Cecal Metabolome Profiles of Turkey Poults in Response to Salmonella Heidelberg Challenge with or Without Turkey-Derived Lactobacillus Probiotic and Trans-Cinnamaldehyde
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.1.1. S. Heidelberg
2.1.2. Lactobacillus salivarius (L. salivarius) and Lactobacillus ingluviei (L. ingluviei)
2.2. Plant-Derived Antimicrobial (PDA)
2.3. Salmonella Challenge Study in Turkey Poults
2.3.1. Ethics Statement
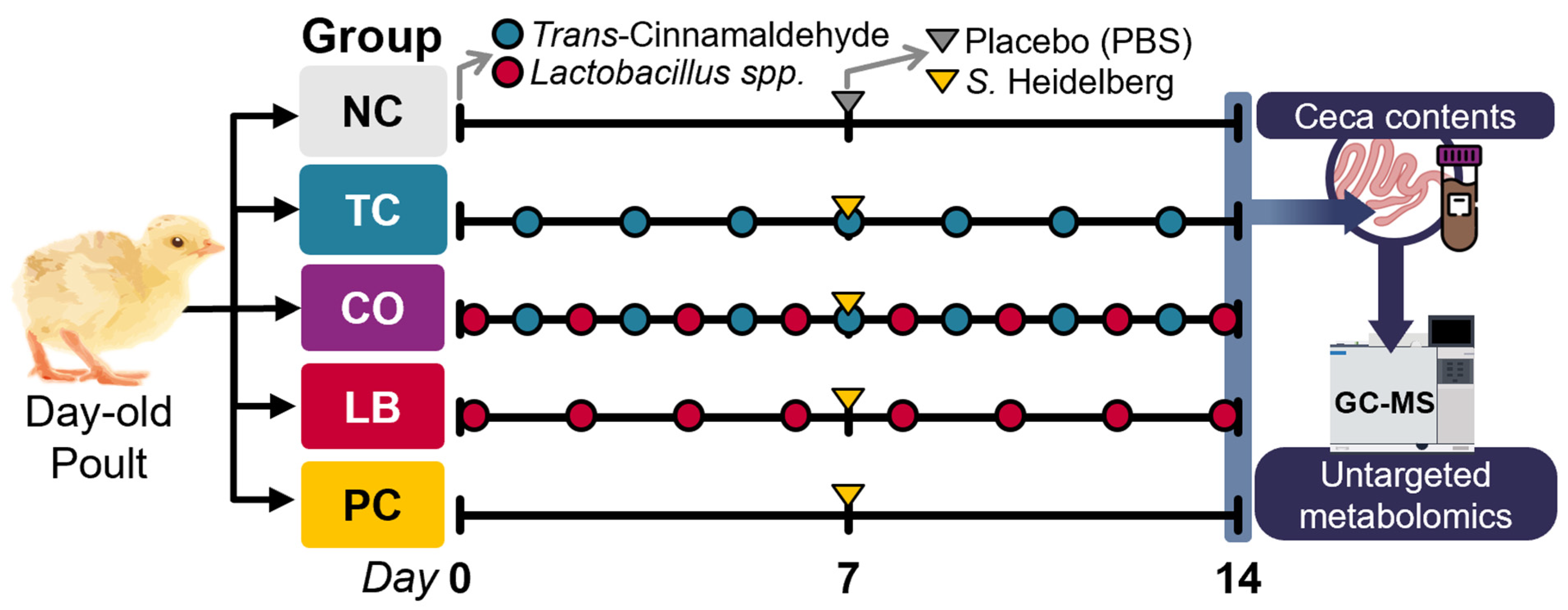
2.3.2. Experimental Design, Poults, and Housing
2.4. Metabolomics Analysis of Cecal Contents
2.5. Statistical Analysis
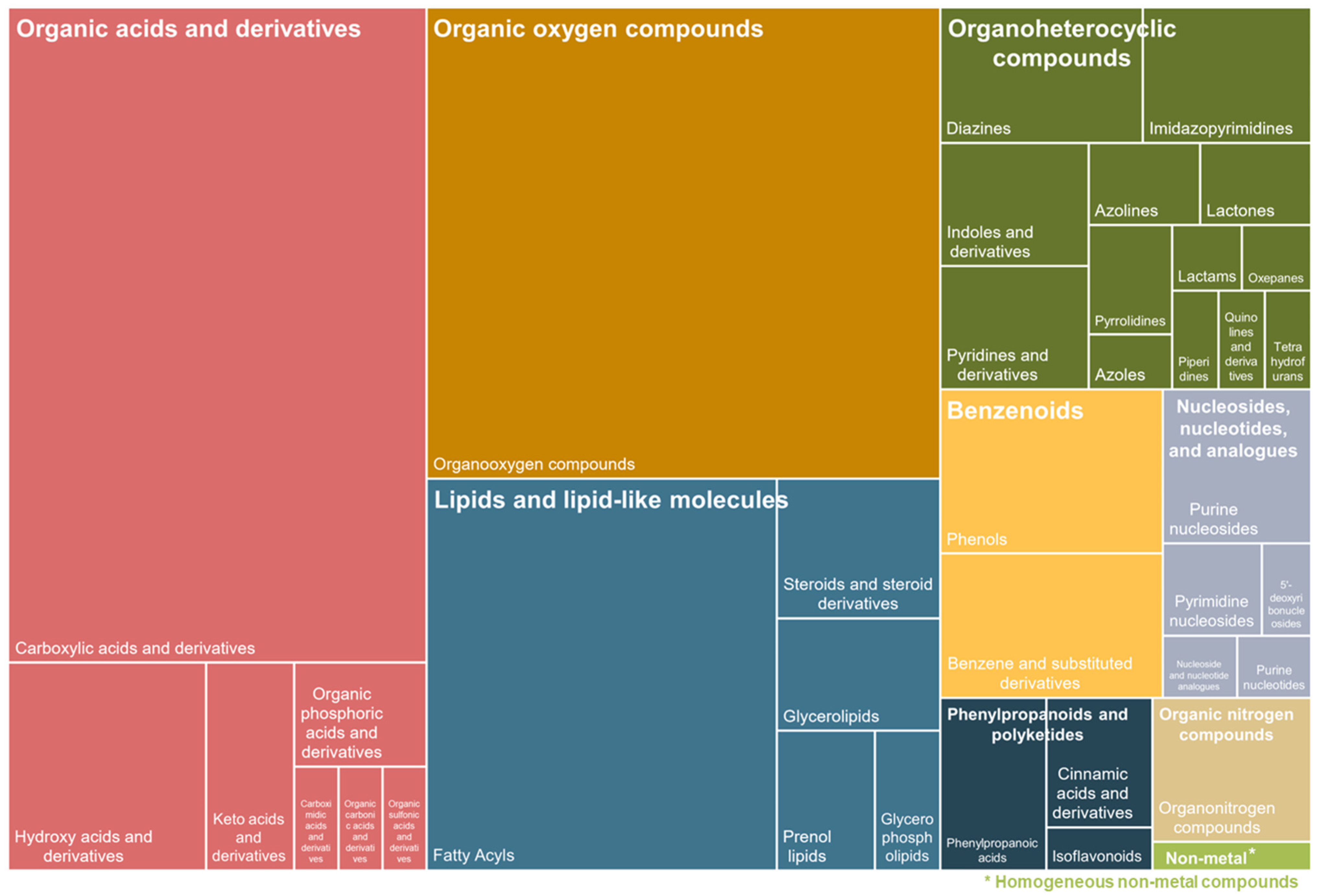
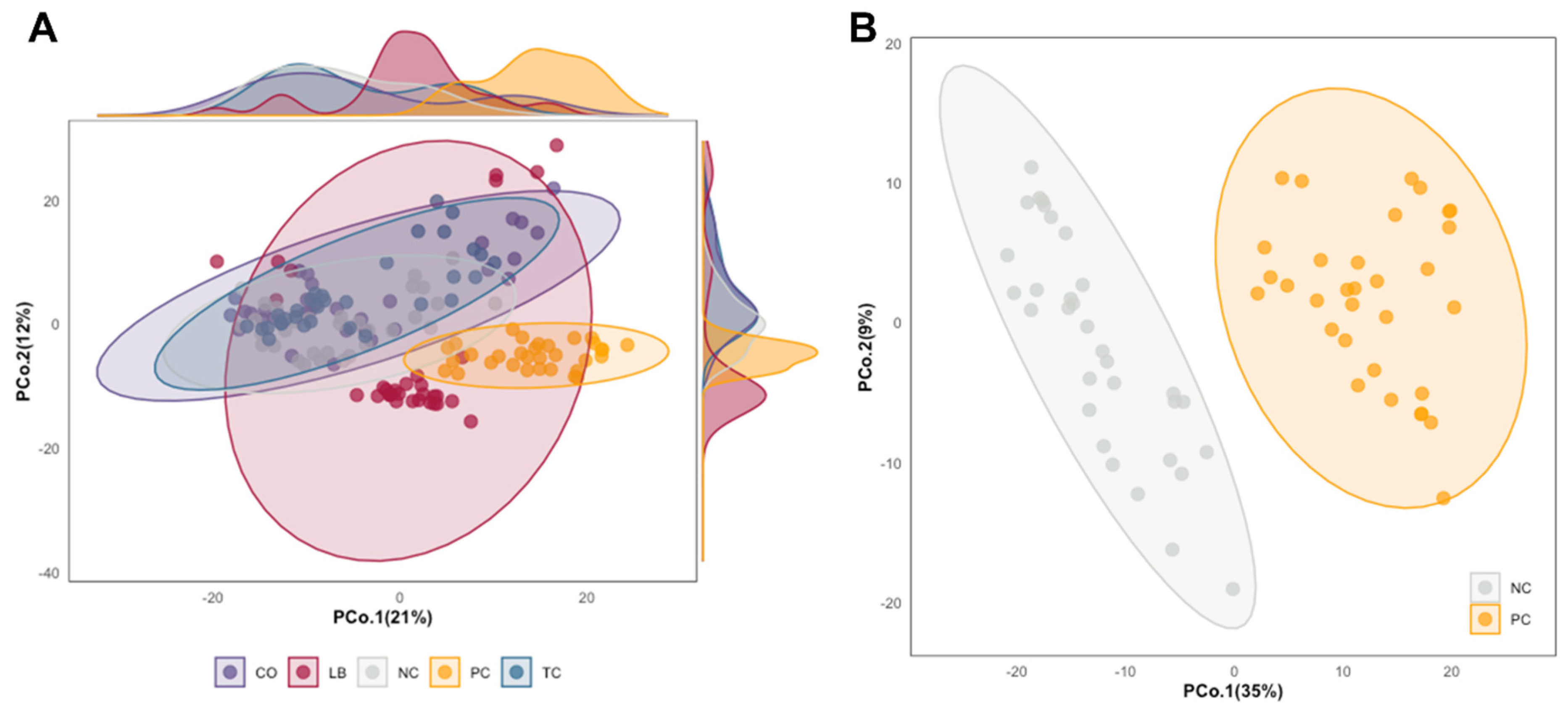
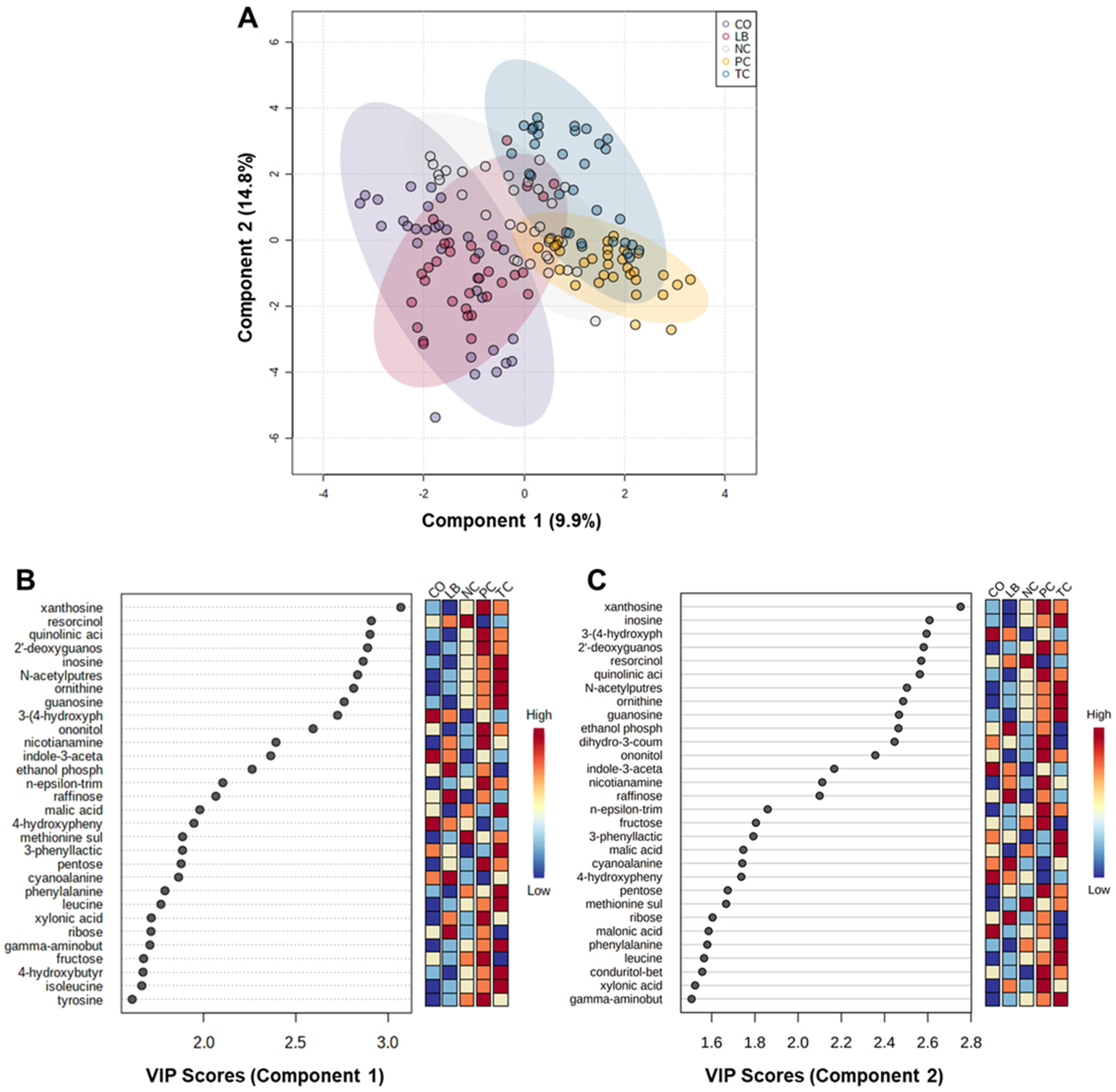
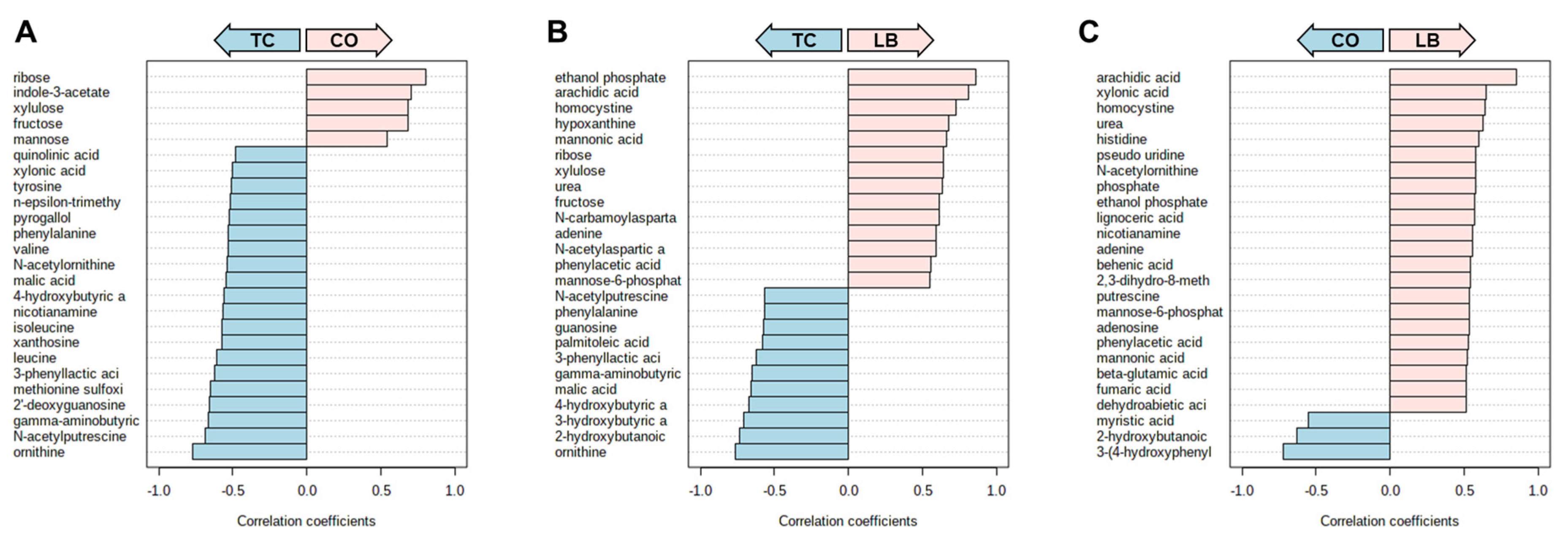
