Home Range and Habitat Selection of Blue-Eared Pheasants Crossoptilon auritum During Breeding Season in Mountains of Southwest China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Satellite-Tracking Dataset
2.3. Vegetation Survey
2.4. Data Analysis
2.4.1. Home Range Estimation
2.4.2. Habitat and Resource Selection Functions
2.4.3. Behavioral States and Activity Rhythms
3. Results
3.1. Home Range Dynamics
3.2. Habitat Selection During the Breeding Season
3.2.1. Habitat Characteristics
3.2.2. Ecological Drivers of Habitat Use
3.2.3. Resource Selection Model
3.3. Female Activity Patterns
3.3.1. Movement Metrics
3.3.2. Behavioral States Analysis via HMM
4. Discussion
4.1. Home Range
4.2. Habitat Selection
4.3. Activity Rhythms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coulson, G.; Cripps, J.K.; Wilson, M.E. Hopping down the main street: Eastern grey kangaroos at home in an urban matrix. Animals 2014, 4, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Andreychev, A.V.; Zhalilov, A.B.; Kuznetsov, V.A. The state of local steepe woodchuck (Marmota bobak) populations in the Republic of Mordovia. Zool. Zhurnal 2015, 94, 723–730. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, X.R.; Pu, G.H.; Yin, H.; Fu, L.; Huang, Y.; Bai, W.; Wang, B.; Zhang, J.; Zhou, C. Occupancy Pattern and Influencing Factors of Tree Representative Forest Pheasants. J. China West Norm. Univ. (Nat. Sci.) 2024, 1–11. Available online: http://kns.cnki.net/kcms/detail/51.1699.N.20240408.1738.004.html (accessed on 23 April 2024).
- Huangfu, B. The Development of Behavioral Research on Crossptilon Auritum. Feed Rev. 2020, 4, 35–38. [Google Scholar]
- Xu, Y.; Guo, L. Research and Prospect on the Discovery and Protection of Key Threatened Birds in Shaanxi Province. J. Green Sci. Technol. 2020, 20, 58–60. [Google Scholar] [CrossRef]
- Yang, N.; Ma, D.Y.; Zhong, X.; Yang, K.; Zhou, Z.; Zhou, H.; Zhou, C.; Wang, B. Habitat suitability assessment of Blue Eared-Pheasant based on MaxEnt Modeling in Wanglang National Nature Reserve, Sichuan Province. Acta Ecol. Sin. 2020, 40, 7064–7072. [Google Scholar]
- Li, H.Q.; Niu, C.H.; Han, P.S.; Liu, X.; Fu, Y. Study on the Suitable Habitat of Western Population of Brown-Eared Pheasent Crossoptilon mantchuricum Based on Max Model. J. Ecol. Rural Environ. 2020, 36, 499–504. [Google Scholar] [CrossRef]
- Ming, F. Study of Bewick’s Swan Cygnus columbianus bewickii Migration Connectivity Based on Molecular Markers and Satellite Tracking. Ph.D. Dissertation, Nanchang University, Nanchang, China, 2023. [Google Scholar] [CrossRef]
- Wang, J.X. Study on Seasonal Home range and Habitat Selection of Wild Boar in Southern Lesser Khingan Mountains Based on GPS Tracking Technology. Ph.D. Dissertation, Northeast Forestry University, Harbin, China, 2021. [Google Scholar] [CrossRef]
- Lu, B.; Li, K.; Wang, C.; Li, S. The application and outlook of wildlife tracking using sensor-based tags in China. Biodivers. Sci. 2024, 32, 173–195. [Google Scholar] [CrossRef]
- Zheng, S.W.; Liao, Y.F. Research on the Habitat, Activities, Diet, and Reproduction of Blue-eared Pheasant. Curr. Zool. 1983, 1, 71–85. [Google Scholar]
- Li, G.Y.; Zhang, R.Y.; Liu, C.Y.; Zhang, Q.M.; Hu, T.Q.; Wang, C.H. Preliminary Observation of Blue-eared Pheasant in Wanglang Nature Reserve, Pingwu, Sichuan. Sichuan J. Zool. 1985, 1, 7–9+33. [Google Scholar]
