Well-Being Indicators in Autistic Children and Therapy Dogs During a Group Intervention: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants: Children and Dogs
2.2. Sample Collection and Cortisol—Oxytocin Measurement in Children and Dogs
2.3. AAS Setting and Structure of the Canine-Assisted Program
- Opening sequence—“High Five” theme song.
- Presentation of the photograph of the four dogs.
- Live presentation of the dogs and first approach.
- Closing sequence—saying goodbye to the dogs, hand cleaning wipes, and theme song.
- Opening sequence—“High Five” theme song.
- Presentation of the activity—showing the photograph of the dogs involved.
- Activity 1 (focus on hyperactivity management)—the subject is asked to follow simple instructions to play with dogs (grab the ball, hold the ball counting to three, throw the ball to the dog, wait for the dog to return it, take the ball).
- Activity 2 (focus on sensory sphere activation)—the subject is asked to touch the dog in specific parts of the body (where is the tail? touch the ear, etc.).
- Activity 3 (focus on hyperactivity management)—the subject is asked to follow simple instructions to feed the dog (take the bowl, put the kibble in the bowl, place the bowl on the ground without dropping it, give the command to access the food).
- Activity 4 (focus on sensory sphere activation)—the subject is asked to take care of dog through epimeletic behavior, such as petting.
- Closing sequence—saying goodbye to the dogs, hand cleaning wipes, and theme song.
- Opening sequence—“High Five” theme song.
- Presentation of the activity—showing the photograph of the dogs.
- Activity 1 (focus on sensory sphere activation)—the subject is asked to take care of dog through epimeletic behaviors, such as feeding, brushing and petting.
- Activity 2 (focus on sensory sphere activation)—the subject is asked to touch the olfactory mat and hide a food reward for dog. Then, the subject observes how the dog searches for food with its sense of smell and can stop to pet it.
- Activity 3 (focus on hyperactivity management)—the subject is asked to follow simple instructions to walk dog on a leash (grab the leash, hold it in your hands, follow the indicated direction, look at dog, you can ask to sit, return the leash).
- Activity 4 (focus on hyperactivity management)—the subject is asked to sit at the entrance of a tunnel, wait for the handler to position dog at the other entrance of the tunnel, say the command to make the dog cross the tunnel and welcome it with a food reward.
- Closing sequence—saying goodbye to the dogs, hand cleaning wipes, and theme song.
- Opening sequence—“High Five” theme song.
- Presentation of the photograph of the dogs.