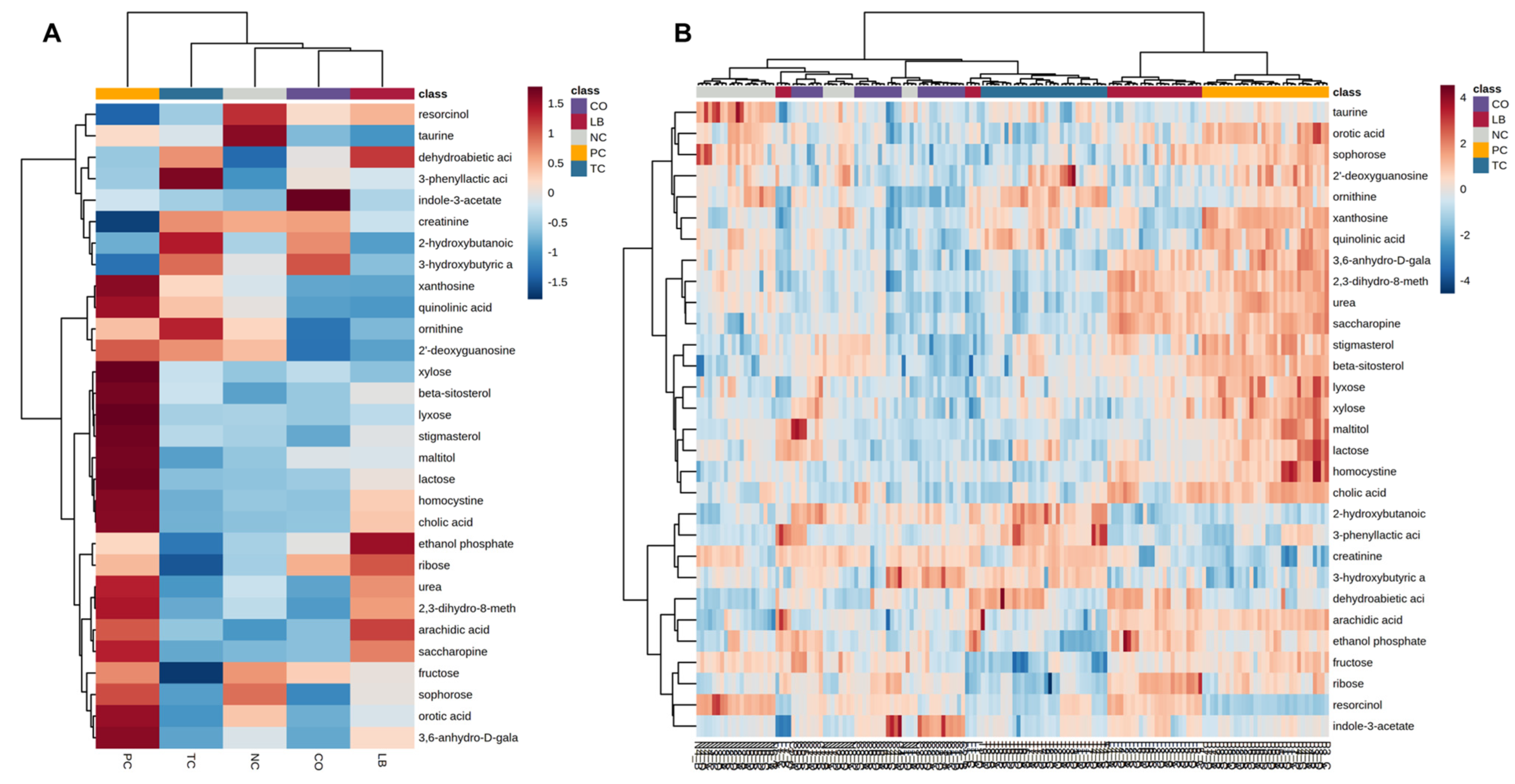
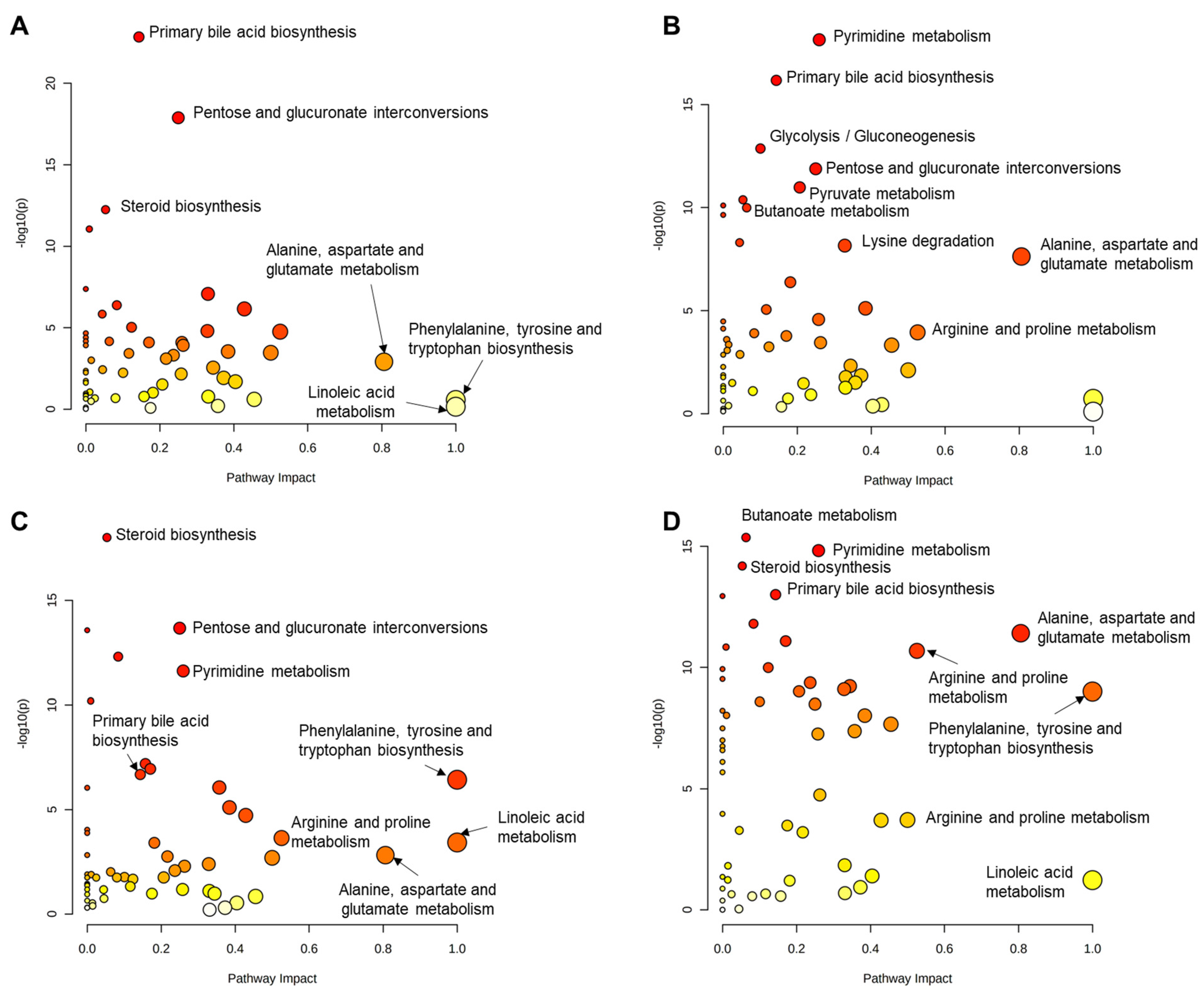
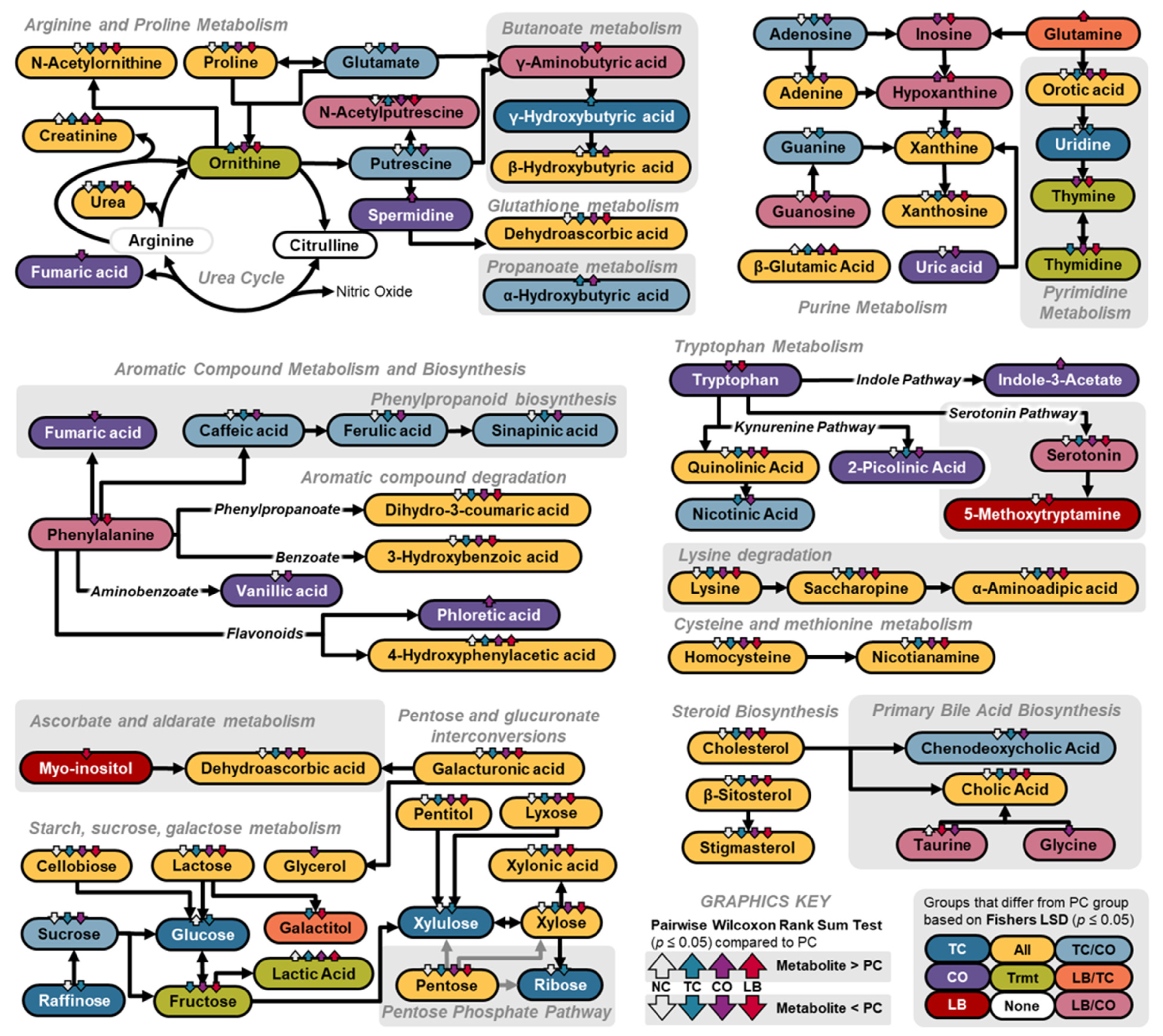
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batz, M.B.; Richardson, L.C.; Bazaco, M.C.; Parker, C.C.; Chirtel, S.J.; Cole, D.; Golden, N.J.; Griffin, P.M.; Gu, W.; Schmitt, S.K.; et al. Recency-Weighted Statistical Modeling Approach to Attribute Illnesses Caused by 4 Pathogens to Food Sources Using Outbreak Data, United States. Emerg. Infect. Dis. 2021, 27, 214–222. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Reduce Infections Caused by Salmonella—FS-04. Healthy People. 2022. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/foodborne-illness/reduce-infections-caused-salmonella-fs-04 (accessed on 25 June 2022).
- CDC. Pathogen Surveillance. Foodborne Diseases Active Surveillance Network. 2022. Available online: https://wwwn.cdc.gov/foodnetfast/ (accessed on 25 June 2022).
- USDA. USDA Launches New Effort to Reduce Salmonella Illnesses Linked to Poultry. Washington. 2021. Available online: https://www.usda.gov/media/press-releases/2021/10/19/usda-launches-new-effort-reduce-salmonella-illnesses-linked-poultry (accessed on 25 June 2022).
