Calcium Metabolism, Immunity and Reproduction in Early Postpartum Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Details
2.2. RNA Extraction and qRT-PCR Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augère-Granier, M.L. The EU Dairy Sector: Main Features, Challenges and Prospects, EPRS: European Parliamentary Research Service. Belgium. 2018. Available online: https://coilink.org/20.500.12592/zwjn5w (accessed on 11 May 2025).
- Chagas, L.; Bass, J.; Blache, D.; Burke, C.; Kay, J.; Lindsay, D.; Lucy, C.; Martin, G.B.; Meier, S.; Rhodes, F.M.; et al. Invited review: New perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows. J. Dairy Sci. 2007, 90, 4022–4032. [Google Scholar] [CrossRef]
- König, S.; Wu, X.; Gianola, D.; Heringstad, B.; Simianer, H. Exploration of relationships between claw disorders and milk yield in holstein cows via recursive linear and threshold models. J. Dairy Sci. 2008, 91, 395–406. [Google Scholar] [CrossRef]
- Pasandideh, M.; Mohammadabadi, M.; Esmailizadeh, A.; Tarang, A. Association of bovine ppargc1a and opn genes with milk production and composition in holstein cattle. Czech. J. Anim. Sci. 2015, 60, 97–104. [Google Scholar] [CrossRef]
- Çobanoğlu, Ö.; Ardiçli, S. Effects of bovine ppargc1a and ltf gene variants on milk yield and composition traits in holstein-friesian and jersey cows. J. Agric. Food Environ. Sci. 2022, 76, 9–20. [Google Scholar] [CrossRef]
- Rearte, R.; Corva, S.; De La Sota, R.; Lacau-Mengido, I.; Giuliodori, M. Associations of somatic cell count with milk yield and reproductive performance in grazing dairy cows. J. Dairy Sci. 2022, 105, 6251–6260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, Y.; Zhao, C.; Bai, Y.; Zhang, F.; Xia, C.; Fu, S.; Zhang, H.; Xu, C.; Wu, L. The relationship of negative energy balance (NEB) and energy metabolism, milk production and reproductive performance during early lactation in dairy cows in Heilongjiang, China. Vet. Arhiv. 2022, 92, 223–232. [Google Scholar] [CrossRef]
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Khan, M.; Manzoor, H.; Asghar, M.A.; Nazeer, H.A.A.; Sadiq, A.B.; Shah, S.H.; Rauf, U.; Zeb, K.; Rehman, M.U. Effect of heat stress on ovaries evolution of repeat breeders in dairy cattle, specifically Holstein Friesian. Biol. Clin. Sci. Res. J. 2024, 1, 737. [Google Scholar] [CrossRef]
- Sigdel, A.; Bisinotto, R.S.; Peñagaricano, F. Genes and pathways associated with pregnancy loss in dairy cattle. Sci. Rep. 2021, 11, 13329. [Google Scholar] [CrossRef]
- Martínez-Moro, Á.; Lamas-Toranzo, I.; González-Brusi, L.; Pérez-Gómez, A.; Padilla-Ruiz, E.; García-Blanco, J.; Bermejo-Álvarez, P. mtDNA content in cumulus cells does not predict development to blastocyst or implantation. Hum. Reprod. Open 2022, 3, hoac029. [Google Scholar] [CrossRef]
