Effect of Roughage Source on the Composition and Colonization of Rumen Bacteria and Methanogens in Dumont and Mongolian Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experiment Design
2.2. Intake, Growth Performance, and Rumen Sample Collection
2.3. Rumen Bacterial and Methanogen DNA Extraction and Analysis
2.4. Data Processing and Analysis
3. Results
3.1. Growth Performance
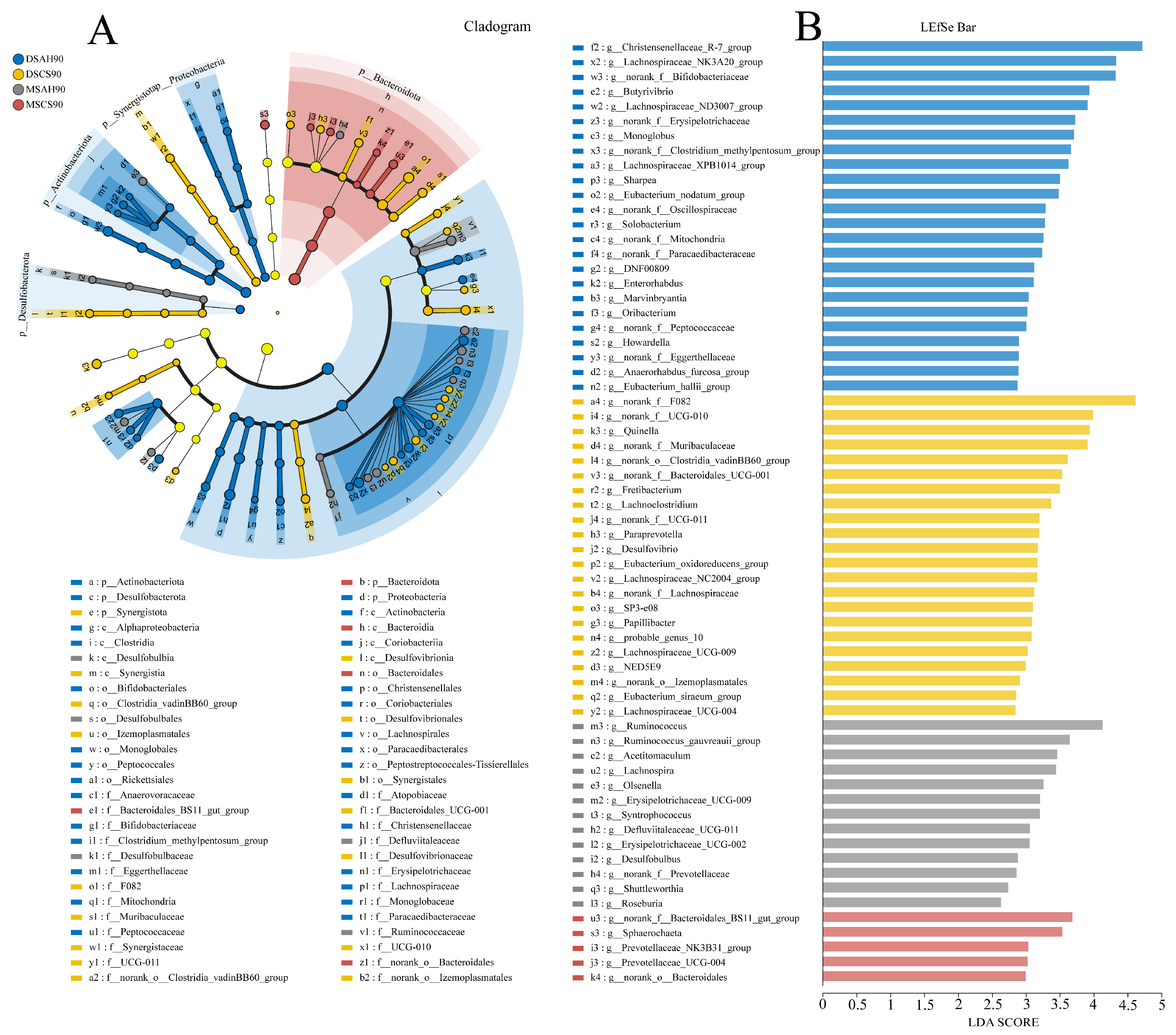
3.2. Bacterial Community Composition
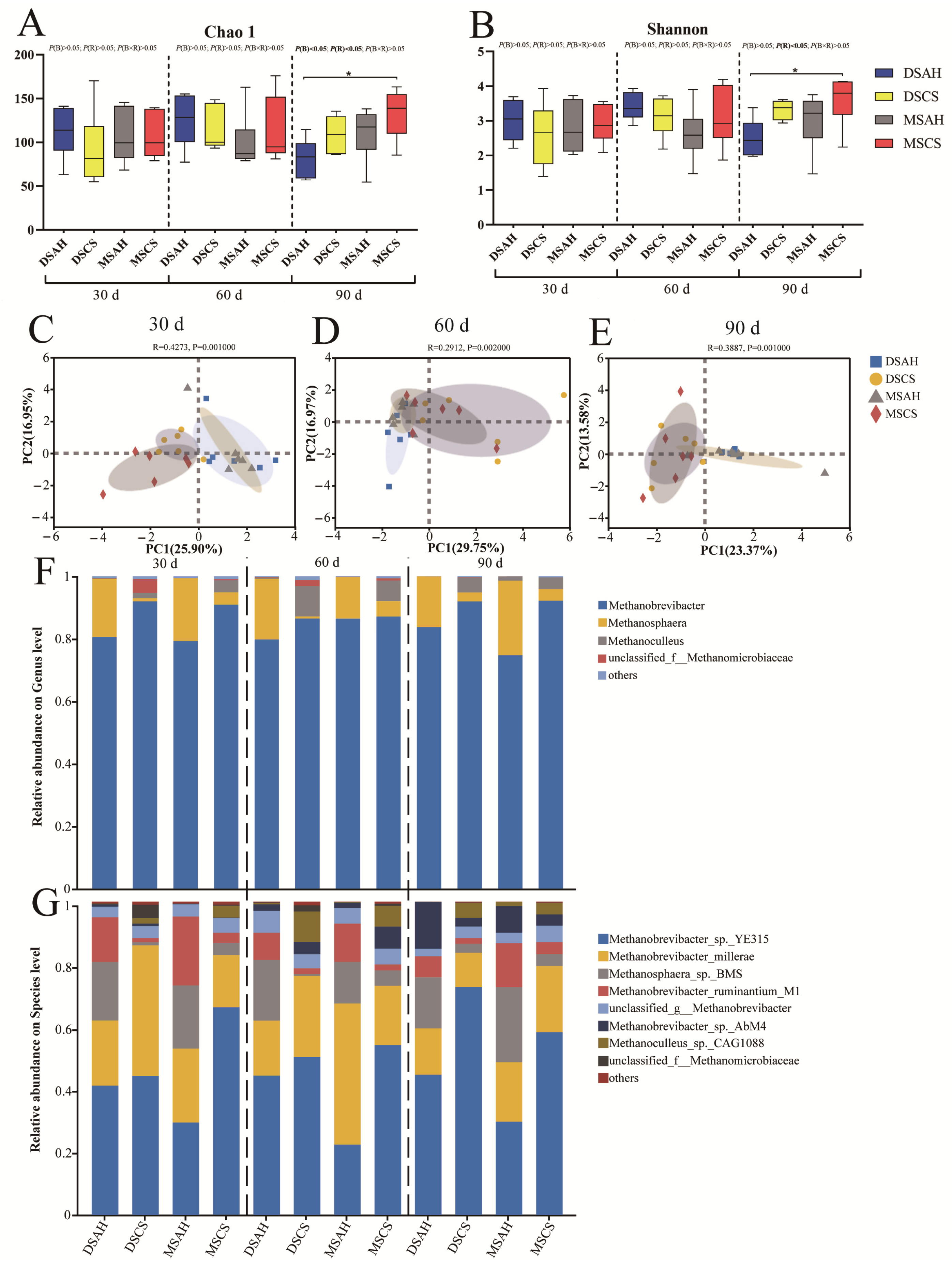
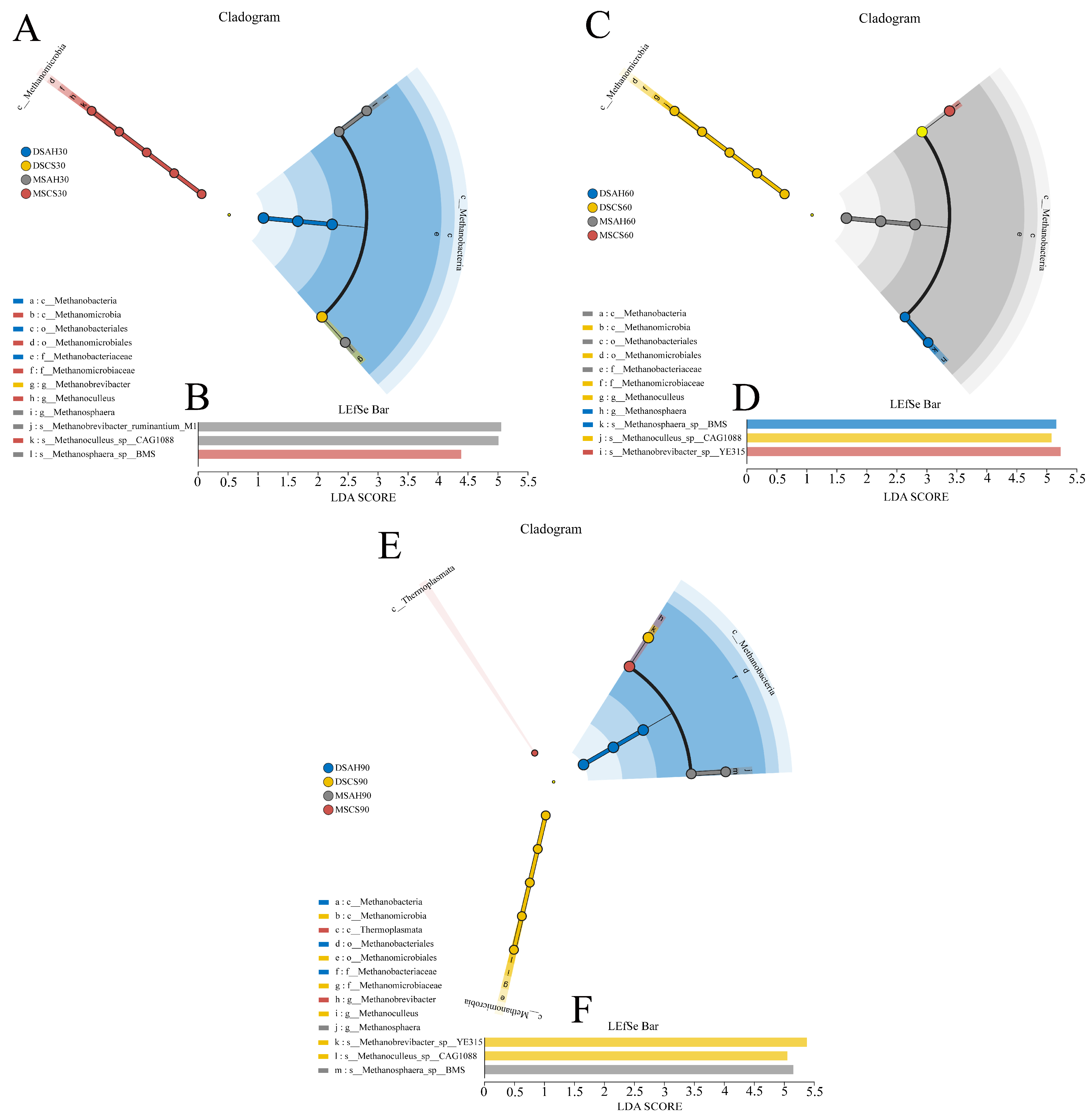
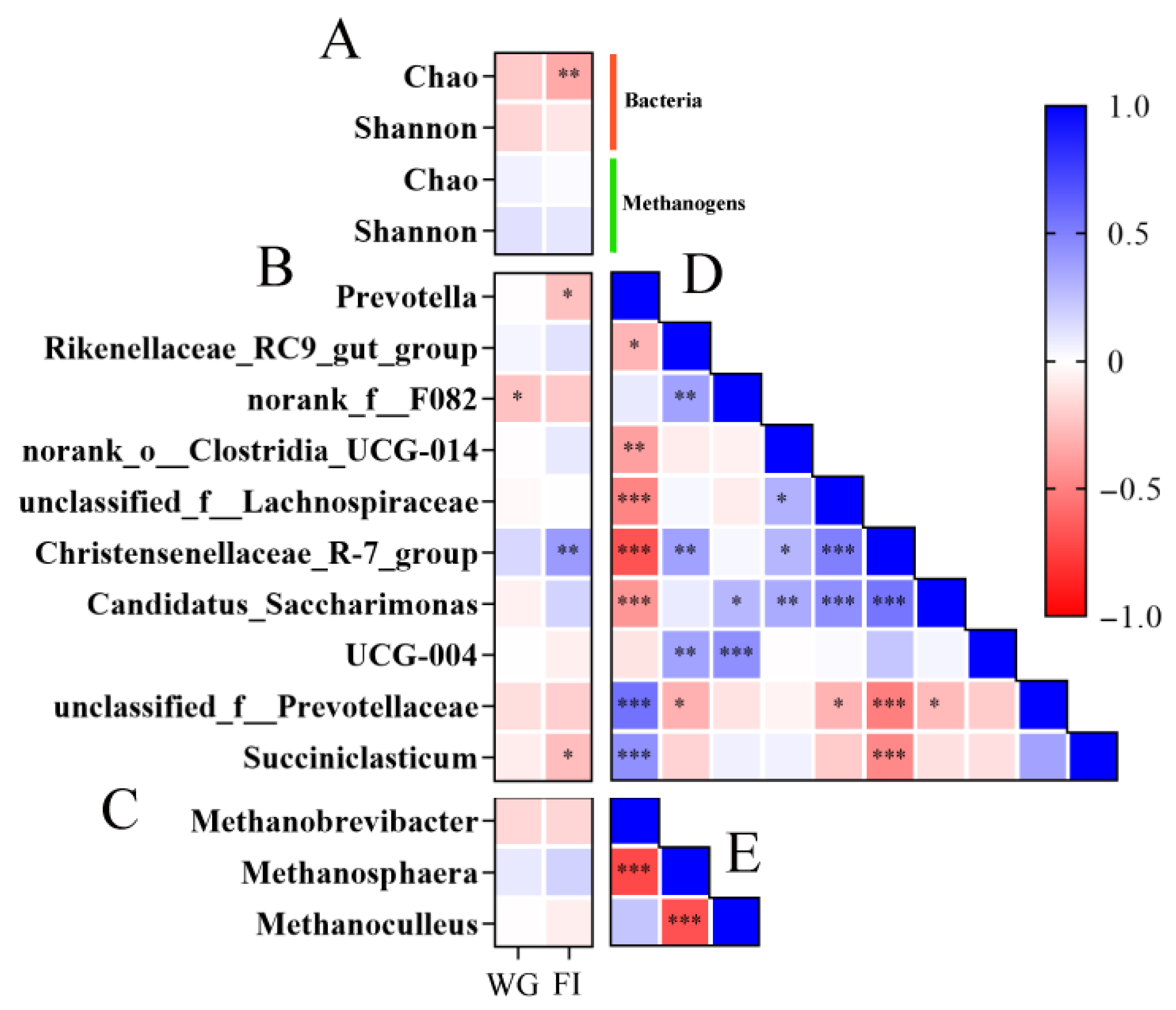
3.3. Ruminal Bacterial Communities
3.4. Ruminal Metabolomics Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AH | Alfalfa hay |
| CS | Corn stalk |
| DSAH | Dumont sheep and alfalfa hay diet group |
| DSCS | Dumont sheep and Corn stalk diet group |
| MSAH | Mongolian sheep and alfalfa hay diet group |
| MSCS | Mongolian sheep and Corn stalk diet group |
| DS | Dumont sheep |
| MS | Mongolian sheep |
References
- Wang, J.; Yang, B.Y.; Zhang, S.J.; Amar, A.; Chaudhry, A.S.; Cheng, L.; Abbasi, I.H.R.; Al-Mamun, M.; Guo, X.F.; Shan, A.S. Using mixed silages of sweet sorghum and alfalfa in total mixed rations to improve growth performance, nutrient digestibility, carcass traits and meat quality of sheep. Animal 2021, 15, 100246. [Google Scholar] [CrossRef] [PubMed]
- Kyawt, Y.Y.; Aung, M.; Xu, Y.; Sun, Z.; Zhou, Y.; Zhu, W.; Padmakumar, V.; Tan, Z.; Cheng, Y. Dynamic changes of rumen microbiota and serum metabolome revealed increases in meat quality and growth performances of sheep fed bio-fermented rice straw. J. Anim. Sci. Biotechnol. 