- Gieraltowski, L.; Higa, J.; Peralta, V.; Green, A.; Schwensohn, C.; Rosen, H.; Libby, T.; Kissler, B.; Marsden-Haug, N.; Booth, H.; et al. National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. PLoS ONE 2016, 11, e0162369. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Gollarza, L.; Sockett, D.; Aulik, N.; Patton, E.; Francois Watkins, L.K.; Gambino-Shirley, K.J.; Folster, J.P.; Chen, J.C.; Tagg, K.A.; et al. Outbreak of Multidrug-Resistant Salmonella Heidelberg Infections Linked to Dairy Calf Exposure, United States, 2015–2018. Foodborne Pathog. Dis. 2022, 19, 199–208. [Google Scholar] [CrossRef]
- Folster, J.P.; Pecic, G.; Singh, A.; Duval, B.; Rickert, R.; Ayers, S.; Abbott, J.; McGlinchey, B.; Bauer-Turpin, J.; Haro, J.; et al. Characterization of Extended-Spectrum Cephalosporin–Resistant Salmonella enterica Serovar Heidelberg Isolated from Food Animals, Retail Meat, and Humans in the United States 2009. Foodborne Pathog. Dis. 2012, 9, 638–645. [Google Scholar] [CrossRef]
- Lynne, A.M.; Kaldhone, P.; David, D.; White, D.G.; Foley, S.L. Characterization of Antimicrobial Resistance in Salmonella enterica Serotype Heidelberg Isolated from Food Animals. Foodborne Pathog. Dis. 2009, 6, 207–215. [Google Scholar] [CrossRef]
- Antony, L.; Behr, M.; Sockett, D.; Miskimins, D.; Aulik, N.; Christopher-Hennings, J.; Nelson, E.; Allard, M.W.; Scaria, J. Genome divergence and increased virulence of outbreak associated Salmonella enterica subspecies enterica serovar Heidelberg. Gut Pathog. 2018, 10, 53. [Google Scholar] [CrossRef]
- CDC. Outbreak of Salmonella Heidelberg infections linked to a single poultry producer—13 states, 2012–2013. Morb. Mortal. Wkly. Rep. 2013, 62, 553–556. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23842445 (accessed on 25 June 2022).
- Dorea, F.C.; Cole, D.J.; Hofacre, C.; Zamperini, K.; Mathis, D.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella Vaccination of Breeder Chickens on Contamination of Broiler Chicken Carcasses in Integrated Poultry Operations. Appl. Environ. Microbiol. 2010, 76, 7820–7825. [Google Scholar] [CrossRef]
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Rissman, T.; Lee, M.D.; Maurer, J.J. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef]
- Gast, R.K.; Stone, H.D.; Holt, P.S. Evaluation of the Efficacy of Oil-Emulsion Bacterins for Reducing Fecal Shedding of Salmonella enteritidis by Laying Hens. Avian Dis. 1993, 37, 1085. [Google Scholar] [CrossRef]
- Avila, L.A.F.; Nascimento, V.P.; Salle, C.T.P.; Moraes, H.L.S. Effects of Probiotics and Maternal Vaccination on Salmonella Enteritidis Infection in Broiler Chicks. Avian Dis. 2006, 50, 608–612. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Sakamoto, K.; Seo, S.-U.; Pickard, J.M.; Gillilland, M.G.; Pudlo, N.A.; Hoostal, M.; Li, X.; Wang, T.D.; Feehley, T.; et al. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 2017, 356, 315–319. [Google Scholar] [CrossRef]
- De Nisco, N.J.; Rivera-Cancel, G.; Orth, K. The Biochemistry of Sensing: Enteric Pathogens Regulate Type III Secretion in Response to Environmental and Host Cues. mBio 2018, 9, e02122-17. [Google Scholar] [CrossRef]
- Dewi, G.; Manjankattil, S.; Peichel, C.; Jia, S.; Nair, D.; Vickers, Z.; Johnson, T.J.; Cardona, C.; Noll, S.; Johny, A.K. Effect of plant-derived antimicrobials against multidrug-resistant Salmonella Heidelberg in ground Turkey. Poult. Sci. 2022, 101, 101581. [Google Scholar] [CrossRef] [PubMed]
- Dewi, G.; Manjankattil, S.; Peichel, C.; Johnson, T.J.; Noll, S.; Cardona, C.; Johny, A.K. Combination of autochthonous Lactobacillus strains and trans-Cinnamaldehyde in water reduces Salmonella Heidelberg in turkey poults. Front. Microbiol. 2024, 15, 1337428. [Google Scholar] [CrossRef]
- Abate, S.; Ahn, Y.G.; Kind, T.; Cataldi, T.R.I.; Fiehn, O. Determination of elemental compositions by gas chromatography/time-of-flight mass spectrometry using chemical and electron ionization. Rapid Commun. Mass Spectrom. 2010, 24, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Fan, S.; Kind, T.; Cajka, T.; Hazen, S.L.; Tang, W.H.W.; Kaddurah-Daouk, R.; Irvin, M.R.; Arnett, D.K.; Barupal, D.K.; Fiehn, O. Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data. Anal. Chem. 2019, 91, 3590–3596. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminformatics 2016, 8, 61. [Google Scholar] [CrossRef]
- Quinn, R.A.; Lim, Y.W.; Mak, T.D.; Whiteson, K.; Furlan, M.; Conrad, D.; Rohwer, F.; Dorrestein, P. Metabolomics of pulmonary exacerbations reveals the personalized nature of cystic fibrosis disease. PeerJ 2016, 4, e2174. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.L.; Peña-Díaz, J.; Finlay, B.B. Chemical communication in the gut: Effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens. Anaerobe 2015, 34, 106–115. [Google Scholar] [CrossRef]
- Duke, G.E.; Eccleston, E.; Kirkwood, S.; Louis, C.F.; Bedbury, H.P. Cellulose Digestion by Domestic Turkeys Fed Low or High Fiber Diets. J. Nutr. 1984, 114, 95–102. [Google Scholar] [CrossRef]
- Hargis, B.M.; Caldwell, D.J.; Brewer, R.L.; Corrier, D.E.; Deloach, J.R. Evaluation of the Chicken Crop as a Source of Salmonella Contamination for Broiler Carcasses. Poult. Sci. 1995, 74, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16S rRNA-Based Analysis of Microbiota from the Cecum of Broiler Chickens. Appl. Environ. Microbiol. 2002, 68, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population Dynamics of Salmonella enterica Serotypes in Commercial Egg and Poultry Production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef]
