Simple Summary
Atrial fibrillation (AF) in horses reduces exercise capacity due to atrial contractile dysfunction, impaired ventricular systolic function, disproportionate ventricular rate response and reduced cardiac output. Atrial dysfunction can persist even after treatment and is considered a risk factor for recurrence. Ambulatory acoustic cardiography (Audicor® (Beaverton, OR, USA)) provides qualitative and quantitative information on hemodynamics and cardiac mechanical function and may aid in the clinical diagnosis of impaired heart function after AF treatment. This study aimed to evaluate the use of an acoustic cardiography monitor to assess cardiac mechanical function and its benefits as an adjunct to echocardiography in horses with AF before and after successful cardioversion to sinus rhythm. Audicor® snapshot analyses provided additional information on left ventricular systolic function and reflected changes seen in echocardiography. However, the Audicor® device did not seem clinically useful as a tool to directly assess left atrial mechanical function after cardioversion to sinus rhythm.
Abstract
Left atrial mechanical dysfunction is common in horses following the treatment of atrial fibrillation (AF). This study aimed to evaluate the use of an acoustic cardiography monitor (Audicor®) in quantifying cardiac mechanical and hemodynamic function in horses with AF before and after treatment and to correlate these findings with echocardiographic measures. Twenty-eight horses with AF and successful transvenous electrical cardioversion were included. Audicor® recordings with concomitant echocardiographic examinations were performed one day before, one day after, and two to seven days after cardioversion. Key variables measured by Audicor® included electromechanical activating time (EMAT), heart rate-corrected EMATc, left ventricular systolic time (LVST), heart rate-corrected LVSTc, systolic dysfunction index (SDI), and intensity and persistence of the third and fourth heart sound (S3, S4). A repeated-measures ANOVA with Tukey’s test was used to compare these variables over time, and linear regression and Bland–Altman analyses were applied to assess associations with echocardiographic findings. Following conversion to sinus rhythm, there was a significant decrease in EMATc and LVSTc (p < 0.0001) and a significant increase in LVST (p = 0.0001), indicating improved ventricular systolic function, with strong agreement between Audicor® snapshot and echocardiographic measures. However, S4 quantification did not show clinical value for assessing left atrial function after conversion.
1. Introduction
Atrial fibrillation (AF) in horses reduces exercise capacity due to atrial contractile dysfunction, decreased ventricular preload and systolic function, disproportionate ventricular rate response, and irregular heart rhythm, resulting in decreased cardiac output at maximum exercise [1,2,3,4]. Echocardiography at rest reveals atrial and ventricular dysfunction, which generally improves 12 to 24 h after conversion of AF to sinus rhythm [3,5,6,7,8]. However, depending on the duration of AF, left atrial (LA) mechanical dysfunction, also termed “LA stunning”, may persist up to seven weeks after conversion, corresponding to AF-induced atrial remodeling [5,9,10,11,12,13,14]. LA stunning is one of several factors associated with the high AF recurrence (rAF) rate of 15–50% after successful cardioversion [15,16,17,18,19].
In a routine clinical setting, echocardiography remains the only established method for assessing ventricular and atrial size and function in horses, including two-dimensional (2DE) and motion-mode (M-mode) echocardiography, tissue Doppler imaging (TDI), and 2D speckle tracking (2DST) imaging [6,9,10,11,17,20,21,22,23,24,25,26,27,28]. Ambulatory acoustic cardiography (Audicor®) is a diagnostic adjunctive to echocardiography in human cardiac patients, offering the advantages of being low in cost, operator-independent, time-efficient, and easy to interpret [29,30]. Several studies in human medicine have confirmed its correlation with echocardiographic indices of cardiac function [29,30,31,32]. Zuber et al. [29] even showed that Audicor® recordings, in particular electro-mechanical activation time (EMAT), were more reproducible in pacemaker-implanted human patients than echocardiographic measurements. Furthermore, EMAT was also an early detector of electromechanical dysfunction before changes in left ventricular ejection fraction (LVEF) became apparent in echocardiography [33]. The Audicor® recording consists of a single-lead surface ECG coupled with a phonocardiogram, allowing quantification of diastolic heart sounds and systolic time intervals [34]. In people, its application ranges from detecting left ventricular (LV) dysfunction [32,34,35,36] and predicting rAF [37,38] to monitoring therapeutic effects [38]. An accentuated third heart sound (S3), an indicator for diastolic function, is considered a risk factor for rAF in humans after successful cardioversion [37]. In contrast to people, both diastolic heart sounds are considered physiological in horses, with S3 being linked to the end of rapid ventricular filling and the fourth heart sound (S4) being linked to atrial contraction [39]. However, heart disease in horses can also cause accentuation or attenuation of diastolic heart sounds, rendering their quantification potentially useful. The feasibility and repeatability of Audicor® analyses in healthy horses as a non-invasive, in-field diagnostic tool to measure cardiac mechanical and hemodynamic function have been previously established [40]. However, the benefits of Audicor® in a clinical setting in horses with heart disease are unclear to date.
This study aimed to examine the use of Audicor® to quantify cardiac mechanical and hemodynamic function in horses with AF before and after successful transvenous electrical cardioversion (TVEC). The objective was to evaluate its use in a clinical setting and its possible benefits as an adjunctive to echocardiography. Specifically, the study investigated whether Audicor® can detect and quantify atrial mechanical dysfunction, impaired ventricular function, as well as recovery thereof after successful restoration of normal sinus rhythm (NSR). To answer these questions, Audicor® recordings and concomitant echocardiographic recordings were compared to evaluate LA and LV function. It was hypothesized that S4 would not be detectable by Audicor® during AF and that the power of S4 would progressively increase upon subsequent follow-up examinations after restoration of NSR, indicating recovery of LA mechanical function. Furthermore, it was postulated that the power of S4 would correlate with echocardiographic variables of LA mechanical function. Lastly, it was hypothesized that Audicor® variables of LV function would improve after cardioversion, mirroring echocardiographic variables of LV function.
2. Materials and Methods
2.1. Study Design
The study was designed as a multicenter, prospective descriptive study.
2.2. Study Sample
Thirty-three horses suffering from AF and successfully treated with TVEC at the Vetsuisse Faculty Zurich (n = 4) and at the Faculty of Veterinary Medicine Ghent University (n = 29) were recruited. Five horses were excluded due to moderate valvular insufficiencies to exclude hemodynamic consequences of valvular regurgitation as confounding factors. The final study sample consisted of 28 horses (24 Warmbloods, 2 Thoroughbreds, 2 Standardbreds), 6 mares, and 22 geldings, ranging from 3 to 16 years of age (10 ± 4 y [mean ± SD] and a body weight of 584 ± 70 kg. The horses showed trace to mild valvular insufficiencies (5 trace, 11 trivial, and 4 mild aortic insufficiencies; 12 trivial and 11 mild tricuspid insufficiencies; 4 trace, 11 trivial, and 8 mild mitral insufficiencies; 3 trace, 7 trivial, and 2 mild pulmonary insufficiencies) with normal atrial and ventricular dimensions. The use of horses spanned from pleasure to sports horses at different levels of showjumping, dressage, eventing, and racing. All horses stayed in NSR for at least 24 h after conversion. For the 2–7-day follow-up period, only data from horses that remained in NSR throughout this time period were included in the analysis, whereas data from horses that reverted to AF before this time point were excluded.
2.3. Study Protocol
All horses underwent a physical examination (demeanor and attitude, body condition score, skin and hair coat, jugular veins, peripheral pulse rate and quality, cardiac auscultation (heart rate (HR), rhythm, murmurs), respiratory rate and lung sounds, mucous membrane color and capillary refill time, gastrointestinal sounds, and rectal temperature), echocardiographic examination, and Audicor® examination at three time points: (1) AF day −1, one day before cardioversion; (2) NSR day 1, one day after cardioversion; and (3) NSR day ≥ 2, two to seven days after cardioversion. Due to the multicenter nature of the study, the third examination was performed at different time points: At the Vetsuisse Faculty Zurich, it was conducted 2 days after cardioversion, while at the Faculty of Veterinary Medicine Ghent University, the time point varied between 3 and 7 days after cardioversion.