- Rupel, I.W.; Bohstedt, G.; Hart, E.B. The role of vitamin D in the nutrition of the dairy calf. J. Anim. Sci. 1932, 1932, 137–141. [Google Scholar]
- Beck, M.R.; Zapalac, D.; Chapman, J.D.; Zanzalari, K.P.; Holub, G.A.; Bascom, S.S.; Engstrom, M.A.; Reuter, R.R.; Foote, A.P. Effect of vitamin D source and dietary cation–anion difference in peripartum dairy cows on calcium homeostasis and milk production. Transl. Anim. Sci. 2022, 6, txac010. [Google Scholar] [CrossRef]
- Nelson, C.D.; Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.E.; Powell, J.L.; Drewnoski, M.E.; O’Neil, M.; Beitz, D.C.; Weiss, W.P. Vitamin D status of dairy cattle: Outcomes of current practices in the dairy industry. J. Dairy Sci. 2016, 99, 10150–10160. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.D.; Reinhardt, T.A.; Thacker, T.C.; Beitz, D.C.; Lippolis, J.D. Modulation of the bovine innate immune response by production of 1α, 25-dihydroxyvitamin D3 in bovine monocytes. J. Dairy Sci. 2010, 93, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.D.; Reinhardt, T.A.; Lippolis, J.D.; Sacco, R.E.; Nonnecke, B.J. Vitamin D signaling in the bovine immune system: A model for understanding human vitamin D requirements. Nutrients 2012, 4, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Rodney, R.; Martínez, N.; Block, E.; Hernandez, L.; Celi, P.; Nelson, C.; Santos, J.E.P.; Lean, I. Effects of prepartum dietary cation-anion difference and source of vitamin D in dairy cows: Vitamin D, mineral, and bone metabolism. J. Dairy Sci. 2018, 101, 2519–2543. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, M.; Kweh, M.; Zimpel, R.; Zuniga, J.; Lopera, C.; Zenobi, M.; Jiang, Y.; Engstrom, M.; Celi, P.; Santos, J.E.P.; et al. Feeding supplemental 25-hydroxyvitamin d3 increases serum mineral concentrations and alters mammary immunity of lactating dairy cows. J. Dairy Sci. 2020, 103, 805–822. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, L.; Zhao, F.; Yan, B. Effects of oral calcium on reproduction and postpartum health in cattle: A meta-analysis and quality assessment. Front. Vet. Sci. 2024, 11, 1357640. [Google Scholar] [CrossRef]
- Kusza, S.; Bagi, Z. A Global Comparative Genomic Analysis of Major Bacterial Pathogens in Bovine Mastitis and Lameness. Animals 2025, 15, 394. [Google Scholar] [CrossRef]
- Starič, J.; Hodnik, J.J. Biochemical bone markers during the transition period are not influenced by parenteral treatment with a high dose of cholecalciferol but can predict milk fever in dairy cows. Front. Vet. Sci. 2021, 7, 591324. [Google Scholar] [CrossRef]
- De Souza, V.P.; Jensen, J.; Whitler, W.; Estill, C.T.; Bishop, C.V. Increasing vitamin D levels to improve fertilization rates in cattle. J. Anim. Sci. 2022, 100, skac168. [Google Scholar] [CrossRef]
- Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.A.; Nonnecke, B.J.; Nelson, C.D. Treatment of an intramammary bacterial infection with 25-hydroxyvitamin D3. PLoS ONE 2011, 6, e25479. [Google Scholar] [CrossRef]
- Martinez, N.; Rodney, R.M.; Block, E.; Hernandez, L.L.; Nelson, C.D.; Lean, I.J.; Santos, J.E.P. Effects of prepartum dietary cation-anion difference and source of vitamin D in dairy cows: Health and reproductive responses. J. Dairy Sci. 2018, 101, 2563–2578. [Google Scholar] [CrossRef]
- Golder, H.M.; McGrath, J.; Lean, I.J. Effect of 25-hydroxyvitamin D3 during prepartum transition and lactation on production, reproduction, and health of lactating dairy cows. J. Dairy Sci. 2021, 104, 5345–5374. [Google Scholar] [CrossRef]
- Sprekeler, N.; Kowalewski, M.P.; Boos, A. TRPV6 and Calbindin-D9k-expression and localization in the bovine uterus and placenta during pregnancy. Rep. Biol. Endocr. 2012, 10, 66. [Google Scholar] [CrossRef]
- Nogueira, M.F.G.; Buratini, J.; Price, C.A.; Castilho, A.C.S.; Pinto, M.G.L.; Barros, C.M. Expression of LH receptor mRNA splice variants in bovine granulosa cells: Changes with follicle size and regulation by FSH in vitro. Mol. Rep. Dev. Incorp. Gam. Res. 2007, 74, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.A.; Satrapa, R.A.; Rosa, F.S.; Piagentini, M.; Castilho, A.C.; Ereno, R.L.; Trinca, L.A.; Nogueira, M.F.G.; Buratini, J.J.; Barros, C.M. Ovulation rate and its relationship with follicle diameter and gene expression of the LH receptor (LHR) in Nelore cows. Theriogenology 2012, 77, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wohlres-Viana, S.; Arashiro, E.K.N.; Machado, M.A.; Camargo, L.S.A.; Siqueira, L.G.B.; Palhao, M.P.; Viana, J.H.M. Intrafollicular oestradiol production, expression of the LH receptor (LHR) gene and its isoforms, and early follicular deviation in Bos indicus. Reprod. Fertility Dev. 2016, 29, 1958–1970. [Google Scholar] [CrossRef]
- Notaro, U.S.; Huber, E.; Stassi, A.F.; Ormaechea, N.E.; Chiaraviglio, J.A.; Baravalle, M.E.; Ortega, H.H.; Rey, F.; Salvetti, N.R. Estrogens receptors, nuclear coactivator 1 and ligand-dependent corepressor expression are altered early during induced ovarian follicular persistence in dairy cattle. Theriogenology 2023, 210, 17–27. [Google Scholar] [CrossRef]
- Rovani, M.T.; Gasperin, B.G.; Ilha, G.F.; Ferreira, R.; Bohrer, R.C.; Duggavathi, R.; Bordignon, V.; Gonçalves, P.B.D. Expression and molecular consequences of inhibition of estrogen receptors in granulosa cells of bovine follicles. J. Ovarian. Res. 2014, 7, 96. [Google Scholar] [CrossRef]
- Ferag, A.; Gherissi, D.E.; Bordja, N.; Boughanem, A.; Moussa, H.H.; Khenenou, T. Monitoring of reproduction activity on algerian dairy cattle farms. Folia. Vet. 2024, 67, 67–78. [Google Scholar] [CrossRef]