- Thomas, D.H. Salt and water excretion by birds: The lower intestine as an integrator of renal and intestinal excretion. Comp. Biochem. Physiol. Part A Physiol. 1982, 71, 527–535. [Google Scholar] [CrossRef]
- Deatherage Kaiser, B.L.; Li, J.; Sanford, J.A.; Kim, Y.-M.; Kronewitter, S.R.; Jones, M.B.; Peterson, C.T.; Peterson, S.N.; Frank, B.C.; Purvine, S.O.; et al. A Multi-Omic View of Host-Pathogen-Commensal Interplay in Salmonella-Mediated Intestinal Infection. PLoS ONE 2013, 8, e67155. [Google Scholar] [CrossRef]
- Bescucci, D.M.; Montina, T.; Boras, V.F.; Inglis, G.D. Infection by Salmonella enterica Serovar Typhimurium DT104 Modulates Immune Responses, the Metabolome, and the Function of the Enteric Microbiota in Neonatal Broiler Chickens. Pathogens 2022, 11, 1257. [Google Scholar] [CrossRef]
- Awad, W.A.; Smorodchenko, A.; Hess, C.; Aschenbach, J.R.; Molnár, A.; Dublecz, K.; Khayal, B.; Pohl, E.E.; Hess, M. Increased intracellular calcium level and impaired nutrient absorption are important pathogenicity traits in the chicken intestinal epithelium during Campylobacter jejuni colonization. Appl. Microbiol. Biotechnol. 2015, 99, 6431–6441. [Google Scholar] [CrossRef]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.M.; Arena, E.T.; Menendez, A.; Han, J.; Ferreira, R.B.R.; Buckner, M.M.C.; Lolić, P.; Madilao, L.L.; Bohlmann, J.; Borchers, C.H.; et al. Impact of Salmonella Infection on Host Hormone Metabolism Revealed by Metabolomics. Infect. Immun. 2011, 79, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Knarreborg, A.; Engberg, R.M.; Jensen, S.K.; Jensen, B.B. Quantitative Determination of Bile Salt Hydrolase Activity in Bacteria Isolated from the Small Intestine of Chickens. Appl. Environ. Microbiol. 2002, 68, 6425–6428. [Google Scholar] [CrossRef]
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile Salt Hydrolase Activity in Probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Tannock, G.W.; Dashkevicz, M.P.; Feighner, S.D. Lactobacilli and bile salt hydrolase in the murine intestinal tract. Appl. Environ. Microbiol. 1989, 55, 1848–1851. [Google Scholar] [CrossRef]
- van Velkinburgh, J.C.; Gunn, J.S. PhoP-PhoQ-Regulated Loci Are Required for Enhanced Bile Resistance in Salmonella spp. Infect. Immun. 1999, 67, 1614–1622. [Google Scholar] [CrossRef]
- Prouty, A.M.; Schwesinger, W.H.; Gunn, J.S. Biofilm Formation and Interaction with the Surfaces of Gallstones by Salmonella spp. Infect. Immun. 2002, 70, 2640–2649. [Google Scholar] [CrossRef]
- Crawford, R.W.; Gibson, D.L.; Kay, W.W.; Gunn, J.S. Identification of a Bile-Induced Exopolysaccharide Required for Salmonella Biofilm Formation on Gallstone Surfaces. Infect. Immun. 2008, 76, 5341–5349. [Google Scholar] [CrossRef]
- Prouty, A.M.; Gunn, J.S. Salmonella enterica Serovar Typhimurium Invasion Is Repressed in the Presence of Bile. Infect. Immun. 2000, 68, 6763–6769. [Google Scholar] [CrossRef]
- Crawford, R.W.; Reeve, K.E.; Gunn, J.S. Flagellated but Not Hyperfimbriated Salmonella enterica Serovar Typhimurium Attaches to and Forms Biofilms on Cholesterol-Coated Surfaces. J. Bacteriol. 2010, 192, 2981–2990. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Stein, K.R.; Hang, H.C. Anti-infective bile acids bind and inactivate a Salmonella virulence regulator. Nat. Chem. Biol. 2023, 19, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.B.M.S.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile Acid Is a Host Factor That Regulates the Composition of the Cecal Microbiota in Rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Pickard, J.M.; Fukuda, S.; Inohara, N.; Núñez, G. An Enteric Pathogen Subverts Colonization Resistance by Evading Competition for Amino Acids in the Gut. Cell Host Microbe 2020, 28, 526–533.e5. [Google Scholar] [CrossRef]
- Mon, K.K.Z.; Zhu, Y.; Chanthavixay, G.; Kern, C.; Zhou, H. Integrative analysis of gut microbiome and metabolites revealed novel mechanisms of intestinal Salmonella carriage in chicken. Sci. Rep. 2020, 10, 4809. [Google Scholar] [CrossRef]
- Mei, X.; Ma, B.; Zhai, X.; Zhang, A.; Lei, C.; Zuo, L.; Yang, X.; Zhou, C.; Wang, H.; Björkroth, J. Florfenicol Enhances Colonization of a Salmonella enterica Serovar Enteritidis floR Mutant with Major Alterations to the Intestinal Microbiota and Metabolome in Neonatal Chickens. Appl. Environ. Microbiol. 2021, 87, e01681-21. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Wink, D.A.; Kasprzak, K.S.; Maragos, C.M.; Elespuru, R.K.; Misra, M.; Dunams, T.M.; Cebula, T.A.; Koch, W.H.; Andrews, A.W.; Allen, J.S.; et al. DNA Deaminating Ability and Genotoxicity of Nitric Oxide and its Progenitors. Science 1991, 254, 1001–1003. [Google Scholar] [CrossRef]
- Chakravortty, D.; Hensel, M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003, 5, 621–627. [Google Scholar] [CrossRef]