2.4. Echocardiography
All studies were conducted in standing, unsedated, restrained horses by multiple experienced operators in Zurich and Ghent. The echocardiograms were performed before, during, or after each Audicor® overnight recording (time between beginning of echocardiogram and beginning of Audicor® recording was −26 to +28 h, median 4 h, quartiles 2 to 7 h). At both centers, transthoracic echocardiography was performed with a GE Vivid E95 echocardiograph with a 4Vc-D probe operated in 2D mode and set at a frequency of 1.4/2.8 MHz (GE Healthcare, Freiburg, Germany).
A single-lead base-apex ECG was recorded simultaneously for timing purposes. Recordings were stored as still images or cine-loops in digital raw format for offline analysis (EchoPac v204, GE Healthcare, Freiburg, Germany). Offline measurements were performed by a single operator (MS) after extensive training. Grading of valvular regurgitation, where present, was confirmed by an experienced echocardiographer (CS). Where possible, three representative non-consecutive or consecutive cycles were recorded, measured, and subsequently averaged for each variable. However, in some instances, fewer than three analyzable cardiac cycles were available due to technical limitations during image acquisition, such as suboptimal image quality. The number of measurements based on fewer than three cardiac cycles is summarized in Supplementary Table S1. Cycles immediately after very long or very short pauses during AF, after sinus pauses, or after second-degree atrioventricular (AV) blocks were excluded from the analyses. Instantaneous HR was calculated based on the RR interval (ms) preceding the respective measurement:
HR = 60,000/RR.
Routine transthoracic 2DE, M-mode, pulse wave TDI (PW TDI) and color TDI (cTDI) echocardiography were performed to assess cardiac structures, valvular competence, chamber dimensions, great vessel dimensions, and LV systolic and diastolic function, by use of standard right and left parasternal long-axis and right parasternal short-axis views [9,20,21,22,25,41,42,43]. The main focus was put on the assessment of LA size and mechanical function using the methods previously described [9,20,22]. Echocardiographic variables and indices used in this study are listed in detail in Table S2. They included the following: maximum left atrial diameters (LADmax and LADllx-max), maximum left atrial areas (LAAmax and LAsxAmax), left atrial active fractional area change (active LA FAC), left atrial reservoir index (LA RI), ratio of active-to-total left atrial area change (active/total LA AC), late-diastolic LV wall motion velocity at the time of atrial contraction (Am), early-diastolic LV wall motion velocity during the phase of rapid ventricular filling (Em), ratio of Em-to-Am (Em/Am), left ventricular diameter at end-diastole (LVIDd), left ventricular volume at end-diastole (LVIVd), relative LV wall thickness at end-diastole (RWTd), left ventricular fractional shortening (LV FS), left ventricular ejection fraction (LV EF), stroke volume (SV), cardiac output (CO), pre-ejection period (PEPm), ejection time (ETm), index of myocardial performance (IMPm), and wall motion velocity during LV ejection (Sm). Measurements of chamber dimensions were corrected for differences in body weight (BWT) according to the principles of allometric scaling [41,42,44,45]. Specifically, the measurements of LA and LV dimensions were normalized to a BWT of 500 kg using the following equations:
Chamber diameter [500] (cm) = Measured chamber diameter/BWT1/3 × 5001/3
Chamber area [500] (cm2) = Measured chamber area/BWT2/3 × 5002/3
Chamber volume [500] (mL) = Measured chamber volume/BWT × 500
To enable direct comparison with heart rate-corrected Audicor® variables, PEPm and ETm were corrected for heart rate, with RR being the electrocardiographic RR interval of the corresponding cardiac cycle, using the following equations:
PEPm-c (%) = PEPm/RR × 100
ETm-c (%) = ETm/RR × 100
2.5. Audicor® Data Recordings
Data recordings were obtained by multiple operators at the Vetsuisse Faculty, University of Zurich, and at the Faculty of Veterinary Medicine, Ghent University, with the Audicor® Dx Patch device (ApoDx Technologies, Taipei, Taiwan) using a previously described method [40]. In brief, the Dx Patch device was placed into a padded holding device and attached to the left side of the thorax using a surcingle. It was positioned between the 5th and 7th intercostal space, in a vertical orientation. Dry ECG electrodes with an Ag/AgCl core (Cognionics Inc., San Diego, CA, USA), wetting of the fur before placement, and tightening of the surcingle ensured a good electrical contact. Connection of the device to a laptop computer was obtained via a proprietary Wi-Fi-type low-power wireless connection. After starting the recording, a good quality, stable ECG and phonocardiogram were visually confirmed on a 10 s snapshot recording. After confirmation of sufficient quality, the wireless connection was discontinued, and the device continued recording to its internal data memory. Three continuous overnight recordings (AF day −1, NSR day 1, NSR day ≥ 2) were conducted in each horse in a standardized way. During the overnight recording, the horses were left undisturbed in the clinic stables. Procedures such as clinical examinations, feeding, and cleaning were continued as usual. After the conclusion of recordings, the raw data files were transferred via USB connection from the Dx Patch device onto a laptop computer.
2.6. Audicor® Data Processing and Analysis
A detailed explanation of the diagnostic algorithm used to process and analyze the ECG data in relation to the heart sound data can be found elsewhere [34,40]. In short, the software algorithm incorporates wavelet-based signal processing techniques and time-frequency analysis for the evaluation of raw data [34] (Figure S1). According to information provided by the manufacturer (Inovise Medical Inc. (Beaverton, OR, USA), personal communication), comparison of the filtered signals from dogs, pigs, horses, and other animals to those of humans does not show any fundamental differences concerning their frequency content and the placement of the relevant fiducial points. However, no experimental data are currently available to validate these cross-species comparisons in horses specifically. Importantly, S4 is not detected or quantified when it occurs in isolation and lacks association with a QRS-T complex (e.g., with a second-degree AV block or in the context of atrial arrhythmia with AV blocks), representing a limitation of the current algorithm.
The data processing and analyses for this study were performed by a single operator (MS) using proprietary analysis software (CA300, Inovise Medical, Inc.) Manual page-by-page verification and correction of the ECG was performed using the scanning function of the program. This step was necessary because the automated detection is based on a human complex morphology algorithm, and it showed a tendency to misinterpret the equine T and P wave as a QRS complex. Artifact detection was performed by the automated proprietary algorithm and by visual control.
For each overnight recording, five consecutive, good-quality, analyzable 10 s snapshot analyses were generated. A snapshot refers to a continuous 10 s segment of the recording during which all heartbeats are automatically detected and processed by the proprietary software. Snapshots were selected based on ECG signal quality within the time frame of 8:00 p.m. and 9:00 p.m. If insufficient diagnostic snapshots were available in this time frame due to suboptimal recording quality, the next best five snapshots from the recording were selected. The following variables were generated for each snapshot analysis: HR, EMAT, heart rate-corrected EMAT (EMATc), left ventricular systolic time (LVST), heart rate-corrected LVST (LVSTc), power (as a function of intensity and persistence) of S3, power of S4, and systolic dysfunction index (SDI, as a function of QRS duration, QT interval, EMATc, and S3) [40] (Table S3). Power is calculated by the software on a relative dimensionless scale from 1 to 10, reflecting both signal intensity and persistence. Importantly, values below 5 for S3 and S4 are considered artefactual and do not correspond to true heart sounds; this threshold was set by the software to ensure optimal accuracy compared to visual overread by phonocardiography experts and to define the actual presence of heart sounds in people (Inovise Medical Inc., personal communication) [35]. It should be noted that these detection algorithms and thresholds were originally developed and validated for human cardiac data and were applied here without species-specific adaptation. To enhance measurement reproducibility, for EMAT, EMATc, LVST, LVSTc, and SDI, the median value across the five selected snapshots was used for further analyses. This approach was chosen based on pilot data from a repeatability study (Table S4) [46,47], which demonstrated improved reproducibility compared to relying on a single snapshot. For the power of S3 and S4, where presence may vary across snapshots, the maximum value across five consecutive snapshots was selected for further analyses.