- Archilia, E.C.; Bello, C.a.P.; Batalha, I.M.; Wulstein, K.; Enriquez, C.; Schütz, L.F. Effects of follicle-stimulating hormone, insulin-like growth factor 1, fibroblast growth factor 2, and fibroblast growth factor 9 on sirtuins expression and histone deacetylase activity in bovine granulosa cells. Theriogenology 2023, 210, 1–8. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Sales, E.T.; Padilha, R.T.; Silva, T.F.P.; Tonioli, R.; Figueiredo, J.R. Hormônio do crescimento (GH) e fator de crescimento semelhante à insulina-I (IGF-I): Importantes reguladores das foliculogêneses in vivo e in vitro. Rev. Brasil. Reprod. Anim. 2012, 36, 32–38. [Google Scholar]
- Gobikrushanth, M.; Purfield, D.C.; Colazo, M.G.; Wang, Z.; Butler, S.T.; Ambrose, D.J. The relationship between serum insulin-like growth factor-1 (IGF-1) concentration and reproductive performance, and genome-wide associations for serum IGF-1 in Holstein cows. J. Dairy Sci. 2018, 101, 9154–9167. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.A.; Newman, M.; Christie, M.F.; Cripps, P.J.; Crowe, M.A.; Smith, R.F.; Dobson, H. The usefulness of a single measurement of insulin-like growth factor-1 as a predictor of embryo yield and pregnancy rates in a bovine MOET program. Theriogenology 2005, 64, 1977–1994. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 83–801. [Google Scholar] [CrossRef]
- Menzies, M.; Ingham, A. Identification and expression of Toll-like receptors 1–10 in selected bovine and ovine tissues. Vet. Immunol. Immunop. 2006, 109, 23–30. [Google Scholar] [CrossRef]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.J.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of Toll-like receptor 4 in the bovine endometrium. Endocrinology 2006, 147, 562–570. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Krakowski, L.; Marczuk, J. Inflammatory cytokine and acute phase protein concentrations in the peripheral blood and uterine washings of cows with subclinical endometritis in the late postpartum period. Vet. Res. Commun. 2015, 39, 143–149. [Google Scholar] [CrossRef]
- Nzeyimana, J.B.; Fan, C.; Zhuo, Z.; Butore, J.; Cheng, J. Heat stress effects on the lactation performance, reproduction, and alleviating nutritional strategies in dairy cattle, a review. J. Anim. Behav. Biomet. 2023, 11, e2023018. [Google Scholar] [CrossRef]
- Joo, S.S.; Lee, S.J.; Park, D.S.; Kim, D.H.; Gu, B.H.; Park, Y.J.; Rim, C.Y.; Kim, M.; Kim, E.T. Changes in blood metabolites and immune cells in Holstein and Jersey dairy cows by heat stress. Animals 2021, 11, 974. [Google Scholar] [CrossRef]
- Sima, N.K.; Akter, M.; Hoque, M.N.; Islam, M.T.; Das, Z.C.; Talukder, A.K. Reproductive management of dairy cows: An existing scenario from urban farming system in Bangladesh. J. Anim. Reprod. Biotechnol. 2023, 38, 215–224. [Google Scholar] [CrossRef]