- Live presentation of the dogs and final farewell.
- Closing sequence—saying goodbye to the dogs, hand cleaning wipes, and theme song.
2.4. Questionnaire About the Impact of AAS on Canines and Children
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Cortisol and Oxytocin Levels in Both Pets and Autistic Children During the AAS
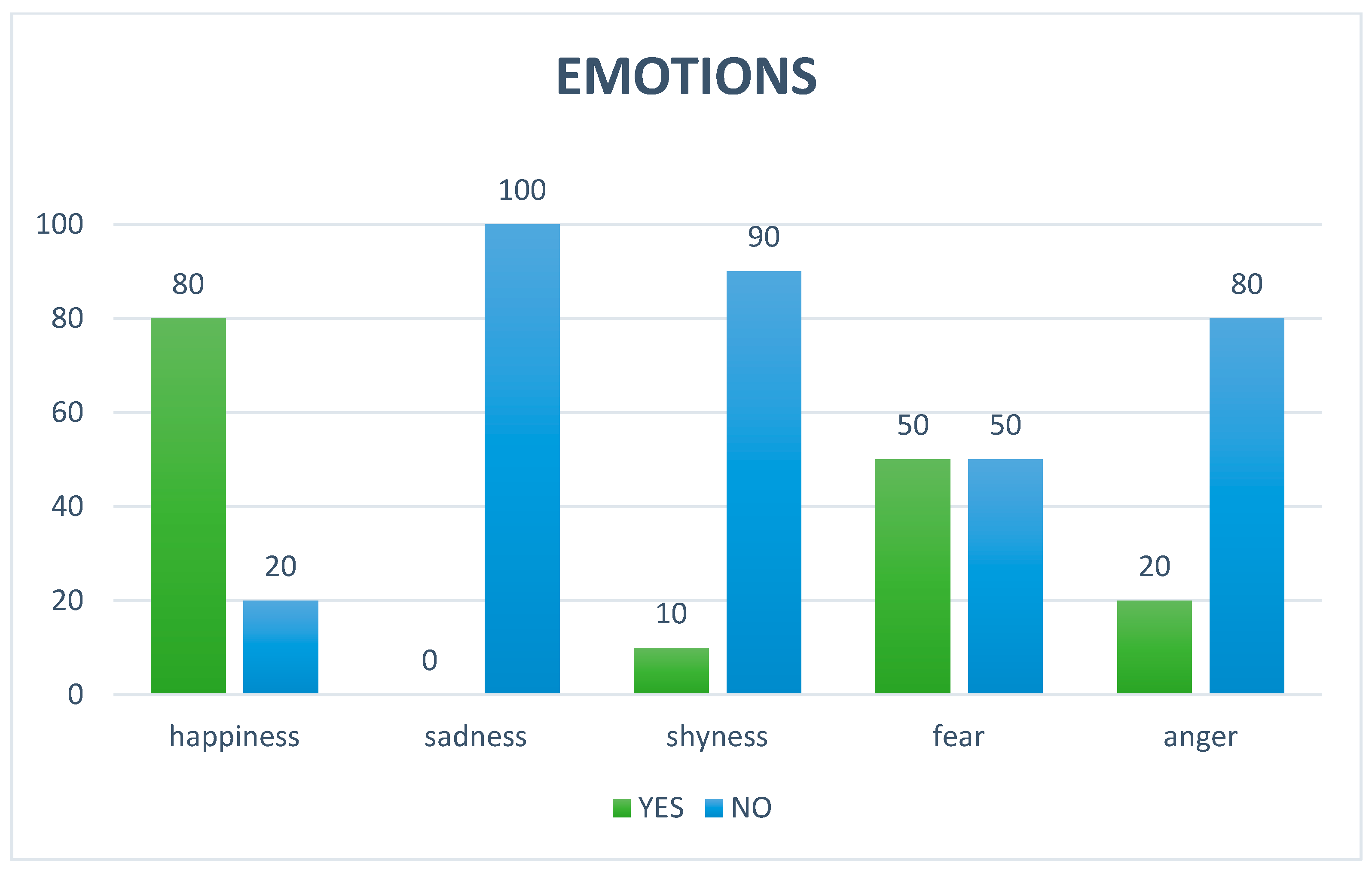
3.2. Parental Opinions About the Impact of AAS on Autistic Children
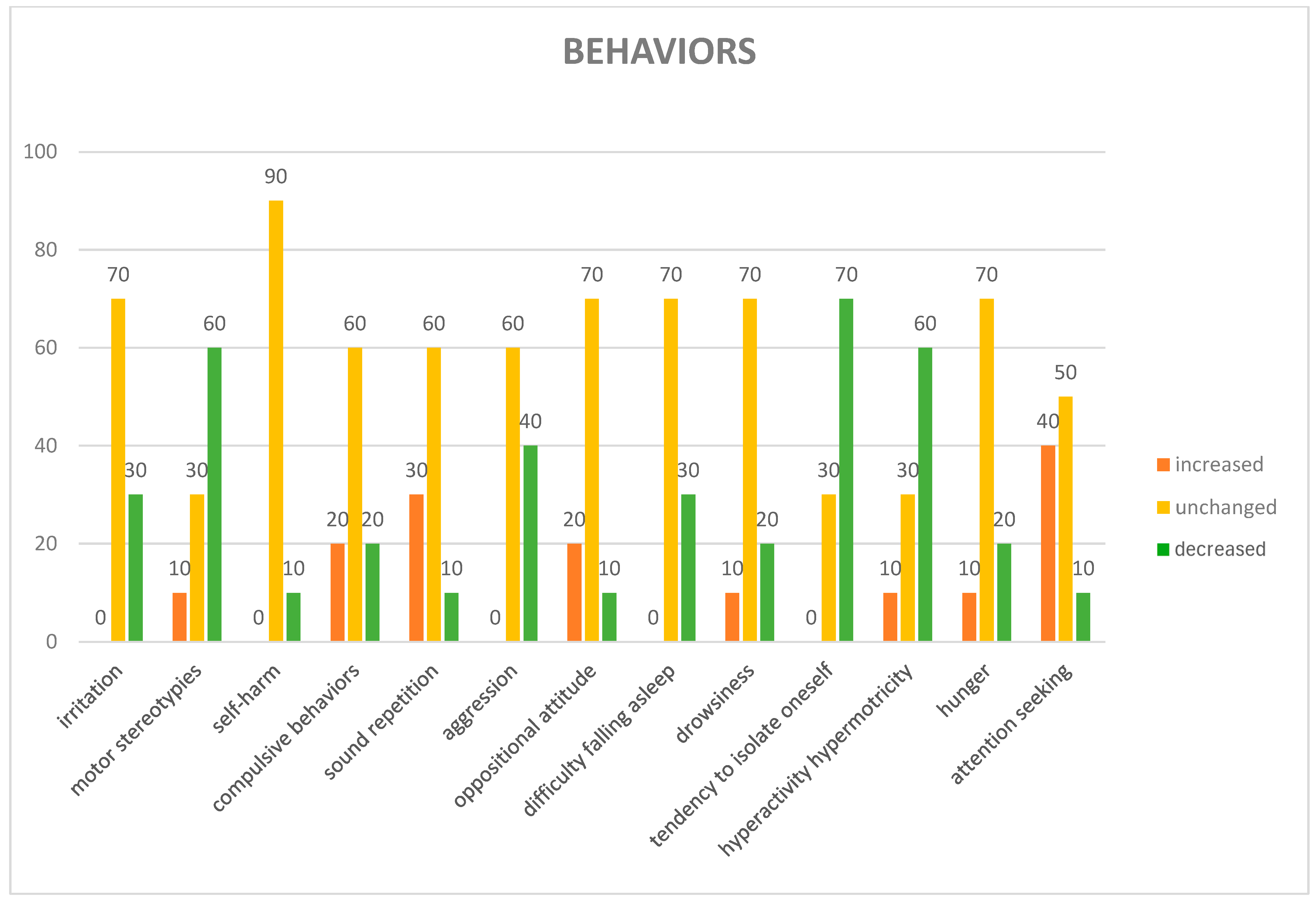
3.3. Opinions of the Dog Handlers About the Impact of AAS on Dogs
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dotson, M.J.; Hyatt, E.M. Understanding dog–human companionship. J. Bus. Res. 2008, 61, 457–466. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Stipo, C.; Quaranta, A. “Like Owner, Like Dog”: Correlation between the Owner’s Attachment Profile and the Owner-Dog Bond. PLoS ONE 2013, 8, e78455. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.A.; Rusu, A.S. Interaction with a Therapy Dog Enhances the Effects of Social Story Method in Autistic Children. Soc. Anim. 2014, 22, 241–261. [Google Scholar] [CrossRef]
- Arsovski, D. The Role of Animal Assisted Therapy in the Rehabilitation of Mental Health Disorders: A Systematic Literature Review. Perspect. Integr. Med. 2024, 3, 142–151. [Google Scholar] [CrossRef]
- Fornefeld, D.; Zellin, U.; Schmidt, P.; Fricke, O. The supporting role of dogs in the inpatient setting: A systematic review of the therapeutic effects of animal-assisted therapy with dogs for children and adolescents in an inpatient setting. Eur. Child Adolesc. Psychiatry 2025, 34, 3–17. [Google Scholar] [CrossRef]
- Nathans-Barel, I.; Feldman, P.; Berger, B.; Modai, I.; Silver, H. Animal-Assisted Therapy Ameliorates Anhedonia in Schizophrenia Patients—A Controlled Pilot Study. Psychother. Psychosom. 2004, 74, 31–35. [Google Scholar] [CrossRef]
- Barker, S.B.; Knisely, J.S.; McCain, K.R.; Best, A.M. Measuring stress and immune system response in healthcare professionals following interaction with a therapy dog. Stress Health 2005, 21, 181–187. [Google Scholar]
- Barker, S.B.; Pandurangi, A.K.; Best, A.M. Effects of animal-assisted therapy on patients with affective disorders. Psychiatry Serv. 2003, 54, 1325–1332. [Google Scholar]
- Shiloh, S.; Sorek, G.; Terkel, J. Reduction of State-Anxiety by Petting Animals in a Controlled Laboratory Experiment. Anxiety Stress Coping Int. J. 2003, 16, 387–395. [Google Scholar] [CrossRef]
- Lang, M.; Jansen, A.T.; Wedekind, D.; Neumann, I.D. Reduced anxiety and improved memory in laboratory rats after intranasal administration of oxytocin. Psychoneuroendocrinology 2010, 35, 849–856. [Google Scholar]