Each overnight recording was also run through the automated acoustic cardiography analysis, which analyzed a fixed time window from 10:00 pm to 04:00 am (maximum analysis period: 6 h) and required at least 4 h of artifact-free ECG and phonocardiogram recordings to generate the so called “cardiac findings report” [40] (Table S3). The variables reported as “cardiac findings” were averaged by the software over the whole analyzed time window and included the following: HR, QRS duration (QRS), heart rate-corrected QT interval (QTc), EMAT, EMATc, LVST, and LVSTc. Additionally, “cardiac findings” also included the percentage of 10 sec segments in the analysis period, in which power of S3 and S4 was ≥5 (on a scale of 0–10), EMAT was ≥15%, and SDI was ≥5 and ≥7.5 (on a scale of 0–10). As with the snapshots, for S3 and S4, the ≥5 threshold is set to provide optimal accuracy compared to visual overread by phonocardiography experts and to define the actual presence of heart sounds in people (Inovise Medical Inc., personal communication) [35]. In case of EMAT (≥15%) and SDI (≥5 and ≥7.5), these cut-off values indicate LV systolic dysfunction in people [31,48,49,50]. SDI ≥ 5 correlates to an ejection fraction (EF) <50% and SDI ≥ 7.5 correlates to an EF < 35% and high left ventricular end-diastolic pressure (LVEDP) [51]. Hence, the respective cut-off values used by the analysis software were those established by the manufacturer and approved by the FDA to guide diagnosis in human patients (Inovise Medical Inc., personal communication); owing to the proprietary system algorithms, they could not be adapted for the purpose of this study.
Table 1 provides an overview of the Audicor® variables and their corresponding echocardiographic measurements, including a brief rationale for their comparison. Further details can be found elsewhere [40].

Table 1.
Comparative table of Audicor® variables and echocardiographic variables.
2.7. Statistical Analysis
All statistical and graphical analyses were performed by commercially available software: Microsoft Excel for Microsoft 365 (Microsoft Corporation, Redmond, WA, USA), GraphPad Prism for Windows, version 9.0.0 (GraphPad Software, San Diego, CA, USA), SigmaPlot for Windows, version 12 (Systat Software Inc., San Jose, CA, USA), and MedCalc for Windows, version 19.2.1 (MedCalc Software Ltd., Ostend, Belgium). The level of significance was set at p < 0.05.
For time points 2–7 d after conversion, the measurements obtained on different days were compared using a one-way analysis of variance (ANOVA). The results did not show any significant differences between time points (F test, p = 0.073 to 0.965). Therefore, recordings from days 2 to 7 were pooled as one time point for subsequent analyses (NSR day ≥ 2).
To investigate the effect of treatment (TVEC) on echocardiographic measurements of LA and LV size and function and on Audicor® variables, all variables were compared between the three time points (AF day −1, NSR day 1, NSR day ≥ 2) using a repeated-measure ANOVA with Tukey’s multiple comparisons test. For the variables not measurable on AF day −1 (i.e., active LA FAC, active/total LA AC, Am, Em/Am), a paired t-test was performed to detect differences between NSR day 1 and NSR day ≥ 2. Homogeneity of variances was assessed by graphical display of the data, and validity of the normality assumption was confirmed by assessment of normal probability plots of residuals. Summary statistics were provided using mean and standard deviation (SD). The differences between time points were reported as the difference of means (dmeans) and the 95% confidence interval (95% CI) of the difference of means. Bonferroni correction was performed to adjust for the family-wise error rate within sets of multiple echocardiographic variables describing LA and LV size and function and Audicor® variables. To assess the association between snapshot Audicor® variables and echocardiographic measurements, multiple linear regression was performed; repeated measures over time in the same horse were accounted for using dummy variables (effects coding). Appropriateness of the linear model was assessed by graphical display of the data and assessment of normal probability plots of the residuals. Agreement between comparable TDI variables and Audicor® variables was evaluated using Bland–Altman analyses.
3. Results
3.1. Echocardiography
Analyzable echocardiograms were obtained in 22/28 (79%) of horses at time point AF day −1, in 21/28 (75%) of horses at time point NSR day 1, and in 17/28 (61%) of horses at time point NSR day ≥ 2. Non-analyzable echocardiograms were primarily attributable to the multicenter study design, which made consistent optimization of image quality across centers challenging.
Table 2 summarizes the comparison of HR and echocardiographic variables of LA size and function between the three time points. Heart rate significantly decreased; LADllx-max (500), LAAmax (500), active LA FAC, LA RI, active/total LA AC, and Am (measured by cTDI) significantly increased; and Em/Am (measured by cTDI) significantly decreased after conversion. After Bonferroni correction to adjust for the family-wise error rate, only changes in HR, active LA FAC, LA RI, active/total LA AC, and Am (measured by cTDI) remained significant.

Table 2.
Summary statistics for heart rate and echocardiographic variables of left atrial size and function, by time point.
Table 3 summarizes the comparison of echocardiographic variables of LV size and function between the three time points. The following indices were significantly altered after conversion: LVIVd (500) significantly increased at time point NSR day ≥ 2 compared to AF day −1; SV significantly increased at time point NSR day ≥ 2 compared to AF day −1 and NSR day 1; CO significantly decreased on NSR day 1 compared to AF day −1 and significantly increased on NSR day ≥ 2 compared to NSR day 1; PEPm (measured by cTDI) significantly increased; PEPm-c (measured by PW TDI) significantly decreased at time point NSR day ≥ 2 compared to AF day −1; PEPm-c (measured by cTDI) and ETm-c (measured by PW TDI and cTDI) significantly decreased at time points NSR day 1 and NSR day ≥ 2 compared to AF day −1. After Bonferroni correction to adjust for the family-wise error rate, only changes in SV and in PEPm-c and ETm-c measured by PW TDI and cTDI remained significant.

Table 3.
Summary statistics for echocardiographic variables of left ventricular size and function, by time point.
3.2. Audicor®
Audicor® recordings were attempted in all horses at all time points, but data quality was not consistent enough to obtain complete datasets in all horses. Motion artifacts, device failure, insufficient contact of the electrodes, and lack of recorded data in the fixed analysis time window (10:00 p.m.–04:00 a.m.) for “cardiac findings” reports were among the reasons for incomplete datasets.
At time point AF day −1, Audicor® raw data recordings allowed extraction of “snapshots” in 22/28 (79%) and calculation of overnight “cardiac findings” reports in 12/28 (43%) of horses. There were missing data because of device failure in six horses, and there were insufficient data for the overnight analysis because of the length or timing of the recording (i.e., no or insufficient data between 10:00 p.m. and 04:00 a.m.) in the other 10 horses. The mean duration of recordings was 10:24 h (SD 5:15 h, range 0:17–17:25 h). The mean duration of analyzed recordings for the “cardiac findings” reports was 5:51 h (SD 00:31 h, range 4:07–6:00 h).
At time point NSR day 1, Audicor® raw data recordings allowed extraction of “snapshots” in 21/28 (75%) and calculation of overnight “cardiac findings” reports in 14/28 (50%) of horses. There were missing data because of device failure in six horses, and there were insufficient data for the overnight analysis because of the length or timing of the recording (i.e., no or insufficient data between 10:00 pm and 04:00 am) in the other eight horses. The mean duration of recordings was 13:40 h (SD 6:15 h, range 0:14–23:32 h). The mean duration of analyzed recordings for the “cardiac findings” reports was 6:00 h (SD 00:01 h, range 5:58–6:00 h).
At time point NSR day ≥ 2, Audicor® raw data recordings allowed extraction of “snapshots” in 21/28 (75%) and calculation of overnight “cardiac findings” reports in 13/28 (46%) of horses. There were missing data because of device failure in seven horses, and there were insufficient data for the overnight analysis because of the length or timing of the recording (i.e., no or insufficient data between 10:00 p.m. and 04:00 a.m.) in the other eight horses. The mean duration of recordings was 13:31 h (SD 05:37 h, range 01:43–20:00 h). The mean duration of analyzed recordings for the “cardiac findings” reports was 05:58 h (SD 00:06 h, range 05:38–06:00 h).
Table 4 summarizes the comparison of “snapshot” analyses between the three time points. The power of S4 was below 5 in all analyzed horses. Several variables significantly changed after treatment: HR, EMATc, LVSTc significantly decreased; LVST and power of S3 significantly increased. After Bonferroni correction to adjust for the family-wise error rate, all changes except the power of S3 remained significant.

Table 4.
Comparison of Audicor® snapshot variables between the three time points.
Table 5 shows the comparison of “cardiac findings” analyses between the three time points. The power of S4 was below 5 in all analyzed horses, resulting in S4 (≥5) equaling zero. Therefore, it was not included in the statistics. Only LVSTc was significantly altered after treatment; however, after Bonferroni correction to adjust for family-wise error rate, it did not remain significant.

Table 5.
Comparison between Audicor® cardiac findings variables between the three time points.