- Shi, D.; Yang, W.; Jiang, S.W.; Yuan, Z.; Lei, X. Preliminary Research on Insects Biodiversity in Wanglang National Nature Reserve. Sichuan J. Zool. 2009, 28, 691–695+699. [Google Scholar]
- Lin, L.; Wang, W.H.; Ran, M.Y.; Geng, S.H.; Wu, W.Q. Evaluation of Giant Panda Habitat Suitability in Wanglang National Nature Reserve. J. Southwest Univ. Sci. Technol. 2021, 36, 57–62. [Google Scholar]
- Li, C.; Li, J.; Liang, C.P.; Zhao, L.; Wang, D.; Jiang, J. The relationship between community structure of ground-dwelling vertebrates and habitat types in the Wanglang Nature Reserve. Acta Ecol. Sin. 2017, 37, 4247–4257. [Google Scholar]
- Lai, Y.D. Study on Participatory Community Grazing Management in Wanglang National Nature Reserve, Sichuan Province. Ph.D. Dissertation, Sichuan Academy of Social Science, Chengdu, China, 2023. [Google Scholar] [CrossRef]
- Zheng, G.M. Pheasants in China; Higher Education Press: Beijing, China, 2015; p. 435. [Google Scholar]
- Shang, X.T.; Fan, F.; Shen, X.L.; Zheng, Y.; Zhao, L.J.; Sun, H.-O.; Li, S.; Zhang, L. Free-ranging livestock reduces the habitat suitability of subalpine pheasants during breeding season in Southwest China. Conserv. Sci. Pract. 2024, 6, e13104. [Google Scholar] [CrossRef]
- Li, V.B.; Pimm, S.; Li, S.; Zhao, L.; Luo, C. Free-ranging livestock threaten the long-term survival of giant pandas. Biol. Conserv. 2017, 216, 18–25. [Google Scholar] [CrossRef]
- Liang, W.; Zheng, G.M.; Ding, C.Q. Habitat use by golden pheasants (Chrysolophus pictus) based on radio-tracking locations. Curr. Zool. 2003, 49, 179–184. [Google Scholar]
- Zhen, W.; Wang, W.; Liu, X.Y.; Du, G.; Zhao, F.D. Field Investigation of Crossoptilon mantchuricum in Xiaowutai Mountain Based on Infrared-Triggered Camera and Artificial Trancect. J. Green Sci. Technol. 2022, 24, 58–60. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, X.Y.; Zhao, S.S.; Song, X.; Guo, R.; Xu, A.C. Activity rhythm and home range of released sika deer (Cervus Nippon kopschi). Acta Theriol. Sin. 2023, 43, 501–512. [Google Scholar] [CrossRef]
- Jin, G.Y.; Yin, D.D.; Liu, K.F.; Yao, Y. Study on the Selection of Winter Habitat of Martes zibellina in Greater Khingan Mountains. J. Anhui Agric. Sci. 2016, 44, 7–10. [Google Scholar] [CrossRef]
- Jin, G.Y. Comparison of Wintering Habitat Selection and Habitat Suitability of Sable Between the Changbai Mountains and the Greater Khingan Mountains in Northeastern China. Ph.D. Dissertation, Northeast Forestry University, Harbin, China, 2017. [Google Scholar]
- Wu, Y.Q.; Liu, N.F. Diurnal Behavior of Blue-eared Pheasant in Winter in Zecha, Gansu. Chin. J. Zool. 2010, 45, 67–71. [Google Scholar] [CrossRef]
- Shao, X.; Lu, Q.; Xiong, M.; Bu, H.; Shi, X.; Wang, D.; Zhao, J.; Li, S.; Yao, M. Prey partitioning and livestock consumption in the world’s richest large carnivore assemblage. Curr. Biol. 2021, 31, 4887–4897. [Google Scholar] [CrossRef] [PubMed]
- Tian, C. Study on Spatial-Temporal Distribution and Factors of Wildlife in Habitat of Giant Pandas. Ph.D. Dissertation, Beijing Forestry University, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Pheasants Forever Inc. Pheasant Facts[DB/OL]. 2024. Available online: https://www.pheasantsforever.org/Habitat/Pheasant-Facts.aspx (accessed on 23 May 2024).