- Nimer, J.; Lundahl, B. Animal-assisted therapy: A meta-analysis. Anthrozoös 2007, 20, 225–238. [Google Scholar] [CrossRef]
- Glenk, L.M. Current perspectives on therapy dog welfare in animal-assisted interventions. Animals 2017, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Mariti, C.; Baragli, P.; Sergi, V.; Gazzano, A. Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results. Animals 2020, 10, 708. [Google Scholar] [CrossRef]
- d’Angelo, D.; d’Ingeo, S.; Ciani, F.; Visone, M.; Sacchettino, L.; Avallone, L.; Quaranta, A. Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study. Animals 2021, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Kohoutková, K.; Machová, K.; Procházková, R.; Makovcová, A.; Zítek, Š.; Svobodová, I. Evaluation of cortisol levels and behavior in dogs during animal-assisted interventions in clinical practice. Appl. Anim. Behav. Sci. 2024, 277, 106321. [Google Scholar]
- Winkle, M.; Johnson, A.; Mills, D. Dog Welfare, Well-Being and Behavior: Considerations for Selection, Evaluation and Suitability for Animal-Assisted Therapy. Animals 2020, 10, 2188. [Google Scholar] [CrossRef]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Salivary cortisol and behavior in therapy dogs during animal-assisted interventions: A pilot study. J. Vet. Behav. 2014, 9, 98–106. [Google Scholar] [CrossRef]
- Foss, C.H.E. Prioritizing Animal Welfare in AAI Hospital Programs. Anim. Behav. Welf. Cases 2023. [Google Scholar] [CrossRef]
- IAHAIO. The IAHAIO Definitions for Animal Assisted Intervention and Guidelines for Wellness of Animals Involved in AAI. White Paper. 2018. Available online: https://iahaio.org/wp/wp-content/uploads/2018/04/iahaio_wp_updated-2018-final.pdf (accessed on 26 May 2025).
- Lerner, H.A. Proposal for a Comprehensive Human–Animal Approach of Evaluation for Animal-Assisted Interventions. Int. J. Environ. Res. Public Health 2019, 16, 4305. [Google Scholar] [CrossRef]
- Binder, A.J.; Parish-Plass, N.; Kirby, M.; Winkle, M.; Skwerer, D.P.; Ackerman, L.; Brosig, C.; Coombe, W.; Delisle, E.; Enders-Slegers, M.-J.; et al. Recommendations for uniform terminology in animal-assisted services (AAS). Hum. Anim. Interact. 2023, 12, e19. [Google Scholar] [CrossRef]
- Simonato, M.; De Santis, M.; Contalbrigo, L.; Benedetti, D.; Finocchi Mahne, E.; Santucci, V.U.; Borrello, S.; Farina, L. The Italian Agreement between the Government and the Regional Authorities: National Guidelines for AAI and Institutional Context. People Anim. Int. J. Res. Pract. 2018, 1, 1. [Google Scholar]
- Marinelli, L.; Normando, S.; Siliprandi, C.; Salvadoretti, M.; Mongillo, P. Dog assisted interventions in a specialized centre and potential concerns for animal welfare. Vet. Res. Commun. 2009, 33, 93–95. [Google Scholar] [CrossRef]