- Ryan, N.J.; Brewer, A.; Chapwanya, A.; O’Farrelly, C.; Williams, E.J.; Evans, A.C.; Beltman, M.E.; Meade, K.G. A preliminary analysis of the variation in circulating 25-hydroxycholecalciferol concentrations in peri-partum spring-calving dairy cows. Vet. Res. Commun. 2023, 47, 311–318. [Google Scholar] [CrossRef]
- Strickland, J.M.; Wisnieski, L.; Mavangira, V.; Sordillo, L.M. Serum vitamin D is associated with antioxidant potential in Peri-parturient cows. Antioxidants 2021, 10, 1420. [Google Scholar] [CrossRef]
- Febrianto, N.; Susilawati, T.; Hartono, B.; Akhiroh, P.; Yekti, A.P.A.; Prafitri, R.; Helmi, M.; Masyithoh, D.; Shamsudin, M.N. Financial Performance and Labour Involvement of Small-Scale Dairy Farmers: A Case Study at the SAE Pujon Dairy Cooperative. J. Ilm-Ilm. Pet. 2023, 33, 428–439. [Google Scholar] [CrossRef]
- Aoki, M. The Role of Single Oral Dose of Excess Vitamin A and/or Vitamin E in Improving Ovarian Function Three Days Post-parturition in Primiparous Dairy Cows. Jpn. Agric. Res. Q. 2023, 57, 321–328. [Google Scholar] [CrossRef]
- Wilkens, M.R.; Nelson, C.D.; Hernandez, L.L.; McArt, J.A. Symposium review: Transition cow calcium homeostasis—Health effects of hypocalcemia and strategies for prevention. J. Dairy Sci. 2020, 103, 2909–2927. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids. Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Wisnieski, L.; Gandy, J.; Norby, B.; Sordillo, L.M. Reduced serum vitamin D concentrations in healthy early-lactation dairy cattle. J. Dairy Sci. 2018, 101, 1488–1494. [Google Scholar] [CrossRef]
- Doepel, L.; Lapierre, H.; Kennelly, J.J. Peripartum performance and metabolism of dairy cows in response to prepartum energy and protein intake. J. Dairy Sci. 2002, 85, 2315–2334. [Google Scholar] [CrossRef]
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef]
- Caputo, M.J.; Li, W.; Kendall, S.J.; Larsen, A.; Weigel, K.A.; White, H.M. Liver and muscle transcriptomes differ in mid-lactation cows divergent in feed efficiency in the presence or absence of supplemental rumen-protected choline. Metabolites 2023, 13, 1023. [Google Scholar] [CrossRef]
- Mahen, P.J.; Williams, H.J.; Smith, R.F.; Grove-White, D. Effect of blood ionised calcium concentration at calving on fertility outcomes in dairy cattle. Vet. Rec. 2018, 183, 263. [Google Scholar] [CrossRef]
- Gráff, M.; Süli, Á.; Szilágyi, S.; Mikó, E. Relationship between body condition and some reproductive parameters of Holstein cattle. Adv. Res. Life Sci. 2017, 1, 59–63. [Google Scholar] [CrossRef]
- Goyal, R.; Billings, T.L.; Mansour, T.; Martin, C.; Baylink, D.J.; Longo, L.D.; Pearce, W.J.; Mata-Greenwood, E. Vitamin D status and metabolism in an ovine pregnancy model: Effect of long-term, high-altitude hypoxia. Am. J. Physiol.-Endoc. Metab. 2016, 310, E1062–E1071. [Google Scholar] [CrossRef] [PubMed]