3.3. Association Between Audicor® and Echocardiography
The associations between Audicor® variables from “snapshot” analyses and echocardiographic indices are summarized in Figure 1, Figure 2 and Figure 3. Linear regression analyses indicated that EMAT and PEPm, EMATc and PEPm-c, and LVSTc and ETm-c were significantly related, with the adjusted R2 ranging between 0.383 and 0.55 for all relationships (Figure 1). No significant relationship was found for LVST and ETm and EMAT/LVST and PEPm/ETm. The mean biases and limits of agreement between corresponding TDI vs. Audicor® variables, expressed as ratios, are shown in Figure 2. Linear regression analyses further indicated that EMAT, EMATc, LVST, LVSTc, and EMAT/LVST were significantly related to LVIVd (500) (adjusted R2 ranging between 0.548 and 0.631 for all relationships) and to SV (adjusted R2 ranging between 0.399 and 0.536 for all relationships) (Figure 3).
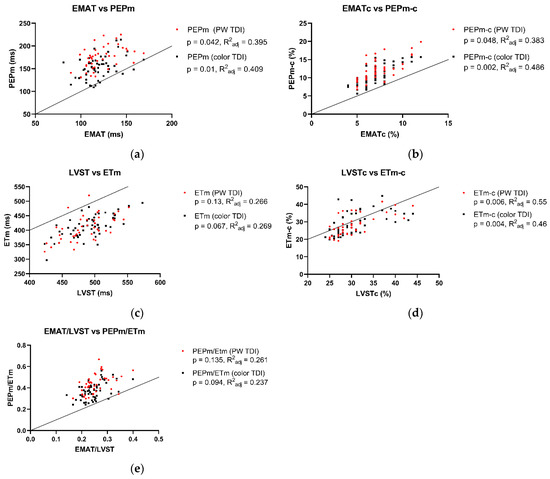
Figure 1.
Association between Audicor® variables from “snapshot” analyses and corresponding tissue Doppler imaging (TDI) variables. The black dots indicate color TDI measurements, and the red dots represent pulsed wave TDI measurements. (a–e): Linear regression analyses of association between Audicor® variables and echocardiographic variables. Solid lines represent lines of identity. P, p value of linear regression statistics; R2adj, adjusted coefficient of determination; PW TDI, pulsed wave TDI; EMAT, electromechanical activation time; EMATc, heart rate-corrected electromechanical activation time; LVST, left ventricular systolic time; LVSTc, heart rate-corrected left ventricular systolic time; EMAT/LVST, ratio of EMAT-to-LVST; PEPm, pre-ejection period; PEPm-c, heart rate-corrected pre-ejection period; ETm, ejection time; ETm-c, heart rate-corrected ejection time; PEPm/ETm, ratio of PEPm-to-ETm. For detailed explanation of echocardiographic indices and Audicor® variables, see Tables S2 and S3.
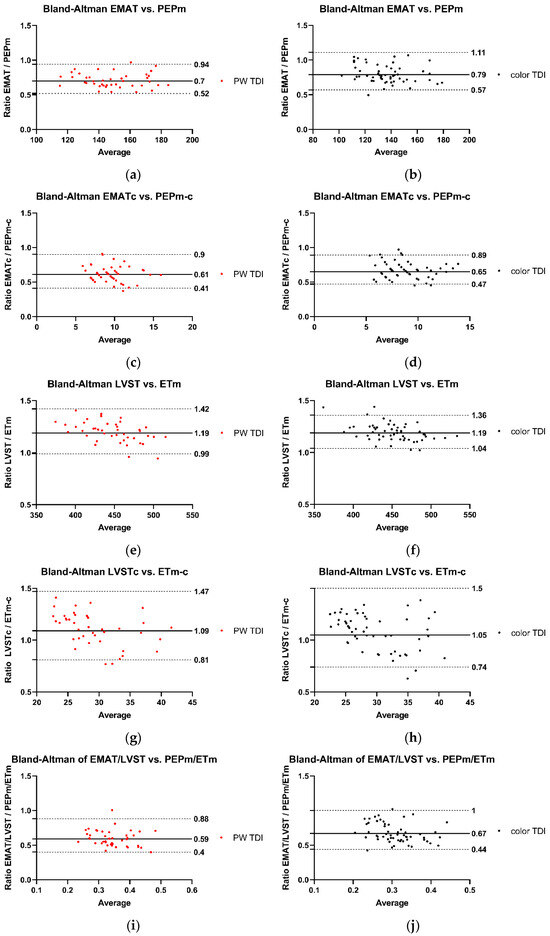
Figure 2.
Agreement between Audicor® variables from “snapshot” analyses and echocardiographic variables. (a–j): Bland–Altman analyses. The solid lines represent the mean bias, the dotted lines illustrate the lower and the upper 95% limit of agreement. The black dots indicate measurements performed with color TDI, and the red dots represent measurements performed with pulsed wave TDI. PW TDI, pulsed wave TDI; EMAT, electromechanical activation time; EMATc, heart rate-corrected electromechanical activation time; LVST, left ventricular systolic time; LVSTc, heart rate-corrected left ventricular systolic time; EMAT/LVST, ratio of EMAT-to-LVST; PEPm, pre-ejection period; PEPm-c, heart rate-corrected pre-ejection period; ETm, ejection time; ETm-c, heart rate-corrected ejection time; PEPm/ETm, ratio of PEPm-to-ETm. For detailed explanation of echocardiographic indices and Audicor® variables, see Tables S2 and S3.
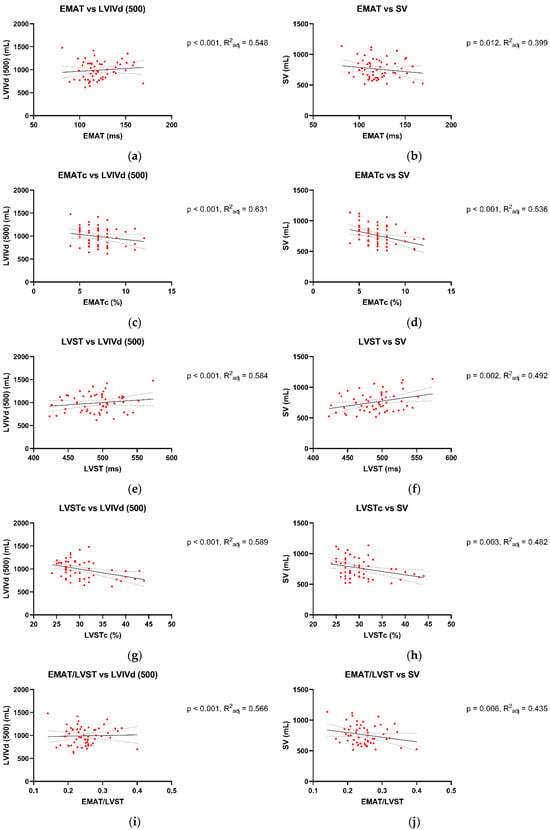
Figure 3.
Association between Audicor® variables from “snapshot” analyses and 2D echocardiographic variables. (a–j): Linear regression analyses of association between Audicor® variables and echocardiographic variables. The solid lines represent the regression line, and the dotted lines illustrate the 95% confidence intervals of the regression lines. P, p value of linear regression statistics; R2adj, coefficient of determination; EMAT, electromechanical activation time; EMATc, heart rate-corrected electromechanical activation time; LVST, left ventricular systolic time; LVSTc, heart rate-corrected left ventricular systolic time; EMAT/LVST, ratio of EMAT-to-LVST; LVIVd, left ventricular volume at end-diastole; SV, stroke volume. For detailed explanation of echocardiographic indices and Audicor® variables, see Tables S2 and S3.
4. Discussion
This is the first study aiming to provide proof of concept for the use of acoustic cardiography to assess LA and LV function in the context of AF in horses.
To compare Audicor® analyses against an established diagnostic method, echocardiographic examinations were performed as a reference to assess LA and LV size and function 1 day before and 1 day and 2–7 days after cardioversion. The echocardiographic studies confirmed previously reported findings of atrial mechanical dysfunction after conversion of AF to NSR and its partial recovery in the days following conversion [9,10,11,13,17,24,52]. Both LA contractile and reservoir function were decreased 1 day after conversion, indicating atrial stunning [9]. Changes in active LA FAC, LA RI, active/total LA AC, and Am (measured by color TDI) indicated an improvement in LA mechanical function from day 1 to 2–7 days after conversion, whilst still remaining below reference values [10,17,41].