- Palestrini, C.; Calcaterra, V.; Cannas, S.; Talamonti, Z.; Papotti, F.; Buttram, D.; Pelizzo, G. Stress level evaluation in a dog during animal-assisted therapy in pediatric surgery. J. Vet. Behav. 2017, 17, 44–49. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; van Hooff, J.A.; de Vries, H.W.; Mol, J.A. Chronic stress in dogs subjected to social and spatial restrictions. II. Hormonal and immunological responses. Physiol. Behav. 1999, 66, 243–254. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; Janssen, N.S.; Mol, J.A. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Horm. Behav. 1996, 30, 272–279. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology 1998, 23.8, 819–835. [Google Scholar] [CrossRef]
- VanFleet, R.; Faa-Thompson, T. The case for using animal assisted play therapy. Br. J. Play Ther. 2010, 6, 4–18. [Google Scholar]
- Odendaal, J.S.; Meintjes, R.A. Effects of positive human–dog interaction on cortisol and oxytocin levels in the dog. J. S. Afr. Vet. Assoc. 2003, 74, 58–62. [Google Scholar]
- Malinowski, K.; Yee, C.; Tevlin, J.M.; Birks, E.K.; Durando, M.M.; Pournajafi-Nazarloo, H.; Cavaiola, A.; McKeever, K.H. The effects of equine assisted therapy on plasma cortisol and oxytocin concentrations and heart rate variability in horses and measures of symptoms of post-traumatic stress disorder in veterans. J. Equine Vet. Sci. 2018, 64, 17–26. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 5. [Google Scholar]
- Gosling, C.J.; Cartigny, A.; Mellier, B.C.; Solanes, A.; Radua, J.; Delorme, R. Efficacy of psychosocial interventions for autism spectrum disorder: An umbrella review. Mol. Psychiatry 2022, 27, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Bottini, S.B.; Morton, H.E.; Buchanan, K.A.; Gould, K. Moving from Disorder to Difference: A Systematic Review of Recent Language Use in Autism Research. Autism Adulthood 2024, 6, 128–140. [Google Scholar] [CrossRef]
- Verheggen, T.; Enders-Slegers, M.J.; Eshuis, J. Enactive Anthrozoology: Toward an integrative theoretical model for understanding the therapeutic relationships between humans and animals. Hum. Anim. Interact. 2017, 5, 13–35. [Google Scholar] [CrossRef]
- Martin, F.; Farnum, J. Animal-assisted therapy for children with pervasive developmental disorders. West. J. Nurs. Res. 2002, 24, 657–670. [Google Scholar] [CrossRef]
- Sams, M.J.; Fortney, E.V.; Willenbring, S. Occupational therapy incorporating animals for children with autism: A pilot investigation. Am. J. Occup. Ther. 2006, 60, 268–274. [Google Scholar] [CrossRef]
- Kapustka, J.; Budzyńska, M. Does animal-assisted intervention work? Research review on the effectiveness of AAI with the use of different animal species. Animals 2020, 10, 135–141. [Google Scholar]