2024, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Shao, C.; Yang, F. Spatial suitability evaluation of the conversion and utilization of crop straw resources in China. Environ. Impact Assess. Rev. 2024, 105, 107438. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, B.; La, S.; Li, D.; Zhu, X.; Sun, H.; Ma, S.; Cui, Y.; Shi, Y. Alfalfa hay substitution for wheat straw improves beef quality via rumen microflora alteration. Heliyon 2023, 9, e20803. [Google Scholar] [CrossRef]
- Knoell, A.; Aluthge, N.; Abbas, W.; Bartenslager, A.; Judy, J.; Morris, D.; Wilson, H.C.; Herrick, K.; Kononoff, P.J.; Fernando, S. 247 The effect of diet on the microbial community structure and composition in lactating Jersey cows consuming a mixture of straw and dry distillers grains plus solubles in replacement of alfalfa hay. J. Anim. Sci. 2020, 98, 138–139. [Google Scholar] [CrossRef]
- Hanlon, M.E.; Moorby, J.M.; McConochie, H.R.; Foskolos, A. Effects of addition of nutritionally improved straw in dairy cow diets at 2 starch levels. J. Dairy Sci. 2020, 103, 10233–10244. [Google Scholar] [CrossRef]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.D.; Kraft, J. Rumen bacterial communities shift across a lactation in Holstein, Jersey and Holstein × Jersey dairy cows and correlate to rumen function, bacterial fatty acid composition and production parameters. FEMS Microbiol. Ecol. 2016, 92, fiw059. [Google Scholar] [CrossRef]
- Elekwachi, C.O.; Wang, Z.; Wu, X.; Rabee, A.; Forster, R.J. Total rRNA-Seq Analysis Gives Insight into Bacterial, Fungal, Protozoal and Archaeal Communities in the Rumen Using an Optimized RNA Isolation Method. Front. Microbiol. 2017, 8, 1814. [Google Scholar] [CrossRef]