In terms of LV function, a significant increase in SV and decrease in HR were observed in the study sample after conversion of AF to NSR, corroborating previous findings of AF’s negative impact on ventricular function and improvement thereof after treatment [6,7,53]. It is likely that the decrease in ventricular performance with AF is concomitant with the atrial mechanical dysfunction, reducing end-diastolic ventricular filling [54], as supported by the lower LVIVd (500) before compared to the days after conversion of AF to NSR. To various extents, an increase in HR related to increased AV nodal conduction is seen in AF at rest, compensating for the reduced SV and maintaining CO [7,13,55]. It is critical to keep in mind that echocardiographic indices of systolic ventricular function are not a direct reflection of contractility, but are impacted by other factors such as preload, afterload, HR, and rhythm [21].
Previous studies have demonstrated changes in systolic time intervals in horses with cardiac disease [25,56,57,58]. In this study, TDI-derived systolic time intervals were not significantly altered after cardioversion. However, rate-corrected PEPm-c and ETm-c decreased significantly after conversion, indicating that lengthening of PEPm and ETm after cardioversion (which was not found to be statistically significant) was disproportional to lengthening of cycle length related to the significant decrease in HR. These rate-corrected systolic time intervals were calculated to enable direct comparison with the respective Audicor® variables. Overall, these findings could further indicate improved LV systolic function after conversion of AF to NSR.
This study demonstrates the feasibility of Audicor® recordings in a clinical setting. However, it was more difficult to acquire analyzable overnight recordings than was expected, given results from a previous experimental study [40]. No significant changes were found in “cardiac findings” variables after conversion. In “snapshot” variables, conversion of AF to NSR was associated with a significant decrease in EMATc and LVSTc as well as a significant increase in LVST. Hence, the “snapshot” analyses proved to be clinically more suitable for use in clinical settings than the analyses that were based on overnight recordings. This could be in part due to the low number of recordings that were sufficient in length and quality to provide the necessary base for the automatic “cardiac findings” analyses.
The EMAT reflects the time required for the LV after electrical activation to generate enough force to close the mitral valve and open the aortic valve. It is influenced by the rate at which sufficient left ventricular pressure develops [34] and has been strongly correlated to the maximum rate of systolic LV pressure rise (LV dP/dtmax): A prolonged EMAT was associated with reduced LV dP/dtmax in people with LV systolic dysfunction [59,60], in both normal sinus rhythm [60] and AF [51]. Furthermore, a link between EMAT and EMATc, respectively, and left ventricular contractility has been proven, as impaired contractility was associated with abnormal EMATc (≥15%) [31,60,61]. Lastly, EMAT has been correlated with LV ejection fraction (LV EF) by echocardiography [32] and angiocardiography [59] and can be used to detect impaired LV systolic function in humans [59,60,62,63]. In the present study, EMATc significantly decreased after conversion, which likely indicates improved ventricular systolic function. This decrease in EMATc can at least partly be explained by the improved atrial function and thus improved LV preload. This effect of AF conversion on EMATc has also been shown in people [38]. However, for this equine study sample, EMATc during AF was still within reference range for horses in NSR [40] and below the human cut-off value (≥15%) for systolic dysfunction [31,48,49,50].
The LVST reflects the time between aortic valve opening and aortic valve closure, hence the time during which the LV is able to produce enough pressure to keep open the aortic valve during systole [59]. It has been related to angiographic LV EF and, thus, systolic function in people and is shortened in patients with LV systolic dysfunction [59]. In this study, LVST significantly increased after conversion of AF to NSR, indicating improved LV systolic function. These changes have also been shown in human patients [38] and are likely linked to improved LV preload because of the decrease in HR and improvement of atrial booster pump function after cardioversion. The LVSTc decreased after conversion, indicating that with decreasing HR, ventricular diastole was disproportionately extended compared to the prolonged LVST. This would also imply the potential for an overall improvement in cardiac function, as a shortened systole-to-diastole ratio improves coronary perfusion. These overall findings confirm the hypothesis that LV function improves after cardioversion.
Both S3 and S4 are considered pathologic in people, and their presence indicates impaired LV function [64]. The S3 is the result of cardiohemic vibrations caused by a sudden deceleration of ventricular inflow in early diastole, while S4 is the result of cardiohemic vibrations caused by a quick deceleration of ventricular inflow initiated by atrial contraction [64]. The use of S3 power as an indicator of LV dysfunction has been extensively investigated in human medicine. A strong correlation between S3 and LVEDP has been observed in patients with LV systolic dysfunction, where those values share a positive linear relationship [59,64]. A previous study in humans showed an increase in both S3 and S4 power with paroxysmal AF [38]. The S3 power has been used as a predictor for AF recurrence after electrical cardioversion [37] and for LV failure [63]. An increase in S4 power has been associated with LV stiffness and increased LVEDP [65], indicating impaired diastolic function [66].
In horses, however, both diastolic heart sounds, S3 and S4, are considered physiologic in most cases [39]. In a previous study, both S3 and S4 were detected using Audicor® in healthy horses, with a prevalence of 0.2–19.7% and 0.1–13.0%, respectively, in overnight recordings [40]. Based on this study, Audicor® quantification of S3 and S4 does not seem to be of clinical value to assess LA and LV function in the context of AF cardioversion. Neither of the diastolic heart sounds showed a significant change after conversion. A more intense S3 during AF, as seen in previous studies [43,67], was not detectable in the present study in horses, and S4 was not detectable above the cut-off of power ≥5 in any recording at any time point. The cut-off of power ≥5 is used in humans to define the actual presence of the heart sounds [35], while its relevance in horses remains unclear, and it is possible that this cut-off is not suitable for horses. Another possible explanation for the lack of S4 in this study sample is the fact that it is associated with atrial contraction [39]. Even though a significant increase in LA mechanical function was detectable in echocardiography, indices associated with LA contraction were still below the reference range after conversion. Therefore, it could be argued that atrial contraction after conversion improved but was still too weak to produce a detectable S4. Furthermore, the missing of S4 in post-conversion recordings could also be explained by the diagnostic algorithm, which neither detects nor quantifies S4 in context with AV blocks. As S4 is most pronounced in horses with slow heart rates and long PQ intervals [43,68,69], this could lead to a reduced number of S4 detected. Finally, placement of the Audicor® device might not have been optimal for quantification of S3 and S4 since it was determined by the placement of the surcingle and, therefore, not optimized for recording of heart sounds.
This study indicates strong agreement between Audicor® variables of systolic function and corresponding echocardiographic variables. Substantial agreement was observed between EMAT and PEPm and between EMATc and PEPm-c. EMAT corresponds to PEPm, but is not identical to it, as EMAT does not contain the isovolumic contraction like PEPm [59]. Similarly, substantial agreement was found between LVSTc and ETm-c. Again, these two values correspond; however, LVSTc is slightly longer, as it contains the isovolumic contraction [36,59]. Lastly, there was also a substantial association between Audicor® variables of systolic function (EMAT, EMATc, LVST, LVSTc, EMAT/LVST) and echocardiographic variables of systolic function (SV) and preload (LVIVd (500)).
In previous studies in people, Audicor® performed well in detecting cardiac function compared to echocardiography [30,32,70]. In some cases, it even outperformed echocardiography in detecting LV systolic and diastolic dysfunction [32,38,60]: for example, in a study investigating LV function in human patients suffering from paroxysmal AF with preserved LVEF, Audicor® was able to detect subclinical LV systolic and diastolic dysfunction, while it remained undetected in echocardiography [38]. In human diagnostic cardiology, Audicor® offers some advantages compared to echocardiography: it is operator-independent, the position of the device is pre-defined, no expertise is needed to perform the recordings, and it can be obtained from a wide variety of positions, such as in a supine, laterally recumbent, or upright position [29]. However, in horses, some of these advantages dissipate, as manual read-through by an expert is required due to the human nature of the algorithm. We could not confirm the hypothesis that S4 would correlate with echocardiographic variables of LA mechanical function. Nevertheless, the hypothesis that certain Audicor® variables correlate well with echocardiographic variables to assess LV systolic function was confirmed, as similar changes were seen in corresponding variables after conversion of AF to NSR.