- Stefanini, M.C.; Bigalli, E.; Tani, F. Study of the acceptance and perceived efficacy of animal assisted therapy (AAT) for parents and nurses in the psychiatry unit of Meyer Children’s Hospital in Florence-Italy. J. Community Med. Health Educ. 2016, 6, 1–4. [Google Scholar]
- Ávila-Álvarez, A.; Pardo-Vázquez, J.; De-Rosende-Celeiro, I.; Jácome-Feijoo, R.; Torres-Tobío, G. Assessing the Outcomes of an Animal-Assisted Intervention in a Paediatric Day Hospital: Perceptions of Children and Parents. Animals 2020, 10, 1788. [Google Scholar] [CrossRef]
- Ang, C.S.; MacDougall, F.A. An Evaluation of Animal-Assisted Therapy for Autism Spectrum Disorders: Therapist and Parent Perspectives. Psychol. Stud. 2022, 67, 72–81. [Google Scholar] [CrossRef]
- Hardy, K.K.; Weston, R.N. Canine-Assisted Therapy for Children with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2020, 7, 197–204. [Google Scholar] [CrossRef]
- Marszałek, A.; Kasperczyk, T.; Walaszek, R. Dog Therapy in Supporting the Rehabilitation Process of Children with Autism. Med. Rehabil. 2022, 26, 58–63. [Google Scholar] [CrossRef]
- Galvany-López, P.; Martí-Vilar, M.; Hidalgo-Fuentes, S.; Cabedo-Peris, J. The Impact of Dog-Assisted Therapy Among Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Children 2024, 11, 1499. [Google Scholar] [CrossRef]
- Rehn, A.K.; Caruso, V.R.; Kumar, S. The effectiveness of animal-assisted therapy for children and adolescents with autism spectrum disorder: A systematic review. Complement. Ther. Clin. Pract. 2023, 50, 101719. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Bagayi, V.; Yang, D.; Huang, X.; Zhong, L.; Kiselev, S.; Chereshnev, V.A. Effectiveness of animal-assisted activities and therapies for autism spectrum disorder: A systematic review and meta-analysis. Front. Vet. Sci. 2024, 11, 1403527. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, K.; Simons, C.; van Amelsvoort, T.; Marcelis, M. Emotional Stress, Cortisol Response, and Cortisol Rhythm in Autism Spectrum Disorders: A Systematic Review. Res. Autism Spectr. Disord. 2022, 98, 102039. [Google Scholar] [CrossRef]
- Schupp, C.W.; Simon, D.; Corbett, B.A. Cortisol Responsivity Differences in Children with Autism Spectrum Disorders During Free and Cooperative Play. J. Autism Dev. Disord. 2013, 43, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Corbett, B.A.; Blain, S.D.; Edmiston, E.K. The Role of Context in Psychosocial Stress Among Adolescents with Autism Spectrum Disorder: Piloting a Semi-Structured, Videogame-Based Paradigm. J. Intellect. Dev. Disabil. 2017, 43, 20–28. [Google Scholar] [CrossRef]