- Wallace, R.J.; Rooke, J.A.; Mckain, N.; Duthie, C.A.; Hyslop, J.J.; Ross, D.W.; Waterhouse, A.; Watson, M.; Roehe, R. The rumen microbial metagenome associated with high methane production in cattle. BMC Genom. 2015, 16, 839. [Google Scholar] [CrossRef]
- Noel, S.J.; Olijhoek, D.W.; Mclean, F.; Løvendahl, P.; Lund, P.; Højberg, O. Rumen and Fecal Microbial Community Structure of Holstein and Jersey Dairy Cows as Affected by Breed, Diet, and Residual Feed Intake. Animals 2019, 9, 498. [Google Scholar] [CrossRef]
- Rira, M.; Morgavi, D.P.; Popova, M.; Marie-Magdeleine, C.; Silou-Etienne, T.; Archimède, H.; Doreau, M. Ruminal methanogens and bacteria populations in sheep are modified by a tropical environment. Anim. Feed Sci. Technol. 2016, 220, 226–236. [Google Scholar] [CrossRef]
- Bohra, V.; Dafale, N.A.; Purohit, H.J. Understanding the alteration in rumen microbiome and CAZymes profile with diet and host through comparative metagenomic approach. Arch. Microbiol. 2019, 201, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Carberry, C.A.; Waters, S.M.; Kenny, D.A.; Creevey, C.J. Rumen methanogenic genotypes differ in abundance according to host residual feed intake phenotype and diet type. Appl. Environ. Microbiol. 2014, 80, 586–594. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Kirs, M.; Ronimus, R.S.; Hoskin, S.O.; Janssen, P.H. Methanogen community structure in the rumens of farmed sheep, cattle and red deer fed different diets. FEMS Microbiol. Ecol. 2011, 76, 311–326. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Zhang, W.; Zhou, J.; Cong, H.; Yang, G.; Liu, G. Rumen microbiota helps Tibetan sheep obtain energy more efficiently to survive in the extreme environment of the Qinghai–Tibet Plateau. Front. Microbiol. 2024, 15, 1431063. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef]
- Sha, Y.; Liu, X.; Li, X.; Wang, Z.; Shao, P.; Jiao, T.; He, Y.; Zhao, S. Succession of rumen microbiota and metabolites across different reproductive periods in different sheep breeds and their impact on the growth and development of offspring lambs. Microbiome 2024, 12, 172. [Google Scholar] [CrossRef]
- Xiang, J.; Zhong, L.; Luo, H.; Meng, L.; Dong, Y.; Qi, Z.; Wang, H. A comparative analysis of carcass and meat traits, and rumen bacteria between Chinese Mongolian sheep and Dorper × Chinese Mongolian crossbred sheep. Animal 2022, 16, 100503. [Google Scholar] [CrossRef]
- King, E.E.; Smith, R.P.; St-Pierre, B.; Wright, A.D. Differences in the rumen methanogen populations of lactating Jersey and Holstein dairy cows under the same diet regimen. Appl. Environ. Microbiol. 2011, 77, 5682–5687. [Google Scholar] [CrossRef]
- Hernandez-Sanabria, E.; Goonewardene, L.A.; Wang, Z.; Zhou, M.; Moore, S.S.; Guan, L.L. Influence of sire breed on the interplay among rumen microbial populations inhabiting the rumen liquid of the progeny in beef cattle. PLoS ONE 2013, 8, e58461. [Google Scholar] [CrossRef]
- Wilde, J.; Slack, E.; Foster, K.R. Host control of the microbiome: Mechanisms, evolution, and disease. Science 2024, 385, eadi3338. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, R.S. Genotype differences and their impact on digestive tract function of ruminants: A review. Aust. J. Exp. Agric. 2004, 44, 459–467. [Google Scholar] [CrossRef]