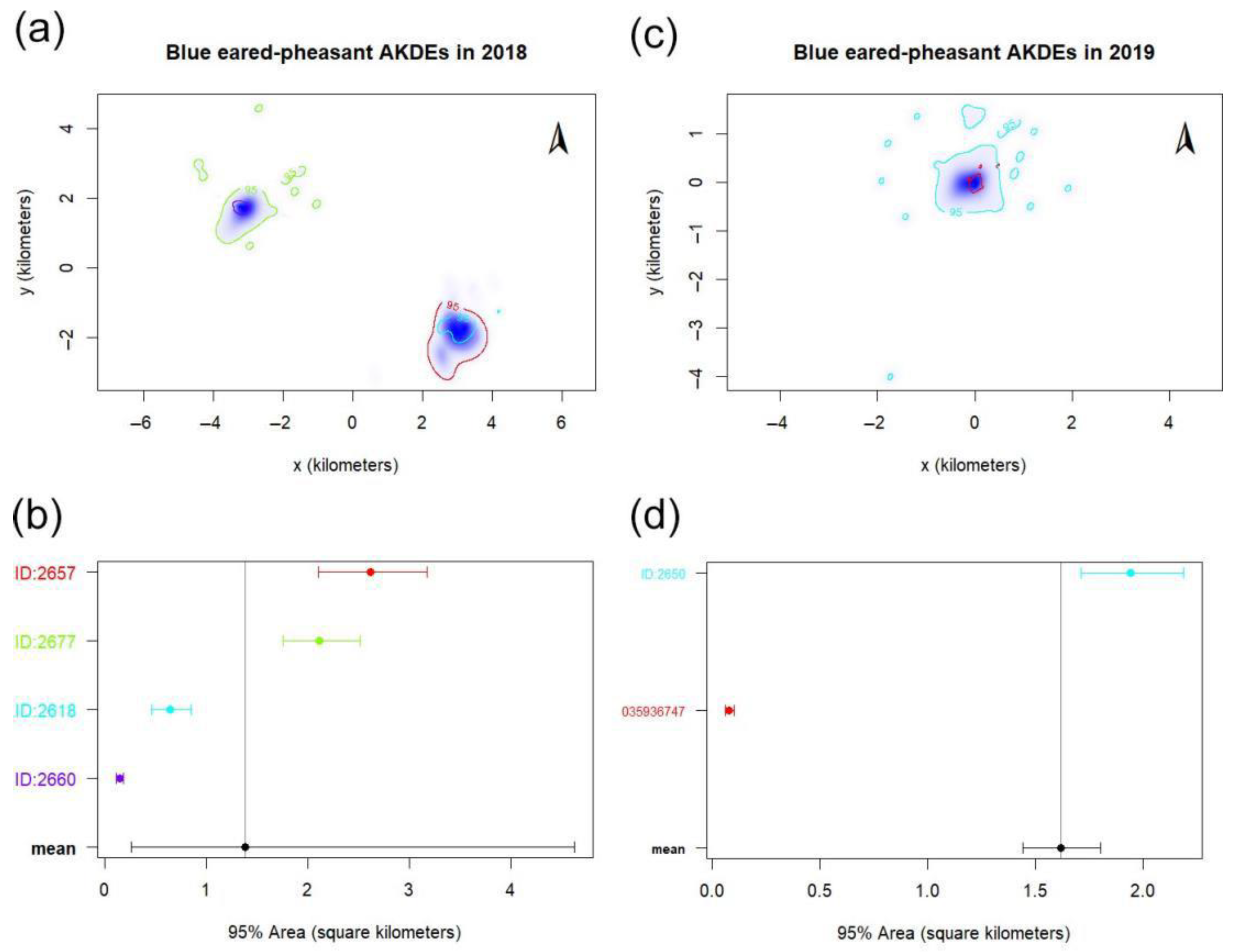
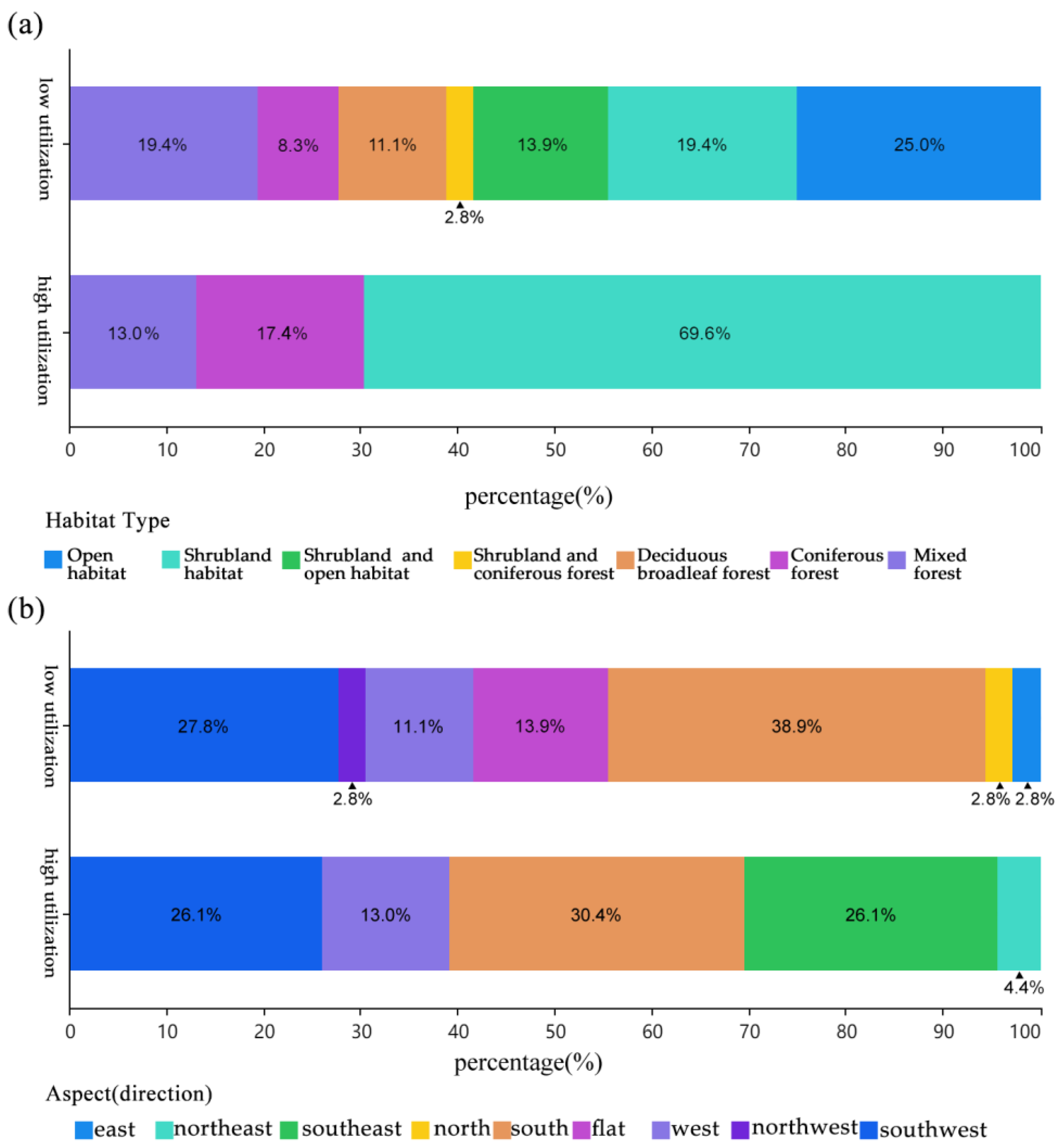
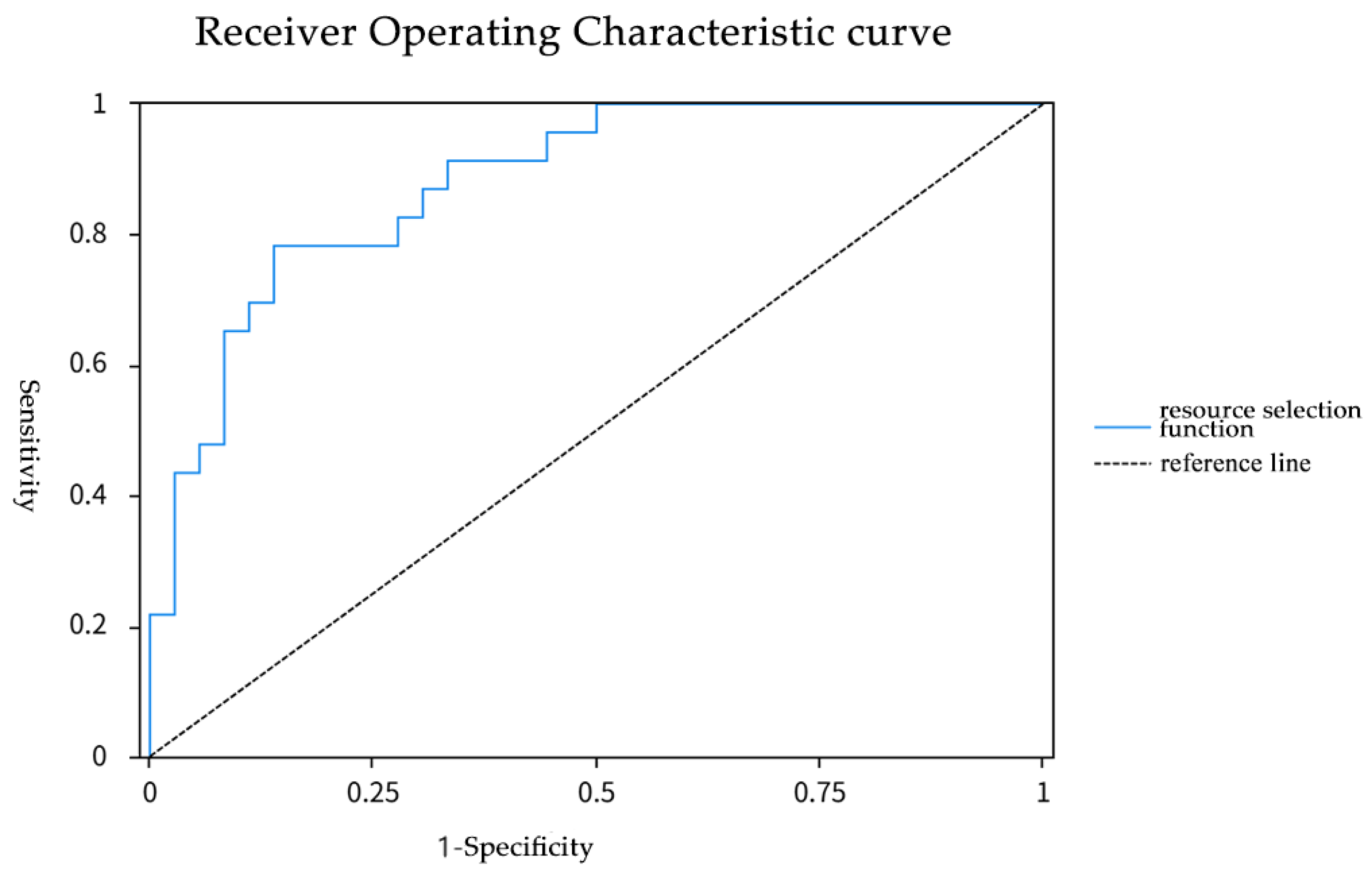
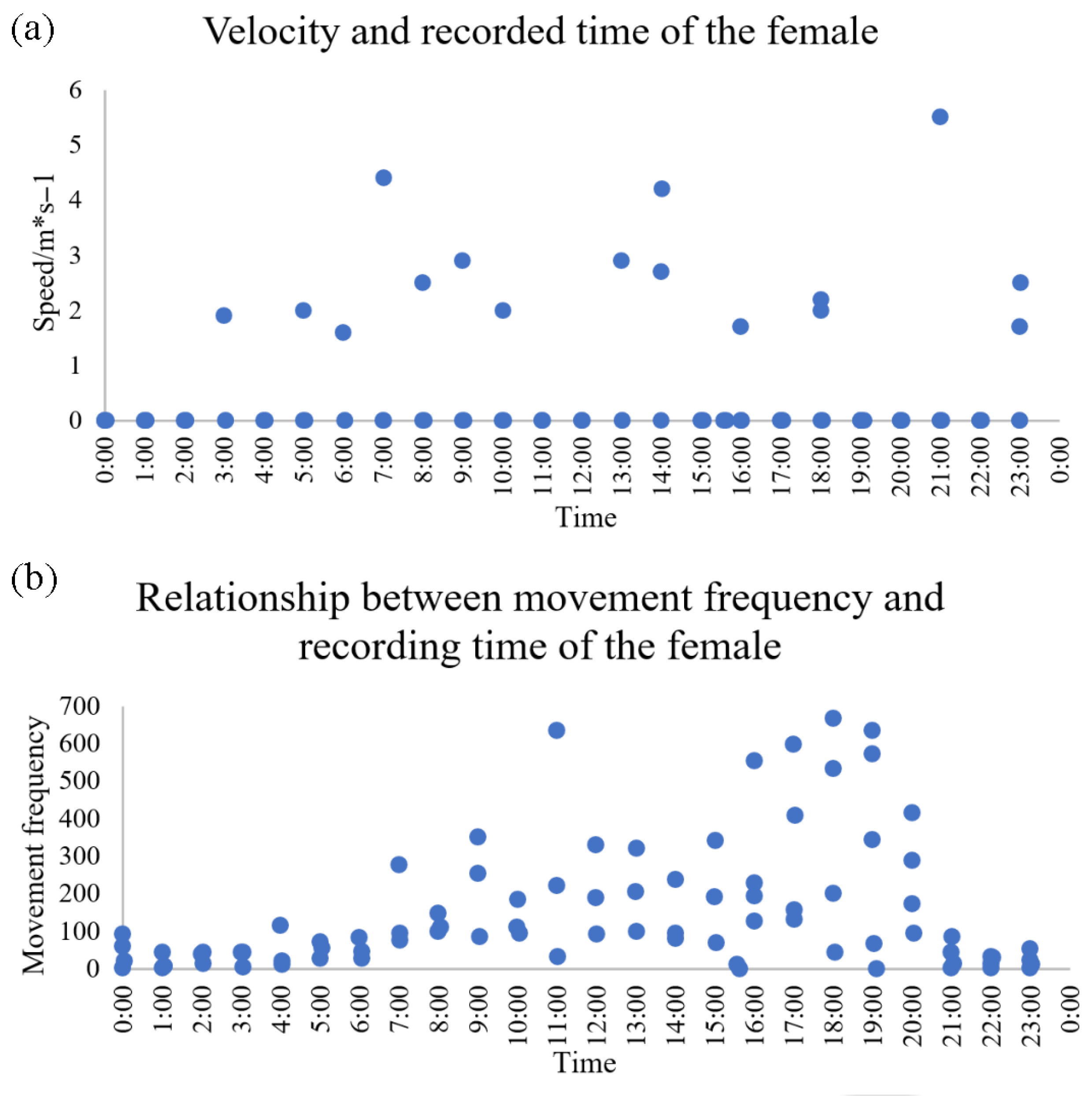

| Area | Sex | ID | Date of Tracking | Effective Tracking Time (Days) | Home Range Distribution Probability | Home Range Area (HRT 2 2.0)/hm2 | Home Range Area (ctmm 2)/hm2 |
|---|---|---|---|---|---|---|---|
| Muyangchang | M 1 | 2618 3 | 9 April 2018–20 April 2018 | 12 | 50% | 11.42 | 67.34 |
| 90% | 49.32 | ||||||
| 95% | 68.35 | ||||||
