) | 90.81 ± 31.64 | 66.74 ± 7.71 | 60.51 ± 6.34 | 6.36 | 0.114 |
| 43 days | |||||
| TCH (mmol/L) | 4.66 ± 1.06 | 3.59 ± 0.79 | 4.38 ± 0.85 | 0.273 | 0.273 |
| ALB (g/L) | 13.01 ± 0.72 | 12.05 ± 0.45 | 12.53 ± 1.47 | 0.282 | 0.425 |
| TP (g/L) | 19.49 ± 0.58 a | 24.9 ± 2.49 b | 22.03 ± 1.51 ab | 0.802 | 0.005 |
| AKP (Kinyoun Units/100 mL) | 69.61 ± 18.31 | 65.23 ± 21.43 | 65.67 ± 17.77 | 5.06 | 0.94 |
| 57 days | |||||
| TCH (mmol/L) | 5.78 ± 0.31 a | 3.82 ± 0.64 b | 4.56 ± 0.35 b | 0.272 | 0.001 |
| ALB (g/L) | 14.55 ± 0.98 | 14.06 ± 0.61 | 15.2 ± 0.25 | 0.227 | 0.113 |
| TP (g/L) | 24.1 ± 0.34 a | 27.01 ± 0.86 b | 27.72 ± 0.84 b | 0.509 | <0.001 |
| AKP (Kinyoun Units/100 mL) | 64.34 ± 14.05 | 56.91 ± 18.24 | 48.31 ± 12.29 | 4.4 | 0.364 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Blank | NSH-L | NSH-H | |||
| Duodenum | |||||
| VH (μm) | 1686.55 ± 25.26 a | 1851.91 ± 73.3 b | 1862.72 ± 49.11 b | 27.97 | 0.002 |
| CD (μm) | 98.75 ± 3.73 | 104.57 ± 14.89 | 114.29 ± 10.6 | 3.412 | 0.176 |
| V:C | 17.09 ± 0.48 | 18 ± 2.77 | 16.42 ± 1.82 | 0.541 | 0.537 |
| Jejunum | |||||
| VH (μm) | 1455.66 ± 20.83 | 1562.04 ±89.94 | 1533.73 ± 53.41 | 21.04 | 0.089 |
| CD (μm) | 121.83 ± 36.48 | 94.96 ± 14.34 | 98.54 ± 6.95 | 6.994 | 0.252 |
| V:C | 12.98 ± 4.73 | 16.8 ± 3.18 | 15.61 ± 0.99 | 0.996 | 0.303 |
| Ileum | |||||
| VH (μm) | 1221.21 ± 48.52 a | 1170.64 ± 76.13 a | 1422.35 ± 44.93 b | 36.13 | <0.001 |
| CD (μm) | 111.77 ± 8.00 | 97.82 ± 17.88 | 99.1 ± 13.58 | 4.063 | 0.33 |
| V:C | 10.98 ± 1.11 | 12.29 ± 2.42 | 14.57 ± 2.08 | 0.678 | 0.077 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Blank | NSH-L | NSH-H | |||
| Slaughter Yield % | 91.3 ± 0.85 | 92.03 ± 3.33 | 89.55 ± 0.76 | 0.617 | 0.258 |
| Semi-eviscerated Yield % | 80.27 ± 1.11 | 80.56 ± 3.43 | 78.38 ± 2.86 | 0.752 | 0.482 |
| Fully Eviscerated Yield% | 65.33 ± 2.21 | 65.46 ± 2.2 | 64.24 ± 1.57 | 0.552 | 0.656 |
| Abdominal Fat Yield% | 2.63 ± 0.07 | 2.39 ± 0.25 | 2.33 ± 0.22 | 0.065 | 0.125 |
| Breast Muscle Yield% | 8.84 ± 0.88 | 9.26 ± 0.86 | 9.58 ± 0.38 | 0.108 | 0.41 |
| Leg Muscle Yield% | 12.5 ± 0.92 a | 14.16 ± 0.68 b | 14.12 ± 0.74 b | 0.156 | 0.024 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| Blank | NSH-L | NSH-H | |||
| Shear force (N) | 4.39 ± 0.73 | 4.78 ± 0.25 | 4.4 ± 0.44 | 0.145 | 0.503 |
| Drip loss 24 h | 2.46 ± 0.45 | 1.83 ± 0.51 | 1.81 ± 0.1 | 0.138 | 0.079 |
| Drip loss 48 h | 6.17 ± 0.57 a | 4.92 ± 0.15 b | 4.61 ± 0.46 b | 0.232 | 0.001 |
| PH45min | 6.22 ± 0.21 | 6.48 ± 0.31 | 6.26 ± 0.17 | 0.071 | 0.297 |
| PH24h | 5.66 ± 0.03 a | 5.71 ± 0.05 ab | 5.79 ± 0.08 b | 0.022 | 0.036 |
| PH48h | 5.59 ± 0.02 a | 5.68 ± 0.03 b | 5.73 ± 0.02 b | 0.182 | <0.001 |
| L45min | 44.38 ± 1.11 | 47.29 ± 2.32 | 47.14 ± 2.1 | 0.644 | 0.105 |
| a45min | 1.89 ± 0.34 | 1.64 ± 0.9 | 1.23 ± 0.29 | 0.172 | 0.318 |
| b45min | 12.32 ± 0.98 a | 9.94 ± 0.78 b | 9.53 ± 0.66 b | 0.427 | 0.002 |
| L24h | 52.76 ± 1.21 | 53.34 ± 1.58 | 51.85 ± 1.09 | 0.389 | 0.316 |
| a24h | 1.46 ± 0.29 | 1.36 ± 0.28 | 1.16 ± 0.21 | 0.078 | 0.33 |
| b24h | 14.15 ± 0.63 a | 12.3 ± 0.71 b | 11.95 ± 0.49 b | 0.332 | 0.001 |
| L48h | 52.95 ± 3.14 | 54.71 ± 1.41 | 52.68 ± 3.23 | 0.762 | 0.545 |
| a48h | 1.22 ± 0.28 | 1.07 ± 0.31 | 1.06 ± 0.1 | 0.069 | 0.625 |
| b48h | 14.67 ± 0.39 a | 12.14 ± 1.34 b | 12.12 ± 1.17 b | 0.454 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Cen, M.; Li, B.; Feng, X.; Cai, H.; Zhang, H. Neutral Sodium Humate Modulates Growth, Slaughter Traits, Antioxidant Status, and Gut Health in Yellow-Feathered Broilers. Animals 2025, 15, 2142. https://doi.org/10.3390/ani15142142
Lv J, Cen M, Li B, Feng X, Cai H, Zhang H. Neutral Sodium Humate Modulates Growth, Slaughter Traits, Antioxidant Status, and Gut Health in Yellow-Feathered Broilers. Animals. 2025; 15(14):2142. https://doi.org/10.3390/ani15142142
Chicago/Turabian StyleLv, Junran, Mingzhu Cen, Benkuan Li, Xin Feng, Hongyu Cai, and Huihua Zhang. 2025. "Neutral Sodium Humate Modulates Growth, Slaughter Traits, Antioxidant Status, and Gut Health in Yellow-Feathered Broilers" Animals 15, no. 14: 2142. https://doi.org/10.3390/ani15142142
APA StyleLv, J., Cen, M., Li, B., Feng, X., Cai, H., & Zhang, H. (2025). Neutral Sodium Humate Modulates Growth, Slaughter Traits, Antioxidant Status, and Gut Health in Yellow-Feathered Broilers. Animals, 15(14), 2142. https://doi.org/10.3390/ani15142142