- Brand, T.S. Grazing behaviour and diet selection by Dorper sheep. Small Rumin. Res. 2000, 36, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Apata, D.F. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture of Lactobacillus bulgaricus. J. Sci. Food Agric. 2010, 88, 1253–1258. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Li, B.; Lu, H.; Zhao, J.; Gao, A.; An, Y.; Yang, J.; Ma, T. Multi-Omics Analysis Reveals the Negative Effects of High-Concentrate Diets on the Colonic Epithelium of Dumont Lambs. Animals 2025, 15, 749. [Google Scholar] [CrossRef]
- Zhou, G.; Li, J.; Liang, X.; Yang, B.; He, X.; Tang, H.; Guo, H.; Liu, G.; Cui, W.; Chen, Y.; et al. Multi-omics revealed the mechanism of feed efficiency in sheep by the combined action of the host and rumen microbiota. Anim. Nutr. 2024, 18, 367–379. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Zhang, X.; Li, C.; Yuan, L.; Zhang, D.; Zhao, Y.; Li, X.; Cheng, J.; Lin, C.; et al. Heritability and recursive influence of host genetics on the rumen microbiota drive body weight variance in male Hu sheep lambs. Microbiome 2023, 11, 197. [Google Scholar] [CrossRef]
- Martinez Boggio, G.; Meynadier, A.; Buitenhuis, A.J.; Marie-Etancelin, C. Host genetic control on rumen microbiota and its impact on dairy traits in sheep. Genet. Sel. Evol. 2022, 54, 77. [Google Scholar] [CrossRef]
- NY/T 816-2021; Nutritional Requirements of Mutton Sheep. Ministry of Agriculture and Rural Affairs of the People’s Republic of China (Publisher Implied by Standard Issuance Context): Beijing, China, 2021.