The main limitation of this study is that the Audicor® device and its proprietary signal processing algorithms were originally developed and validated for use in humans. Detection thresholds, such as the cut-off of 5 for S3 and S4 power, were derived from human validation studies and may not directly translate to horses. In particular, low-frequency components of equine heart sounds may require dedicated research to establish appropriate detection thresholds. Similarly, SDI, which was developed for use in human patients, was included in this study to allow initial exploration of its behavior in horses. However, its diagnostic value in the equine setting remains uncertain and warrants further investigation. The lack of equine-specific algorithm development limits the generalizability of the findings and may have affected the accuracy and sensitivity of heart sound detection and systolic time interval measurements. The application of human-derived algorithms also made the preparation of the recordings for analysis time-intensive, as manual page-by-page verification and correction of the ECG were necessary to ensure correct analysis. Furthermore, overnight recordings proved difficult to obtain in a clinical setting, as the use of the device on horses caused substantial motion artifacts, leading to a substantial loss of data. However, “snapshot” recordings were feasible to obtain efficiently in a clinical setting and were easily analyzable. Finally, as mentioned above, the position of the Audicor® device was not optimized for the points of maximum intensity of the heart sounds in individual horses.
Further studies in other settings are needed to establish Audicor® as a potentially useful adjunctive to echocardiography in horses with cardiac disease. While this study focused on comparative assessments, future mechanistic investigations evaluating the relationship between heart sounds and underlying hemodynamic events will be essential to provide the physiological basis for species-specific algorithm development. The generation of equine-adapted algorithms may ultimately improve diagnostic accuracy and optimize the clinical utility of acoustic cardiography in this species. In addition, future studies will be conducted using the next generation Audicor® device, allowing optimized positioning and recording location for “snapshot” recordings, possibly improving data quality for detection of heart sounds, derivation of systolic time intervals, and quantification of heart sound intensity.
5. Conclusions
This study provided proof of concept for the use of Audicor® in a clinical setting in the context of equine AF. “Snapshot” recordings proved to be feasible to obtain in a clinical setting, and the analyses provided additional information on LV systolic function, reflecting changes seen in echocardiography. However, the Audicor® device under investigation does not seem to be clinically useful as a tool to directly assess LA mechanical function after conversion of AF to NSR, at least not within the time frame up to 7 days after successful cardioversion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15131993/s1, Table S1: List of number echocardiographic variables analyzed based on fewer than three cardiac cycles; Table S2: List of echocardiographic variables; Figure S1: Illustration of Audicor®; Table S3: List of Audicor® acoustic cardiography variables; Table S4: Reproducibility of “snapshot” variables.
Author Contributions
Conceptualization, M.J.S., G.v.L. and C.C.S.; data curation, M.J.S., I.L.P., H.K.J., I.V. and G.v.S.; supervision, C.C.S.; project administration, M.J.S., G.v.L. and C.C.S.; writing—original draft preparation, M.J.S.; writing—review and editing, M.J.S. and C.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
The SHK “Stiftung für Herz- und Kreislaufkrankheiten” (Hergiswil, Switzerland) supported the study with an unrestricted grant (2019).
Institutional Review Board Statement
The study was approved by the district veterinary office of the Canton of Zurich (ZH113/19).
Informed Consent Statement
All owners signed an informed consent statement.
Data Availability Statement
The data are available from the authors upon reasonable request.
Acknowledgments
The authors thank the staff of the Equine Hospital involved in the collection of the data. The authors are especially grateful to Christina Eberhardt, who performed some of the echocardiographic examinations. We also thank Tim Wheeler and Peter Bauer for the technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| Am | Late diastolic LV wall motion velocity at the time of atrial contraction |
| Active LA FAC | Left-atrial active fractional area change |
| active/total LA AC | Ratio of active-to-total left atrial area change |
| AF | Atrial fibrillation |
| ANOVA | One-way analysis of variance |
| AV | Atrioventricular |
| BWT | Body weight |
| CO | Cardiac output |
| cTDI | Color tissue Doppler imaging |
| dmeans | Difference of means |
| EF | Ejection fraction |
| Em | Early-diastolic LV wall motion velocity during the phase of rapid ventricular filling |
| Em/Am | Ratio of Em-to-Am |
| EMAT | Electromechanical activation time |
| EMATc | Heart rate-corrected electromechanical activation time |
| ETm | Ejection time |
| ETm-c | Heart rate-corrected ejection time |
| HR | Heart rate |
| IMPm | Index of myocardial performance |
| LA | Left atrium |
| LAAmax | Internal left atrial area measured during maximum atrial filling (right parasternal long-axis view) |
| LAsxAmax | Internal area of the left atrium during maximum atrial filling (right parasternal short-axis view) |
| LADllx-max | Internal left atrial diameter during maximum atrial filling (left parasternal long-axis view) |
| LADmax | Internal left atrial diameter during maximum atrial filling (right parasternal long-axis view) |
| LA RI | Left atrial reservoir index |
| LV | Left ventricular |
| LV dP/dtmax | maximum rate of systolic LV pressure rise |
| LV EF | Left ventricular ejection fraction |
| LVEDP | Left ventricular end-diastolic pressure |
| LV FS | Left ventricular fractional shortening |
| LVIDd | Left ventricular diameter at end-diastole |
| LVIVd | Left ventricular volume at end-diastole |
| LVST | Left ventricular systolic time |
| LVSTc | Heart rate-corrected LVST |
| M-Mode | Motion mode |
| NSR | Normal sinus rhythm |
| PEPm | Pre-ejection period |
| PEPm-c | Heart rate-corrected pre-ejection period |
| PW TDI | Pulse wave tissue Doppler imaging |
| QRS | QRS duration |
| QTc | Heart rate-corrected QT interval |
| rAF | Atrial fibrillation recurrence |
| RWTd | Relative LV wall thickness at end-diastole |
| SD | Standard deviation |
| SDI | Systolic dysfunction index |
| Sm | Wall motion velocity during LV ejection |
| SV | Stroke volume |
| S3 | Third heart sound |
| S4 | Fourth heart sound |
| TDI | Tissue Doppler imaging |
| 2DE | Two-dimensional echocardiography |
| 2DST | 2D speckle tracking |
| 95% CI | 95% confidence interval |
References
- Reef, V.B.; Bonagura, J.; Buhl, R.; McGurrin, M.K.J.; Schwarzwald, C.C.; van Loon, G.; Young, L.E. Recommendations for management of equine athletes with cardiovascular abnormalities. J. Vet. Intern. Med. 2014, 28, 749–761. [Google Scholar] [CrossRef]
- Buntenkötter, S.; Deegen, E. Behaviour of the heart rate of horses with auricular fibrillation during exercise and after treatment. Equine Vet. J. 1976, 8, 26–29. [Google Scholar]
- Reef, V.B.; Reimer, J.M.; Spencer, P.A. Treatment of atrial fibrillation in horses: New perspectives. J. Vet. Intern. Med. 1995, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, H.; Bubeck, K.; Rohn, K.; Stadler, P. Pulmonary artery wedge pressure during treadmill exercise in warmblood horses with atrial fibrillation. Res. Vet. Sci. 2006, 81, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Hesselkilde, E.Z.; Carstensen, H.; Flethøj, M.; Fenner, M.; Kruse, D.D.; Sattler, S.M.; Tfelt-Hansen, J.; Pehrson, S.; Braunstein, T.H.; Carlson, J.; et al. Longitudinal study of electrical, functional and structural remodelling in an equine model of atrial fibrillation. BMC Cardiovasc. Disord. 2019, 19, 228. [Google Scholar] [CrossRef] [PubMed]