| Jincaopo | M | 2660 | 22 April 2018–23 May 2018 | 32 | 50% | 2.52 | 14.38 |
| 90% | 9.82 | ||||||
| 95% | 14.59 | ||||||
| Muyangchang | M | 2657 | 18 May 2018–13 August 2018 | 88 | 50% | 46.17 | 258.19 |
| 90% | 171.41 | ||||||
| 95% | 253.33 | ||||||
| Jincaopo | M | 2677 | 25 May 2018–5 July 2018 | 42 | 50% | 25.98 | 212.05 |
| 90% | 124.98 | ||||||
| 95% | 241.75 | ||||||
| Muyangchang | M | 2650 3 | 18 May 2019–14 August 2019 | 89 | 50% | 23.54 | 195.22 |
| 90% | 97.54 | ||||||
| 95% | 213.49 | ||||||
| Muyangchang | F 1 | IMEI:8613 59035936 747SN:HQP830 | 12 May 2019–15 May 2019 | 4 | 50% | 1.18 | 7.87 |
| 90% | 7.00 | ||||||
| 95% | 10.01 |
| ID (Sex 3, Year) | 2618 (M, 2018) | 2660 (M, 2018) | 2657 (M, 2018) | 2677 (M, 2018) | 2650 2 (Mx, 2019) |
|---|---|---|---|---|---|
| 2618 (M, 2018) | / | 0 | 0.49 | 0 | 0.54 |
| 2660 (M, 2018) | 0 | / | 0 | 0.25 | 0 |
| 2657 (M, 2018) | 64.64 | 0 | / | 0 | 0.64 |
| 2677 (M, 2018) | 0 | 14.58 | 0 | / | 0 |
| 2650 2 (M, 2019) | 65.19 | 0 | 148.66 | 0 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Shang, X.; Fan, F.; Zheng, Y.; Zhao, L.; Li, S.; Liu, Y.; Zhang, L. Home