- Guo, W.; Na, M.; Liu, S.; Li, K.; Du, H.; Zhang, J.; Zhang, Y.; Na, R.; Liu, Y. Relationships between rumen methanogens and fungal communities and their response to changes in alfalfa forms and starch in sheep diets. Front. Microbiomes 2025, 4, 1567462. [Google Scholar] [CrossRef]
- Cao, X.; Zuo, S.; Lin, Y.; Cai, R.; Yang, F.; Wang, X.; Xu, C. Expansion Improved the Physical and Chemical Properties and In Vitro Rumen Digestibility of Buckwheat Straw. Animals 2023, 14, 29. [Google Scholar] [CrossRef]
- Matar, A.M.; Abdelrahman, M.M.; Alhidary, I.A.; Ayadi, M.A.; Alobre, M.M.; Aljumaah, R.S. Effects of Roughage Quality and Particle Size on Rumen Parameters and Fatty Acid Profiles of Longissimus Dorsi Fat of Lambs Fed Complete Feed. Animals 2020, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, G.; Gao, Q.; Liu, Z.; Jia, J.; Xu, Y.; Chen, Z.; Li, B.; Li, C. Microbial insights into ruminal fiber degradation and feed efficiency of Hu sheep. Front. Microbiol. 2025, 16, 1561336. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Costa-Roura, S.; Balcells, J.; Fuente, L.D.G.; Jesús, M.G.; Núria, L.; Daniel, V. Nutrient utilization efficiency, ruminal fermentation and microbial community in Holstein bulls fed concentrate-based diets with different forage source. Anim. Feed Sci. Technol. 2020, 269, 114662. [Google Scholar] [CrossRef]
- Hao, Y.; Gong, Y.; Huang, S.; Ji, S.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. Effects of Age, Diet CP, NDF, EE, and Starch on the Rumen Bacteria Community and Function in Dairy Cattle. Microorganisms 2021, 9, 1788. [Google Scholar] [CrossRef]
- An, X.; Zhang, L.; Luo, J.; Zhao, S.; Jiao, T. Effects of Oat Hay Content in Diets on Nutrient Metabolism and the Rumen Microflora in Sheep. Animals 2020, 10, 2341. [Google Scholar] [CrossRef]
- Guo, W.; Na, M.; Liu, S.; Li, K.; Du, H.; Zhang, J.; Na, R. Rumen-Degradable Starch Improves Rumen Fermentation, Function, and Growth Performance by Altering Bacteria and Its Metabolome in Sheep Fed Alfalfa Hay or Silage. Animals 2024, 15, 34. [Google Scholar] [CrossRef]
- Dai, X.; Tian, Y.; Li, J.; Su, X.; Wang, X.; Zhao, S.; Liu, L.; Luo, Y.; Liu, D.; Zheng, H.; et al. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386. [Google Scholar] [CrossRef]
- Hou, L.; Duan, P.; Yang, Y.; Shah, A.M.; Li, J.; Xu, C.; Guo, T. Effects of residual black wolfberry fruit on growth performance, rumen fermentation parameters, microflora and economic benefits of fattening sheep. Front. Vet. Sci. 2025, 11, 1528126. [Google Scholar] [CrossRef]
- Battelli, M.; Colombini, S.; Parma, P.; Galassi, G.; Crovetto, G.M.; Spanghero, M.; Pravettoni, D.; Zanzani, S.A.; Manfredi, M.T.; Rapetti, L. In vitro effects of different levels of quebracho and chestnut tannins on rumen methane production, fermentation parameters, and microbiota. Front. Vet. Sci. 2023, 10, 1178288. [Google Scholar] [CrossRef]
- Jize, Z.; Zhuoga, D.; Xiaoqing, Z.; Na, T.; Jiacuo, G.; Cuicheng, L.; Bandan, P. Different feeding strategies can affect growth performance and rumen functions in Gangba sheep as revealed by integrated transcriptome and microbiome analyses. Front. Microbiol. 2022, 13, 908326. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zhang, N.; Bi, Y.; Ma, T.; Paengkoum, P.; Xin, J.; Xiao, W.; Zhao, Y.; Yuan, C.; Wang, D.; et al. Partially substituting roughage with traditional Chinese herbal medicine residues in the diet of goats improved feed quality, growth performance, hematology, and rumen microbial profiles. BMC Vet. Res. 2024, 20, 576. [Google Scholar] [CrossRef]