- Marr, C.M.; Reef, V.B.; Reimer, J.M.; Sweeney, R.W.; Reid, S.W. An echocardiographic study of atrial fibrillation in horses: Before and after conversion to sinus rhythm. J. Vet. Intern. Med. 1995, 9, 336–340. [Google Scholar] [CrossRef]
- Kubo, K.; Senta, T.; Sugimoto, O. Changes in cardiac output with experimentally induced atrial fibrillation in the horse. Exp. Rep. Equine Health Lab. 1975, 12, 101–108. [Google Scholar]
- Stadler, P.; Deegen, E.; Kroker, K. Echocardiography and therapy of atrial fibrillation in horses. Dtsch. Tierarztl. Wochenschr. 1994, 101, 190–194. [Google Scholar]
- Schwarzwald, C.C.; Schober, K.E.; Bonagura, J.D. Echocardiographic evidence of left atrial mechanical dysfunction after conversion of atrial fibrillation to sinus rhythm in 5 horses. J. Vet. Intern. Med. 2007, 21, 820–827. [Google Scholar] [CrossRef]
- Decloedt, A.; Verheyen, T.; Van Der Vekens, N.; Sys, S.; De Clercq, D.; van Loon, G. Long-term follow-up of atrial function after cardioversion of atrial fibrillation in horses. Vet. J. 2013, 197, 583–588. [Google Scholar] [CrossRef]
- De Clercq, D.; van Loon, G.; Tavernier, R.; Duchateau, L.; Deprez, P. Atrial and Ventricular Electrical and Contractile Remodeling and Reverse Remodeling Owing to Short-Term Pacing-Induced Atrial Fibrillation in Horses. J. Vet. Intern. Med. 2008, 22, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, G.; Duytschaever, M.; Tavernier, R.; Fonteyne, W.; Jordaens, L.; Deprez, P. An equine model of chronic atrial fibrillation: Methodology. Vet. J. 2002, 164, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, A.; Van Steenkiste, G.; Vera, L.; Buhl, R.; van Loon, G. Atrial fibrillation in horses part 1: Pathophysiology. Vet. J. 2020, 263, 105521. [Google Scholar] [CrossRef]
- Manning, W.J.; Silverman, D.I.; Katz, S.E.; Riley, M.F.; Come, P.C.; Doherty, R.M.; Munson, J.T.; Douglas, P.S. Impaired left atrial mechanical function after cardioversion: Relation to the duration of atrial fibrillation. J. Am. Coll. Cardiol. 1994, 23, 1535–1540. [Google Scholar] [CrossRef]
- McGurrin, M.K.; Physick-Sheard, P.W.; Kenney, D.G. Transvenous electrical cardioversion of equine atrial fibrillation: Patient factors and clinical results in 72 treatment episodes. J. Vet. Intern. Med. 2008, 22, 609–615. [Google Scholar] [CrossRef]
- De Clercq, D.; Decloedt, A.; Sys, S.U.; Verheyen, T.; Van Der Vekens, N.; van Loon, G. Atrial fibrillation cycle length and atrial size in horses with and without recurrence of atrial fibrillation after electrical cardioversion. J. Vet. Intern. Med. 2014, 28, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, A.; Schwarzwald, C.C.; De Clercq, D.; Van Der Vekens, N.; Pardon, B.; Reef, V.B.; van Loon, G. Risk factors for recurrence of atrial fibrillation in horses after cardioversion to sinus rhythm. J. Vet. Intern. Med. 2015, 29, 946–953. [Google Scholar] [CrossRef]
- Mitchell, K.J.; De Clercq, D.; Stirn, M.; van Loon, G.; Schwarzwald, C.C. Plasma homocysteine concentrations in healthy horses and horses with atrial fibrillation. J. Vet. Cardiol. 2018, 20, 276–284. [Google Scholar] [CrossRef]
- Vernemmen, I.; De Clercq, D.; Decloedt, A.; Vera, L.; Van Steenkiste, G.; van Loon, G. Atrial premature depolarisations five days post electrical cardioversion are related to atrial fibrillation recurrence risk in horses. Equine Vet. J. 2020, 52, 374–378. [Google Scholar] [CrossRef]
- Schwarzwald, C.C.; Schober, K.E.; Bonagura, J.D. Methods and reliability of echocardiographic assessment of left atrial size and mechanical function in horses. Am. J. Vet. Res. 2007, 68, 735–747. [Google Scholar] [CrossRef]
- Schwarzwald, C.C. Equine Echocardiography. Vet. Clin. N. Am. Equine Pract. 2019, 35, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Schwarzwald, C.C.; Schober, K.E.; Bonagura, J.D. Methods and reliability of tissue Doppler imaging for assessment of left ventricular radial wall motion in horses. J. Vet. Intern. Med. 2009, 23, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, C.; Mitchell, K.J.; Schwarzwald, C.C. Quantification of left atrial wall motion in healthy horses using two-dimensional speckle tracking. J. Vet. Cardiol. 2020, 30, 32–43. [Google Scholar] [CrossRef]
- Eberhardt, C.; Schwarzwald, C.C. Quantification of left atrial contractile function using two-dimensional speckle tracking echocardiography in horses after conversion of atrial fibrillation to sinus rhythm. J. Vet. Cardiol. 2021, 35, 108–120. [Google Scholar] [CrossRef]
- Koenig, T.R.; Mitchell, K.J.; Schwarzwald, C.C. Echocardiographic Assessment of Left Ventricular Function in Healthy Horses and in Horses with Heart Disease Using Pulsed-Wave Tissue Doppler Imaging. J. Vet. Intern. Med. 2017, 31, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, A.; Verheyen, T.; Sys, S.; De Clercq, D.; van Loon, G. Two-dimensional speckle tracking for quantification of left ventricular circumferential and radial wall motion in horses. Equine Vet. J. 2013, 45, 47–55. [Google Scholar] [CrossRef]
- Decloedt, A.; Verheyen, T.; Sys, S.; De Clercq, D.; van Loon, G. Quantification of left ventricular longitudinal strain, strain rate, velocity, and displacement in healthy horses by 2-dimensional speckle tracking. J. Vet. Intern. Med. 2011, 25, 330–338. [Google Scholar] [CrossRef]
- Decloedt, A.; Verheyen, T.; Sys, S.; De Clercq, D.; van Loon, G. Evaluation of tissue Doppler imaging for regional quantification of radial left ventricular wall motion in healthy horses. Am. J. Vet. Res. 2013, 74, 53–61. [Google Scholar] [CrossRef]
- Zuber, M.; Toggweiler, S.; Quinn-Tate, L.; Brown, L.; Amkieh, A.; Erne, P. A comparison of acoustic cardiography and echocardiography for optimizing pacemaker settings in cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2008, 31, 802–811. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, N.; Arand, P.; Michaels, A.D. Computerized acoustic cardiography correlates with echocardiography and invasive haemodynamics after percutaneous transvenous mitral commissurotomy. J. Med. Eng. Technol. 2011, 35, 59–64. [Google Scholar] [CrossRef]
- Efstratiadis, S.; Michaels, A.D. Computerized acoustic cardiographic electromechanical activation time correlates with invasive and echocardiographic parameters of left ventricular contractility. J. Card. Fail. 2008, 14, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Odermatt, Y.; Brauchlin, A.; Zander, T.; Müller, A.; Zuber, M.; Winterhalder, R.; Erne, P. The clinical value of echocardiography and acoustic cardiography to monitor patients undergoing anthracycline chemotherapy. Clin. Cardiol. 2013, 36, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Walia, R.; Chang, S.L.; Lin, Y.J.; Lo, L.W.; Hu, Y.F.; Chao, T.F.; Chung, F.P.; Liao, J.N.; Lin, C.Y.; Chang, Y.T.; et al. Early detection of electromechanical dysfunction in patients with idiopathic premature ventricular contractions. Pacing Clin. Electrophysiol. 2019, 42, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Erne, P. Beyond auscultation--acoustic cardiography in the diagnosis and assessment of cardiac disease. Swiss Med. Wkly. 2008, 138, 439–452. [Google Scholar]
- Dillier, R.; Zuber, M.; Arand, P.; Erne, S.; Erne, P. Assessment of systolic and diastolic function in asymptomatic subjects using ambulatory monitoring with acoustic cardiography. Clin. Cardiol. 2011, 34, 384–388. [Google Scholar] [CrossRef]
- Shah, S.J.; Michaels, A.D. Hemodynamic correlates of the third heart sound and systolic time intervals. Congest. Heart Fail. 2006, 12, 8–13. [Google Scholar] [CrossRef]