- Sandri, M.; Colussi, A.; Perrotta, M.G.; Stefanon, B. Salivary Cortisol Concentration in Healthy Dogs Is Affected by Size, Sex, and Housing Context. J. Vet. Behav. 2015, 10, 302–306. [Google Scholar] [CrossRef]
- Wenger-Riggenbach, B.; Boretti, F.S.; Quante, S.; Schellenberg, S.; Reusch, C.E.; Sieber-Ruckstuhl, N.S. Salivary Cortisol Concentrations in Healthy Dogs and Dogs with Hypercortisolism. J. Vet. Intern. Med. 2010, 24, 551–556. [Google Scholar] [CrossRef]
- Di Nardo, F.; Anfossi, L.; Ozella, L.; Saccani, A.; Giovannoli, C.; Spano, G.; Baggiani, C. Validation of a qualitative immunochromatographic test for the noninvasive assessment of stress in dogs. J. Chromatogr. B 2016, 1028, 192–198. [Google Scholar] [CrossRef]
- Ng, Z.Y.; Pierce, B.J.; Otto, C.M.; Buechner-Maxwell, V.A.; Siracusa, C.; Werre, S.R. The effect of dog–human interaction on cortisol and behavior in registered animal-assisted activity dogs. Appl. Anim. Behav. Sci. 2014, 159, 69–81. [Google Scholar] [CrossRef]
- Haubenhofer, D.K.; Kirchengast, S. Physiological Arousal for Companion Dogs Working with Their Owners in Animal-Assisted Activities and Animal-Assisted Therapy. J. Appl. Anim. Welf. Sci. 2006, 9, 165–172. [Google Scholar] [CrossRef]
- King, C.; Watters, J.; Mungre, S. Effect of a time-out session with working animal-assisted therapy dogs. J. Vet. Behav. 2011, 6, 232–238. [Google Scholar] [CrossRef]
- Buttner, A.P.; Thompson, B.; Strasser, R.; Santo, J. Evidence for a synchronization of hormonal states between humans and dogs during competition. Physiol. Behav. 2015, 147, 54–62. [Google Scholar] [CrossRef]
- Fureix, C.; Jego, P.; Sankey, C.; Hausberger, M. How horses (Equus caballus) see the world: Humans as significant “objects”. Anim. Cogn. 2009, 12, 643–654. [Google Scholar] [CrossRef]
- Glenk, L.M.; Foltin, S. Therapy Dog Welfare Revisited: A Review of the Literature. Vet. Sci. 2021, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Toutain, M.; Dollion, N.; Henry, L.; Grandgeorge, M. How Do Children and Adolescents with ASD Look at Animals? A Scoping Review. Children 2024, 11, 211. [Google Scholar] [CrossRef]
- Cortesi, B.C.; Palestrini, C.; Buttram, D.; Mazzola, S.; Cannas, S. Stress and burnout in dogs involved in animal assisted interventions: A survey of Italian handlers’ opinion. J. Vet. Behav. 2025, 78, 63–69. [Google Scholar] [CrossRef]
- Haubenhofer, D.K.; Kirchengast, S. Dog Handlers’ and Dogs’ Emotional and Cortisol Secretion Responses Associated with Animal-Assisted Therapy Sessions. Soc. Anim. 2007, 15, 127–150. [Google Scholar] [CrossRef]
- Pop, D.; Rusu, A.; Pop-Vancia, V.; Papuc, I.; Constantinescu, R.; Mireşan, V. Physiological Effects of Human-Animal Positive Interaction in Dogs -Review of the Literature. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2014, 71, 102. [Google Scholar] [CrossRef]