- De Groote, M.A.; Testerman, T.; Xu, Y.; Stauffer, G.; Fang, F.C. Homocysteine Antagonism of Nitric Oxide-Related Cytostasis in Salmonella typhimurium. Science 1996, 272, 414–417. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Behnsen, J.; Jellbauer, S.; Wong, C.P.; Edwards, R.A.; George, M.D.; Ouyang, W.; Raffatellu, M. The Cytokine IL-22 Promotes Pathogen Colonization by Suppressing Related Commensal Bacteria. Immunity 2014, 40, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4 + CD8αα + T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.A.; Crowley, J.R.; Sambandam, N.; Muegge, B.D.; Costello, E.K.; Hamady, M.; Knight, R.; Gordon, J.I. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl. Acad. Sci. USA 2009, 106, 11276–11281. [Google Scholar] [CrossRef]
- Fujisaka, S.; Avila-Pacheco, J.; Soto, M.; Kostic, A.; Dreyfuss, J.M.; Pan, H.; Ussar, S.; Altindis, E.; Li, N.; Bry, L.; et al. Diet, Genetics, and the Gut Microbiome Drive Dynamic Changes in Plasma Metabolites. Cell Rep. 2018, 22, 3072–3086. [Google Scholar] [CrossRef]
- Kumar, A.; Russell, R.M.; Pifer, R.; Menezes-Garcia, Z.; Cuesta, S.; Narayanan, S.; MacMillan, J.B.; Sperandio, V. The Serotonin Neurotransmitter Modulates Virulence of Enteric Pathogens. Cell Host Microbe 2020, 28, 41–53.e8. [Google Scholar] [CrossRef]
- Linden, D.R.; Foley, K.F.; Mcquoid, C.; Simpson, J.; Sharkey, K.A.; Mawe, G.M. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol. Motil. 2005, 17, 565–574. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Mailer, R.; Pabst, O.; Weier, G.; Sedlik, W.; Li, Z.; Chen, J.J.; Murphy, D.L.; Gershon, M.D. Role of serotonin in intestinal inflammation: Knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol. Liver Physiol. 2009, 296, G685–G695. [Google Scholar] [CrossRef]
- Ghia, J.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El–Sharkawy, R.T.; Côté, F.; Mallet, J.; Khan, W.I. Serotonin Has a Key Role in Pathogenesis of Experimental Colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef]
- Kidd, M.; Gustafsson, B.I.; Drozdov, I.; Modlin, I.M. IL1β- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol. Motil. 2009, 21, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Bogomolnaya, L.; McClelland, M.; Andrews-Polymenis, H. De novo pyrimidine synthesis is necessary for intestinal colonization of Salmonella Typhimurium in chicks. PLoS ONE 2017, 12, e0183751. [Google Scholar] [CrossRef] [PubMed]
- González-Segura, L.; Witte, J.F.; McClard, R.W.; Hurley, T.D. Ternary Complex Formation and Induced Asymmetry in Orotate Phosphoribosyltransferase. Biochemistry 2007, 46, 14075–14086. [Google Scholar] [CrossRef]
- Chaudhuri, R.R.; Morgan, E.; Peters, S.E.; Pleasance, S.J.; Hudson, D.L.; Davies, H.M.; Wang, J.; Van Diemen, P.M.; Buckley, A.M.; Bowen, A.J.; et al. Comprehensive Assignment of Roles for Salmonella Typhimurium Genes in Intestinal Colonization of Food-Producing Animals. PLoS Genet. 2013, 9, e1003456. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; Missotten, J.A.M.; Fremaut, D.; De Smet, S.; Dierick, N.A. In vitro characterisation of the antimicrobial activity of selected essential oil components and binary combinations against the pig gut flora. Anim. Feed. Sci. Technol. 2009, 151, 111–127. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Puupponen-Pimiä, R.; Aura, A.-M.; Helander, I.M.; Nohynek, L.; Oksman-Caldentey, K.-M.; Saarela, M. Weakening of Salmonella with Selected Microbial Metabolites of Berry-Derived Phenolic Compounds and Organic Acids. J. Agric. Food Chem. 2007, 55, 3905–3912. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial Succession in the Broiler Gastrointestinal Tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef]
- Adhikari, B.; Kwon, Y.M. Characterization of the Culturable Subpopulations of Lactobacillus in the Chicken Intestinal Tract as a Resource for Probiotic Development. Front. Microbiol. 2017, 8, 1389. [Google Scholar] [CrossRef]
- Stecher, B.; Chaffron, S.; Käppeli, R.; Hapfelmeier, S.; Freedrich, S.; Weber, T.C.; Kirundi, J.; Suar, M.; McCoy, K.D.; von Mering, C.; et al. Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria. PLoS Pathog. 2010, 6, e1000711. [Google Scholar] [CrossRef]
- Endt, K.; Stecher, B.; Chaffron, S.; Slack, E.; Tchitchek, N.; Benecke, A.; Van Maele, L.; Sirard, J.-C.; Mueller, A.J.; Heikenwalder, M.; et al. The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Salmonella Diarrhea. PLoS Pathog. 2010, 6, e1001097. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewi, G.; Ramanathan, R.; Kollanoor Johny, A. Cecal Metabolome Profiles of Turkey Poults in Response to Salmonella Heidelberg Challenge with or Without Turkey-Derived Lactobacillus Probiotic and Trans-Cinnamaldehyde. Animals 2025, 15, 2016. https://doi.org/10.3390/ani15142016
Dewi G, Ramanathan R, Kollanoor Johny A. Cecal Metabolome Profiles of Turkey Poults in Response to Salmonella Heidelberg Challenge with or Without Turkey-Derived Lactobacillus Probiotic and Trans-Cinnamaldehyde. Animals. 2025; 15(14):2016. https://doi.org/10.3390/ani15142016
Chicago/Turabian StyleDewi, Grace, Ranjith Ramanathan, and Anup Kollanoor Johny. 2025. "Cecal Metabolome Profiles of Turkey Poults in Response to Salmonella Heidelberg Challenge with or Without Turkey-Derived Lactobacillus Probiotic and Trans-Cinnamaldehyde" Animals 15, no. 14: 2016. https://doi.org/10.3390/ani15142016
APA StyleDewi, G., Ramanathan, R., & Kollanoor Johny, A. (2025). Cecal Metabolome Profiles of Turkey Poults in Response to Salmonella Heidelberg Challenge with or Without Turkey-Derived Lactobacillus Probiotic and Trans-Cinnamaldehyde. Animals, 15(14), 2016. https://doi.org/10.3390/ani15142016