- Ashley, B.; Simner, C.; Manousopoulou, A.; Jenkinson, C.; Hey, F.; Frost, J.M.; Rezwan, F.I.; White, C.H.; Lofthouse, E.M.; Hyde, E.; et al. Placental uptake and metabolism of 25(OH)vitamin D determine its activity within the fetoplacental unit. eLife 2022, 11, e71094. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Jeung, E.B. Uterine TRPV6 expression during the estrous cycle and pregnancy in a mouse model. Am. J. Physiol.-Endoc. Metab. 2007, 293, E132–E138. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; An, B.; Choi, K.; Jeung, E. Change of genes in calcium transport channels caused by hypoxic stress in the placenta, duodenum, and kidney of pregnant rats1. Biol. Reprod. 2012, 88, 30–31. [Google Scholar] [CrossRef]
- Emam, M.A.; Abouelroos, M.E.; Gad, F.A. Expression of calbindin-D9k and vitamin D receptor in the uterus of Egyptian buffalo during follicular and luteal phases. Acta Histochem. 2016, 118, 471–477. [Google Scholar] [CrossRef]
- Zheng, W.; Xie, Y.; Li, G.; Kong, J.; Feng, J.Q.; Li, Y.C. Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. J. Biol. Chem. 2004, 279, 52406–52413. [Google Scholar] [CrossRef]
- Sirajudeen, S.; Shah, I.; Al Menhali, A. A narrative role of vitamin D and its receptor: With current evidence on the gastric tissues. Int. J. Mol. Sci. 2019, 20, 3832. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.V.; Cruze, L.; Wei, W.; Gehris, J.; Wagner, C.L. Maternal vitamin D sufficiency and reduced placental gene expression in angiogenic biomarkers related to comorbidities of pregnancy. J. Ster. Biochem. Mol. Biol. 2017, 173, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, H.; Kim, E.Y.; Moon, S.; Lee, S.; Cho, M.; Cho, H.J.; Ko, J.J.; Chang, E.M.; Lee, K.A.; et al. Knockdown of PRKAR2B results in the failure of oocyte maturation. Cell. Physiol. Biochem. 2018, 45, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, D.W.; Hudgins-Spivey, S.; Krust, A.; Lee, E.Y.; Koo, Y.; Cheon, Y.; Gye, M.C.; Chambon, P.; Ko, C.M. Theca-specific estrogen receptor-α knockout mice lose fertility prematurely. Endocrinology 2009, 150, 3855–3862. [Google Scholar] [CrossRef]
- Chen, Y.H.; Liu, Z.B.; Ma, L.; Zhang, Z.C.; Fu, L.; Yu, Z.; Chen, W.; Song, Y.P.; Wang, P.; Wang, W.; et al. Gestational vitamin D deficiency causes placental insufficiency and fetal intrauterine growth restriction partially through inducing placental inflammation. J. Ster. Biochem. Mol. Biol. 2020, 203, 105733. [Google Scholar] [CrossRef]