- Shabat, S.K.B.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. Int. Soc. Microb. Ecol. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, L.; Tang, G.; Yu, J.; Chen, J.; Li, Z.; Cao, Y.; Lei, X.; Deng, L.; Wu, S.; et al. Multi-omics revealed the long-term effect of ruminal keystone bacteria and the microbial metabolome on lactation performance in adult dairy goats. Microbiome 2023, 11, 215. [Google Scholar] [CrossRef]
- Min, B.R.; Wang, W.; Pitta, D.W.; Indugu, N.; Patra, A.K.; Wang, H.H.; Abrahamsen, F.; Hilaire, M.; Puchala, R. Characterization of the ruminal microbiota in sheep and goats fed different levels of tannin-rich Sericea lespedeza hay. J. Anim. Sci. 2024, 102, skae198. [Google Scholar] [CrossRef]
- Hook, S.E.; Wright, A.D.; McBride, B.W. Methanogens: Methane producers of the rumen and mitigation strategies. Archaea 2010, 2010, 945785. [Google Scholar] [CrossRef]
- Franzolin, R.; St-Pierre, B.; Northwood, K.; Wright, A.D. Analysis of rumen methanogen diversity in water buffaloes (Bubalus bubalis) under three different diets. Microb. Ecol. 2012, 64, 131–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Chen, Y.; Guan, L.L. The Effects of Breed and Residual Feed Intake Divergence on the Abundance and Active Population of Rumen Microbiota in Beef Cattle. Animals 2022, 12, 1966. [Google Scholar] [CrossRef]
- Wallace, R.J.; Sasson, G.; Garnsworthy, P.C.; Tapio, I.; Gregson, E.; Bani, P.; Huhtanen, P.; Bayat, A.R.; Strozzi, F.; Biscarini, F.; et al. A heritable subset of the core rumen microbiome dictates dairy cow productivity and emissions. Sci. Adv. 2019, 5, eaav8391. [Google Scholar] [CrossRef]
- Dong, L.F.; Ma, N.J.; Tu, Y.; Diao, Q.Y. Weaning methods affect ruminal methanogenic archaea composition and diversity in Holstein calves. J. Integr. Agric. 2019, 18, 1080–1092. [Google Scholar] [CrossRef]
- Savin, K.W.; Moate, P.J.; Williams, S.R.O.; Bath, C.; Hemsworth, J.; Wang, J.H.; Ram, D.; Zawadzki, J.; Rochfort, S.; Cocks, B.G. Dietary wheat and reduced methane yield are linked to rumen microbiome changes in dairy cows. PLoS ONE 2022, 17, e0268157. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hitch, T.C.A.; Chen, Y.; Creevey, C.J.; Guan, L.L. Comparative metagenomic and metatranscriptomic analyses reveal the breed effect on the rumen microbiome and its associations with feed efficiency in beef cattle. Microbiome 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ji, S.; Yan, H.; Hao, Y.; Zhang, J.; Wang, Y.; Cao, Z.; Li, S. The day-to-day stability of the ruminal and fecal microbiota in lactating dairy cows. Microbiologyopen 2020, 9, e990. [Google Scholar] [CrossRef] [PubMed]
- Perea, K.; Perz, K.; Olivo, S.K.; Williams, A.; Lachman, M.; Ishaq, S.L.; Thomson, J.; Yeoman, C.J. Feed efficiency phenotypes in lambs involve changes in ruminal, colonic, and small-intestine-located microbiota. J. Anim. Sci. 2017, 95, 2585–2592. [Google Scholar] [CrossRef][Green Version]
- Garcia, J.L.; Patel, B.K.; Ollivier, B. Taxonomic, phylogenetic, and ecological diversity of methanogenic Archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef]
- Qiu, X.; Qin, X.; Chen, L.; Chen, Z.; Hao, R.; Zhang, S.; Yang, S.; Wang, L.; Cui, Y.; Li, Y.; et al. Serum Biochemical Parameters, Rumen Fermentation, and Rumen Bacterial Communities Are Partly Driven by the Breed and Sex of Cattle when Fed High-Grain Diet. Microorganisms 2022, 10, 323. [Google Scholar] [CrossRef]
- GB/T 35892-2018; Laboratory Animal Guideline for Ethical Review of Animal Welfare. Standardization Administration of China (SAC): Beijing, China, 2018.