- Chmelíková, E.; Bolechová, P.; Chaloupková, H.; Svobodová, I.; Jovičić, M.; Sedmíková, M. Salivary Cortisol as a Marker of Acute Stress in Dogs: A Review. Domest. Anim. Endocrinol. 2020, 72, 106428. [Google Scholar] [CrossRef]
- Van der Steen, S.; Heineman, M.M.P.; Ernst, M.J.A. Evaluating animal-assisted interventions: An empirical illustration of differences between outcome measures. Animals 2019, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L. Bias in clinical intervention research. Am. J. Epidemiol. 2006, 163, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Van de Mortel, T.F. Faking it: Social desirability response bias in self- report research. Aust. J. Adv. Nurs. 2008, 25, 40–48. [Google Scholar]
- Heimlich, K. Animal-assisted therapy and the severely disabled child: A quantitative study. J. Rehabil. 2001, 67, 48–54. [Google Scholar]
- Hatch, A. The view from all fours: A look at an animal-assisted activity program from the animals’ perspective. Anthrozoös 2007, 20, 37–50. [Google Scholar] [CrossRef]
- Contalbrigo, L.; Walsh, E.A.; Meers, L.L.; Benedetti, D.; De Santis, M.; Bassan, E.; Normando, S. The Selection and Training of Shelter Dogs for Involvement in Canine-Assisted Interventions: What Are the Ethical Issues? Vet. Sci. 2025, 12, 497. [Google Scholar] [CrossRef]
- Haverbeke, A.; Diederich, C.; Depiereux, E.; Giffroy, J.M. Cortisol and Behavioral Responses of Working Dogs to Environmental Challenges. Physiol. Behav. 2008, 93, 59–67. [Google Scholar] [CrossRef]
- Fine, A.H. The IAHAIO definitions for animal-assisted intervention and guidelines for wellness of animals involved. In Handbook on Animal-Assisted Therapy: Foundations and Guidelines for Animal-Assisted Interventions, 5th ed.; Elsevier: San Diego, CA, USA, 2019. [Google Scholar]



| CORT Levels (ng/mL) at Different Time Points | ||||||
|---|---|---|---|---|---|---|
| ID | T0-Pre | T0-Post | T45-Pre | T45-Post | T90-Pre | T90-Post |
| French Bulldog (FB) | 3.46 | 0.73 | 9.53 | 499.13 | 4.69 | 50.54 |
| German Shepherd (GS) | 5.96 | 43.60 | 13.39 | 9.21 | 5.95 | 6.94 |
| Border Collie (BC) | 6.28 | 15.90 | 14.98 | 18.67 | 6.04 | 17.01 |
| Mixed-breed (MB) | 6.76 | 6.28 | 6.07 | 3.93 | 4.56 | 22.30 |
| OT levels (ng/mL) at Different Time Points | ||||||
| ID | T0-Pre | T0-Post | T45-Pre | T45-Post | T90-Pre | T90-Post |
| French Bulldog (FB) | 3.36 | 74.30 | 3.61 | 193.53 | 3.37 | 36.80 |
| German Shepherd (GS) | 20.91 | 5.40 | 17.57 | 3.51 | 23.70 | 8.61 |
| Border Collie (BC) | 10.47 | 15.68 | 5.30 | 33.00 | 4.66 | 15.84 |
| Mixed-breed (MB) | 14.35 | 16.20 | 8.23 | 6.32 | 3.56 | 12.10 |
| CORT Levels (ng/mL) at Different Time Points | ||||||