Range and Habitat Selection of Blue-Eared Pheasants Crossoptilon auritum During Breeding Season in Mountains of Southwest China. Animals 2025, 15, 2015. https://doi.org/10.3390/ani15142015
Peng J, Shang X, Fan F, Zheng Y, Zhao L, Li S, Liu Y, Zhang L. Home Range and Habitat Selection of Blue-Eared Pheasants Crossoptilon auritum During Breeding Season in Mountains of Southwest China. Animals. 2025; 15(14):2015. https://doi.org/10.3390/ani15142015
Chicago/Turabian StylePeng, Jinglin, Xiaotong Shang, Fan Fan, Yong Zheng, Lianjun Zhao, Sheng Li, Yang Liu, and Li Zhang. 2025. "Home Range and Habitat Selection of Blue-Eared Pheasants Crossoptilon auritum During Breeding Season in Mountains of Southwest China" Animals 15, no. 14: 2015. https://doi.org/10.3390/ani15142015
APA StylePeng, J., Shang, X., Fan, F., Zheng, Y., Zhao, L., Li, S., Liu, Y., & Zhang, L. (2025). Home Range and Habitat Selection of Blue-Eared Pheasants Crossoptilon auritum During Breeding Season in Mountains of Southwest China. Animals, 15(14), 2015. https://doi.org/10.3390/ani15142015