| Ingredients | Treatment 1 | Nutrient | Treatment | ||
|---|---|---|---|---|---|
| AH | CS | AH | CS | ||
| Corn stalk | 0 | 50 | Dry matter | 87.46 | 87.40 |
| Alfalfa hay | 50 | 0 | Digestible energy, MJ/kg | 12.03 | 12.05 |
| Corn | 28.2 | 26.5 | Crude protein | 13.23 | 13.13 |
| Rapeseed meal | 0 | 5 | Starch | 20.35 | 20.47 |
| Soybean meal | 0 | 15.1 | Acid detergent fiber | 20.79 | 27.82 |
| Corn husk | 12.5 | 0 | Neutral detergent fiber | 35.61 | 45.13 |
| Wheat bran | 6 | 0 | Ether extract | 3.68 | 3.67 |
| Soybean oil | 0.3 | 0.4 | Feed cost, USD/t | 450 | 290 |
| Salt | 0.5 | 0.5 | Rumen degradable protein | 6.4 | 5.2 |
| Premix 2 | 2.5 | 2.5 | |||
| Items | Treatments 1 | SEM | p-Value 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dumont Sheep | Mongolian Sheep | T | B | R | B × R | ||||
| AH | CS | AH | CS | ||||||
| Weight gain, kg | |||||||||
| 30 d | 2.30 | 2.01 | 2.12 | 1.89 | 0.24 | 0.673 | 0.548 | 0.292 | 0.896 |
| 60 d | 5.30 | 4.95 | 5.02 | 4.04 | 0.58 | 0.466 | 0.314 | 0.268 | 0.594 |
| 90 d | 5.89 a | 4.86 ab | 5.23 a | 3.92 b | 0.49 | 0.026 | 0.072 | 0.012 | 0.746 |
| Feed intake, kg | |||||||||
| 30 d | 24.66 a | 23.59 a | 24.35 a | 21.88 b | 0.57 | 0.002 | 0.016 | 0.002 | 0.080 |
| 60 d | 35.53 a | 34.59 a | 34.65 a | 29.20 b | 1.46 | 0.002 | 0.010 | 0.009 | 0.053 |
| 90 d | 40.45 a | 37.29 b | 39.36 ab | 32.58 c | 1.49 | 0.001 | 0.003 | 0.001 | 0.049 |
| Feed efficiency | |||||||||
| 30 d | 0.09 | 0.08 | 0.09 | 0.09 | 0.01 | 0.917 | 0.798 | 0.551 | 0.798 |
| 60 d | 0.15 | 0.14 | 0.14 | 0.14 | 0.02 | 0.927 | 0.641 | 0.652 | 0.926 |
| 90 d | 0.15 | 0.13 | 0.13 | 0.12 | 0.01 | 0.419 | 0.311 | 0.196 | 0.769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Liu, H.; Wang, Y.; Na, M.; Zhang, R.; Na, R. Effect of Roughage Source on the Composition and Colonization of Rumen Bacteria and Methanogens in Dumont and Mongolian Sheep. Animals 2025, 15, 2079. https://doi.org/10.3390/ani15142079
Guo W, Liu H, Wang Y, Na M, Zhang R, Na R. Effect of Roughage Source on the Composition and Colonization of Rumen Bacteria and Methanogens in Dumont and Mongolian Sheep. Animals. 2025; 15(14):2079. https://doi.org/10.3390/ani15142079
Chicago/Turabian StyleGuo, Wenliang, Hongyang Liu, Yue Wang, Meila Na, Ran Zhang, and Renhua Na. 2025. "Effect of Roughage Source on the Composition and Colonization of Rumen Bacteria and Methanogens in Dumont and Mongolian Sheep" Animals 15, no. 14: 2079. https://doi.org/10.3390/ani15142079
APA StyleGuo, W., Liu, H., Wang, Y., Na, M., Zhang, R., & Na, R. (2025). Effect of Roughage Source on the Composition and Colonization of Rumen Bacteria and Methanogens in Dumont and Mongolian Sheep. Animals, 15(14), 2079. https://doi.org/10.3390/ani15142079