|---|---|---|---|---|---|---|
| ID | T0-Pre | T0-Post | T45-Pre | T45-Post | T90-Pre | T90-Post |
| 1-AFP | 3.50 | 3.51 | 3.52 | 16.63 | 5.87 | 4.19 |
| 2-DSD | 197.50 | 3.51 | 3.50 | 115.68 | 26.68 | 2648.22 |
| 3-GR | 9.63 | 4.38 | 3.86 | 114.96 | 4.97 | 92.06 |
| 4-NF | 5.54 | 27.70 | 479.60 | 581.31 | 181.25 | 3409.70 |
| 5-PG | 69.96 | 4.31 | 3.69 | 11.33 | 1544.25 | 165.08 |
| 6-RS | 3.57 | 4.96 | 3.50 | 4.41 | 37,918.89 | 4.95 |
| 7-SJ | 23.40 | 41.72 | 3.49 | 23.91 | 5155.83 | 391.55 |
| 8-SD | 14.64 | 6.80 | 3.50 | 33.68 | 8.91 | 169.77 |
| 9-ZA | 36.54 | 112.05 | 5.66 | 4.59 | 3.91 | 1892.92 |
| 10-MSF | 3.60 | 18.20 | 3.61 | 26.77 | 111.50 | 201.81 |
| OT Levels (ng/mL) at Different Time Points | ||||||
| ID | T0-Pre | T0-Post | T45-Pre | T45-Post | T90-Pre | T90-Post |
| 1-AFP | 1.09 | 1.25 | 10.38 | 3.29 | 1.89 | 1.84 |
| 2-DSD | 62.46 | 95.14 | 1.12 | 14.11 | 8.44 | 1.87 |
| 3-GR | 11.02 | 21.59 | 72.05 | 44.21 | 1.86 | 1.92 |
| 4-NF | 10.05 | 65.64 | 111.50 | 88.40 | 211.64 | 495.70 |
| 5-PG | 28.77 | 20.21 | 7.58 | 1.98 | 151.46 | 2.59 |
| 6-RS | 14.30 | 6.64 | 4.18 | 1.96 | 29.51 | 1.92 |
| 7-SJ | 17.76 | 25.56 | 0.42 | 142.12 | 62.01 | 4.32 |
| 8-SD | 20.62 | 7.73 | 1.98 | 6.43 | 2.34 | 35.38 |
| 9-ZA | 21.37 | 43.28 | 8.73 | 1.93 | 1.85 | 5.69 |
| 10-MSF | 2.91 | 13.80 | 6.57 | 26.18 | 3.55 | 73.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, V.O.; Sacchettino, L.; Rusu, A.S.; Ciccarelli, D.; Gazzano, V.; de Cesare, M.; Visone, M.; Mizzoni, V.; Napolitano, F.; d’Angelo, D. Well-Being Indicators in Autistic Children and Therapy Dogs During a Group Intervention: A Pilot Study. Animals 2025, 15, 2032. https://doi.org/10.3390/ani15142032
Giuliano VO, Sacchettino L, Rusu AS, Ciccarelli D, Gazzano V, de Cesare M, Visone M, Mizzoni V, Napolitano F, d’Angelo D. Well-Being Indicators in Autistic Children and Therapy Dogs During a Group Intervention: A Pilot Study. Animals. 2025; 15(14):2032. https://doi.org/10.3390/ani15142032
Chicago/Turabian StyleGiuliano, Viviana Orsola, Luigi Sacchettino, Alina Simona Rusu, Davide Ciccarelli, Valentina Gazzano, Martina de Cesare, Michele Visone, Vincenzo Mizzoni, Francesco Napolitano, and Danila d’Angelo. 2025. "Well-Being Indicators in Autistic Children and Therapy Dogs During a Group Intervention: A Pilot Study" Animals 15, no. 14: 2032. https://doi.org/10.3390/ani15142032
APA StyleGiuliano, V. O., Sacchettino, L., Rusu, A. S., Ciccarelli, D., Gazzano, V., de Cesare, M., Visone, M., Mizzoni, V., Napolitano, F., & d’Angelo, D. (2025). Well-Being Indicators in Autistic Children and Therapy Dogs During a Group Intervention: A Pilot Study. Animals, 15(14), 2032. https://doi.org/10.3390/ani15142032