- Liu, N.Q.; Ouyang, Y.; Bulut, Y.; Lagishetty, V.; Chan, S.Y.; Hollis, B.W.; Wagner, C.; Equils, O.; Hewison, M. Dietary vitamin D restriction in pregnant female mice is associated with maternal hypertension and altered placental and fetal development. Endocrinology 2013, 154, 2270–2280. [Google Scholar] [CrossRef]
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43, 260–267. [Google Scholar] [CrossRef]
- Vestergaard, A.L.; Justesen, S.; Volqvartz, T.; Aagaard, S.K.; Andreasen, M.F.; Lesnikova, I.; Uldbjerg, N.; Larsen, A.; Bor, P. Vitamin D insufficiency among Danish pregnant women—Prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet. Gyn. Scan. 2021, 100, 480–488. [Google Scholar] [CrossRef]
- Magiełda-Stola, J.; Kurzawińska, G.; Ożarowski, M.; Bogacz, A.; Wolski, H.; Drews, K.; Karpiński, T.M.; Wolek, M.; Seremak-Mrozikiewicz, A. Placental mRNA and protein expression of VDR, CYP27B1 and CYP2R1 genes related to vitamin D metabolism in preeclamptic women. Appl. Sci. 2021, 11, 11880. [Google Scholar] [CrossRef]
- Dionne, S.; Calderon, M.R.; White, J.H.; Memari, B.; Elimrani, I.; Adelson, B.; Piccirillo, C.; Seidman, E.G. Differential effect of vitamin D on NOD2-and TLR-induced cytokines in Crohn’s disease. Mucosal. Immunol. 2014, 7, 1405–1415. [Google Scholar] [CrossRef]
- Liu, N.Q.; Kaplan, A.T.; Lagishetty, V.; Ouyang, Y.B.; Ouyang, Y.; Simmons, C.F.; Equils, O.; Hewison, M. Vitamin D and the regulation of placental inflammation. J. Immunol. 2011, 186, 5968–5974. [Google Scholar] [CrossRef]
- Bikle, D. Nonclassic actions of Vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef]
- Shin, J.S.; Choi, M.Y.; Longtine, M.S.; Nelson, D.M. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef]
- Arbel, R.; Bigun, Y.; Ezra, E.; Sturman, H.; Hojman, D. The effect of extended calving intervals in high lactating cows on milk production and profitability. J. Dairy Sci. 2001, 84, 600–608. [Google Scholar] [CrossRef]
- Tóth, V.; Heinc, E.; Mikó, E.; Csendes, T.; Bánhelyi, B. Profitability optimization of dairy farms: The effect of pregnancy rate and culling decision. Animals 2023, 14, 18. [Google Scholar] [CrossRef]
| Main Role | Gene | Forward (5′-3′) | Reverse (5′-3′) | |
|---|---|---|---|---|
| HG | GAPDH | CCACGAGAAGTATAACAACACC | GTCATAAGTCCCTCCACGAT | |
| GOI | Calcium signaling | CaBP-9k | TCTCCAGAAGAACTGAAGGGC | CCAACACCTGGAATTCTTCG |
| TRPV6 | CAAGGAGCCCATGACATCTGA | CAGGGCTTTCACGAGGTTCA | ||
| Feto-placental growth | CD45 | CTCGATGTTAAGCGAGAGGAAT | TCTTCATCTTCCACGCAGTCTA | |
| ESR1 | CCAACCAGTGCACGATTGAT | TTCCGTATTCCGCCTTTCAT | ||
| GJA1 | GTCTTCGAGGTGGCCTTCTTG | AGTCCACCTGATGTGGGCAG | ||
| IGF1 | AGCAGTCTTCCAACCCAA | AGATGCGAGGAGGATGTG | ||
| LHR | AAACTTGCCAACAAACGA | ATAGCAAGTCTTGTCCAGGA | ||
| MRO | CCCACTTACAGGACAGGAATCC | TGGAAGCTGTAGTCCTTGCTTTG | ||
| PRKAR2β | GGGCATTCAACGCTCCAGTA | CTGGATTCAGCATCATCTTCTTCTT | ||
| PTGER2 | GTTCCACGTGTTGGTGACAG | ACTCGGCGCTGGTAGAAGTA | ||