- Erne, P.; Resink, T.J.; Mueller, A.; Coslovsky, M.; Kobza, R.; Conen, D.; Bauer, P.; Arand, P. of acoustic cardiography immediately following electrical cardioversion to predict relapse of atrial fibrillation. J. Atr. Fibrillation 2017, 10, 1527. [Google Scholar] [CrossRef]
- Pan, K.L.; Chang, S.L.; Lin, Y.J.; Lo, L.W.; Hu, Y.F.; Chung, F.P.; Chao, T.F.; Liao, J.N.; Lin, C.Y.; Lin, C.H.; et al. Left Ventricular Electromechanical Remodeling Detected by Acoustic Cardiography in Paroxysmal Atrial Fibrillation. J. Cardiovasc. Transl. Res. 2020, 13, 362–371. [Google Scholar] [CrossRef]
- Keen, J.A. Examination of Horses with Cardiac Disease. Vet. Clin. N. Am. Equine Pract. 2019, 35, 23–42. [Google Scholar] [CrossRef]
- Zuber, N.; Zuber, M.; Schwarzwald, C.C. Assessment of systolic and diastolic function in clinically healthy horses using ambulatory acoustic cardiography. Equine Vet. J. 2019, 51, 391–400. [Google Scholar] [CrossRef]
- Huesler, I.M.; Mitchell, K.J.; Schwarzwald, C.C. Echocardiographic Assessment of Left Atrial Size and Function in Warmblood Horses: Reference Intervals, Allometric Scaling, and Agreement of Different Echocardiographic Variables. J. Vet. Intern. Med. 2016, 30, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, D.; Schwarzwald, C.C. Echocardiographic assessment of left ventricular size and systolic function in Warmblood horses using linear measurements, area-based indices, and volume estimates: A retrospective database analysis. J. Vet. Intern. Med. 2021, 35, 504–520. [Google Scholar] [CrossRef]
- Schwarzwald, C.C. Disorders of the Cardiovascular System. In Equine Internal Medicine; Reed, S.M., Bayly, W.M., Sellon, D.C., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2018; pp. 387–541. [Google Scholar]
- Brown, D.J.; Rush, J.E.; MacGregor, J.M.; Ross, J.N.; Brewer, B.; Rand, W.M. M-mode echocardiographic ratio indices in normal dogs, cats, and horses: A novel quantitative method. J. Vet. Intern. Med. 2003, 17, 653–662. [Google Scholar] [PubMed]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar]
- Bland, M. Clinical measurement. In An Introduction to Medical Statistics; Bland, M., Ed.; Oxford University Press: New York, NY, USA, 2000; pp. 268–293. [Google Scholar]
- Abbott, J.A.; Gentile-Solomon, J.M. Measurement Variation and Repeatability of Echocardiographic Variables Used to Estimate Pulmonary Artery Pressure in Dogs. J. Vet. Intern. Med. 2017, 31, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, M.; Fang, F.; Shang, Q.; Sun, J.P.; Sanderson, J.E.; Yu, C.M. Prognostic value of acoustic cardiography in patients with chronic heart failure. Int. J. Cardiol. 2016, 219, 121–126. [Google Scholar] [CrossRef]
- Wang, S.; Fang, F.; Liu, M.; Lam, Y.Y.; Wang, J.; Shang, Q.; Sun, J.P.; Sanderson, J.E.; Yu, C.M. Rapid bedside identification of high-risk population in heart failure with reduced ejection fraction by acoustic cardiography. Int. J. Cardiol. 2013, 168, 1881–1886. [Google Scholar] [CrossRef]
- Wen, Y.N.; Lee, A.P.; Fang, F.; Jin, C.N.; Yu, C.M. Beyond auscultation: Acoustic cardiography in clinical practice. Int. J. Cardiol. 2014, 172, 548–560. [Google Scholar] [CrossRef]
- Dillier, R.; Kobza, R.; Erne, S.; Zuber, M.; Arand, P.; Erne, P. Noninvasive detection of left-ventricular systolic dysfunction by acoustic cardiography in atrial fibrillation. Cardiol. Res. Pract. 2011, 2011, 173102. [Google Scholar] [CrossRef]
- De Clercq, D.; Decloedt, A.; Tavernier, R.; Deprez, P.; van Loon, G. Atrial and ventricular electrical and contractile remodelling and reverse remodelling due to chronic pacing-induced atrial fibrillation in horses: Preliminary results. Vlaams Diergeneeskd. Tijdschr. 2019, 88, 269–277. [Google Scholar][Green Version]
- van Loon, G. Atrial pacing and Experimental Atrial Fibrillation in Equines. In Veterinary Sciences; Ghent University: Ghent, Belgium, 2001; pp. 161–206. [Google Scholar]
- van Loon, G. Cardiac Arrhythmias in Horses. Vet. Clin. N. Am. Equine Pract. 2019, 35, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Reef, V.B.; Levitan, C.W.; Spencer, P.A. Factors affecting prognosis and conversion in equine atrial fibrillation. J. Vet. Intern. Med. 1988, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, A.; Verheyen, T.; Sys, S.; De Clercq, D.; van Loon, G. Tissue Doppler imaging and 2-dimensional speckle tracking of left ventricular function in horses exposed to lasalocid. J. Vet. Intern. Med. 2012, 26, 1209–1216. [Google Scholar] [CrossRef]
- Schefer, K.D.; Hagen, R.; Ringer, S.K.; Schwarzwald, C.C. Laboratory, electrocardiographic, and echocardiographic detection of myocardial damage and dysfunction in an Arabian mare with nutritional masseter myodegeneration. J. Vet. Intern. Med. 2011, 25, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, A.; Verheyen, T.; Sys, S.; De Clercq, D.; Bijnens, B.; van Loon, G. Influence of atrioventricular interaction on mitral valve closure and left ventricular isovolumic contraction measured by tissue Doppler imaging. Circ. Cardiovasc. Imaging 2013, 6, 109–116. [Google Scholar] [CrossRef][Green Version]
- Roos, M.; Toggweiler, S.; Zuber, M.; Jamshidi, P.; Erne, P. Acoustic cardiographic parameters and their relationship to invasive hemodynamic measurements in patients with left ventricular systolic dysfunction. Congest. Heart Fail. 2006, 12, 19–24. [Google Scholar] [CrossRef]
- Roos, M.; Toggweiler, S.; Jamshidi, P.; Zuber, M.; Kobza, R.; Meier, R.; Erne, P. Noninvasive detection of left ventricular systolic dysfunction by acoustic cardiography in cardiac failure patients. J. Card. Fail. 2008, 14, 310–319. [Google Scholar] [CrossRef]
- Jamshidi, P.; Kobza, R.; Toggweiler, S.; Arand, P.; Zuber, M.; Erne, P. Impact of preload changes on positive and negative left ventricular dP/dt and systolic time intervals: Preload changes on left ventricular function. Indian. Heart J. 2012, 64, 314–318. [Google Scholar] [CrossRef]
- Zuber, M.; Kipfer, P.; Attenhofer Jost, C. Systolic dysfunction: Correlation of acoustic cardiography with Doppler echocardiography. Congest. Heart Fail. 2006, 12, 14–18. [Google Scholar] [CrossRef]
- Shapiro, M.; Moyers, B.; Marcus, G.M.; Gerber, I.L.; McKeown, B.H.; Vessey, J.C.; Jordan, M.V.; Huddleston, M.; Foster, E.; Chatterjee, K.; et al. Diagnostic characteristics of combining phonocardiographic third heart sound and systolic time intervals for the prediction of left ventricular dysfunction. J. Card. Fail. 2007, 13, 18–24. [Google Scholar] [CrossRef]
- Marcus, G.M.; Gerber, I.L.; McKeown, B.H.; Vessey, J.C.; Jordan, M.V.; Huddleston, M.; McCulloch, C.E.; Foster, E.; Chatterjee, K.; Michaels, A.D. Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. JAMA 2005, 293, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Nakamura, K.; Marcus, G.M.; Gerber, I.L.; McKeown, B.H.; Jordan, M.V.; Huddleston, M.; Foster, E.; Michaels, A.D. Association of the fourth heart sound with increased left ventricular end-diastolic stiffness. J. Card. Fail. 2008, 14, 431–436. [Google Scholar] [CrossRef]
- Zuber, M.; Attenhofer Jost, C.H.; Kipfer, P.; Collins, S.P.; Michota, F.; Peacock, W.F. Acoustic cardiography augments prolonged QRS duration for detecting left ventricular dysfunction. Ann. Noninvasive Electrocardiol. 2007, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Smetzer, D.L.; Smith, C.R. Diastolic Heart Sounds of Horses. J. Am. Vet. Med. Assoc. 1965, 146, 937–944. [Google Scholar] [PubMed]
- Smetzer, D.L.; Smith, C.R.; Hamlin, R.L. The fourth heart sound in the equine. Ann. N. Y. Acad. Sci. 1965, 127, 306–321. [Google Scholar] [CrossRef]
- Patteson, M.W. Equine Cardiology; Blackwell Science: Oxford, UK, 1996. [Google Scholar]
- Hasan, A.; Abraham, W.T.; Quinn-Tate, L.; Brown, L.; Amkieh, A. Optimization of cardiac resynchronization devices using acoustic cardiography: A comparison to echocardiography. Congest. Heart Fail. 2006, 12, 25–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).