| TGFβR1 | CAGGTTTACCATTGCTTGTTCA | TGCCATTGTCTTTATTGTCTGC | ||
| Immune response | CD14 | GGGTACTCTCTGCTCAAGGAAC | CTTGGGCAATGTTCAGCAC | |
| IFNα | AGAGCCTCCTGGACAAGCTAC | CATGACTTCTGCTCTGACAACC | ||
| IL10 | TACTCTGTTGCCTGGTCTTCCT | AGTAAGCTGTGCAGTTGGTCCT | ||
| IL1α | AGAGGATTCTCAGCTTCCTGTG | ATTTTTCTTGCTTTGTGGCAAT | ||
| IL1β | GAGGAGCATCCTTTCATTCATC | TTCCTCTCCTTGTACGAAGCTC | ||
| IL1R2 | ATCCCATGTAAGGTGTTTCTGG | TGACAGGATCAAAAATCAGTGG | ||
| IL6 | ATGACTTCTGCTTTCCCTACCC | GCTGCTTTCACACTCATCATTC | ||
| MD2 | GGGAAGCCGTGGAATACTCTAT | CCCCTGAAGGAGAATTGTATTG | ||
| NOD1 | GTCACTCACATCCGAAACACTC | CCTGAGATCCACATAAGCGTCT | ||
| NOS2 | GGACAGTAAAGACGTCTCCAGA | TATGGTCAAACTTTTGGGGTTC | ||
| TLR1 | CCCACAGGAAAGAAATTCCA | GGAGGATCGTGATGAAGGAA | ||
| TNF | ACTCAGGTCCTCTTCTCAAGCC | ATGATCCCAAAGTAGACCTGCC |
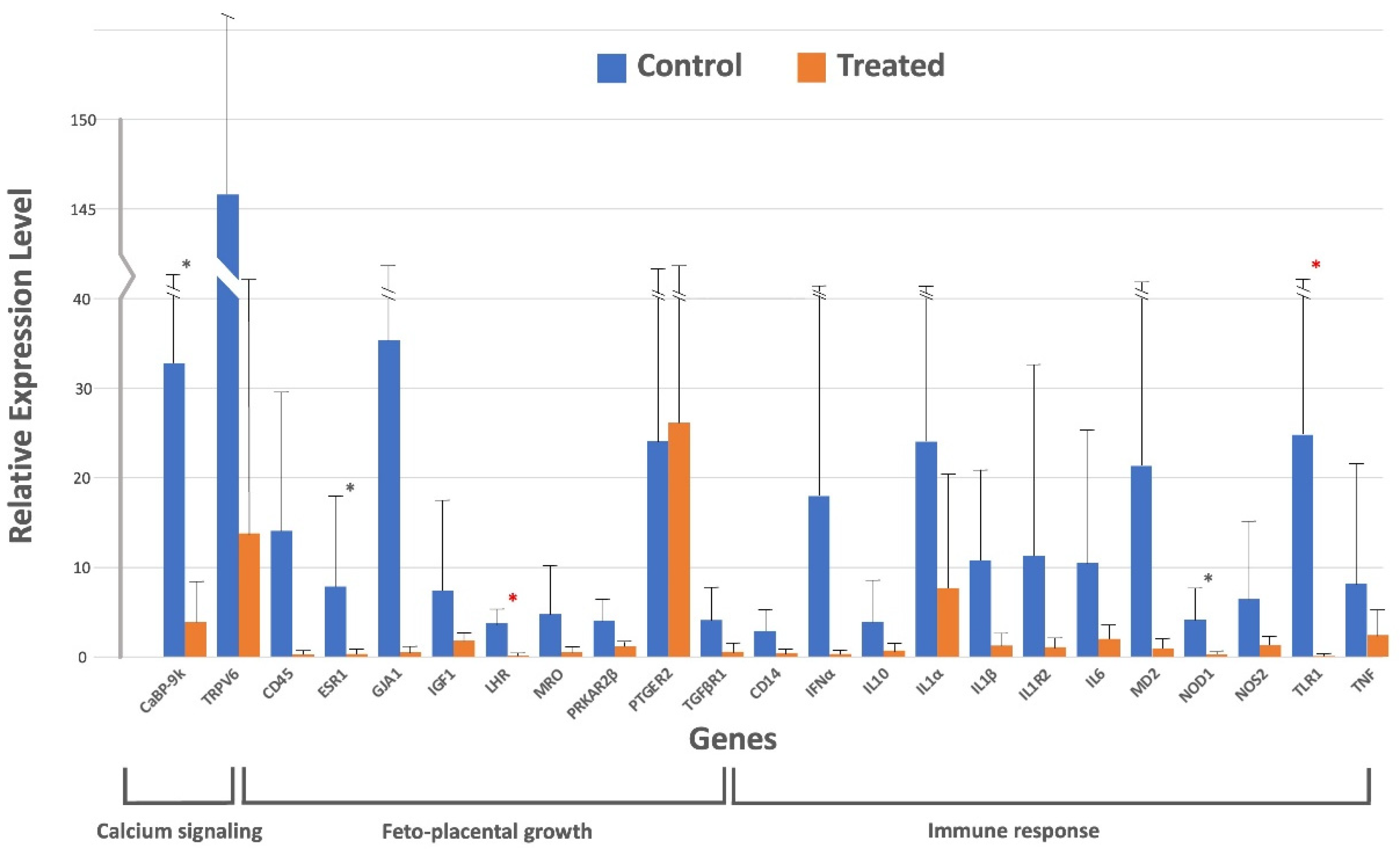
| Gene | Control Group (Mean ± SD) | Treated Group (Mean ± SD) |
|---|---|---|
| CaBP-9k | 32.80 ± 91.50 | 3.90 ± 8.54 |
| CD14 | 2.95 ± 5.48 | 0.41 ± 0.45 |
| CD45 | 14.08 ± 29.19 | 0.28 ± 0.38 |
| ESR1 | 7.89 ± 17.87 | 0.34 ± 0.34 |
| GJA1 | 35.37 ± 101.87 | 0.49 ± 0.76 |
| IFNα | 17.96 ± 51.38 | 0.33 ± 0.32 |
| IGF1 | 7.40 ± 17.22 | 1.81 ± 1.85 |
| IL10 | 3.94 ± 8.49 | 0.67 ± 0.68 |
| IL1α | 24.06 ± 51.76 | 7.70 ± 20.01 |
| IL1β | 10.84 ± 21.92 | 1.26 ± 2.02 |
| IL1R2 | 11.34 ± 31.86 | 1.08 ± 1.32 |
| IL6 | 10.49 ± 24.86 | 2.03 ± 2.49 |
| LHR | 3.75 ± 5.45 | 0.13 ± 0.17 |
| MD2 | 21.34 ± 60.14 | 0.92 ± 0.97 |
| MRO | 4.78 ± 10.24 | 0.49 ± 0.61 |
| NOD1 | 4.21 ± 7.00 | 0.25 ± 0.30 |
| NOS2 | 6.47 ± 15.92 | 1.32 ± 1.90 |
| PRKAR2β | 4.09 ± 6.66 | 1.18 ± 1.40 |
| PTGER2 | 24.11 ± 68.56 | 26.36 ± 75.45 |
| TGFβR1 | 4.12 ± 7.21 | 0.53 ± 0.71 |
| TLR1 | 24.80 ± 61.45 | 0.07 ± 0.08 |
| TNF | 8.19 ± 22.27 | 2.43 ± 5.19 |
| TRPV6 | 145.64 ± 430.47 | 13.89 ± 42.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusza, S.; Bagi, Z.; Astuti, P.K.; Wanjala, G.; Szenci, O.; Bajcsy, Á.C. Calcium Metabolism, Immunity and Reproduction in Early Postpartum Dairy Cows. Animals 2025, 15, 2103. https://doi.org/10.3390/ani15142103
Kusza S, Bagi Z, Astuti PK, Wanjala G, Szenci O, Bajcsy ÁC. Calcium Metabolism, Immunity and Reproduction in Early Postpartum Dairy Cows. Animals. 2025; 15(14):2103. https://doi.org/10.3390/ani15142103
Chicago/Turabian StyleKusza, Szilvia, Zoltán Bagi, Putri Kusuma Astuti, George Wanjala, Ottó Szenci, and Árpád Csaba Bajcsy. 2025. "Calcium Metabolism, Immunity and Reproduction in Early Postpartum Dairy Cows" Animals 15, no. 14: 2103. https://doi.org/10.3390/ani15142103
APA StyleKusza, S., Bagi, Z., Astuti, P. K., Wanjala, G., Szenci, O., & Bajcsy, Á. C. (2025). Calcium Metabolism, Immunity and Reproduction in Early Postpartum Dairy Cows. Animals, 15(14), 2103. https://doi.org/10.3390/ani15142103






