Seasonal Spatial Distribution Characteristics and Patterns of the Squid Uroteuthis duvauceli, Uroteuthis edulis, Loliolus sumatrensis, and Loliolus japonica in the Southern Yellow and East China Seas: Predictions Under Different Climate Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
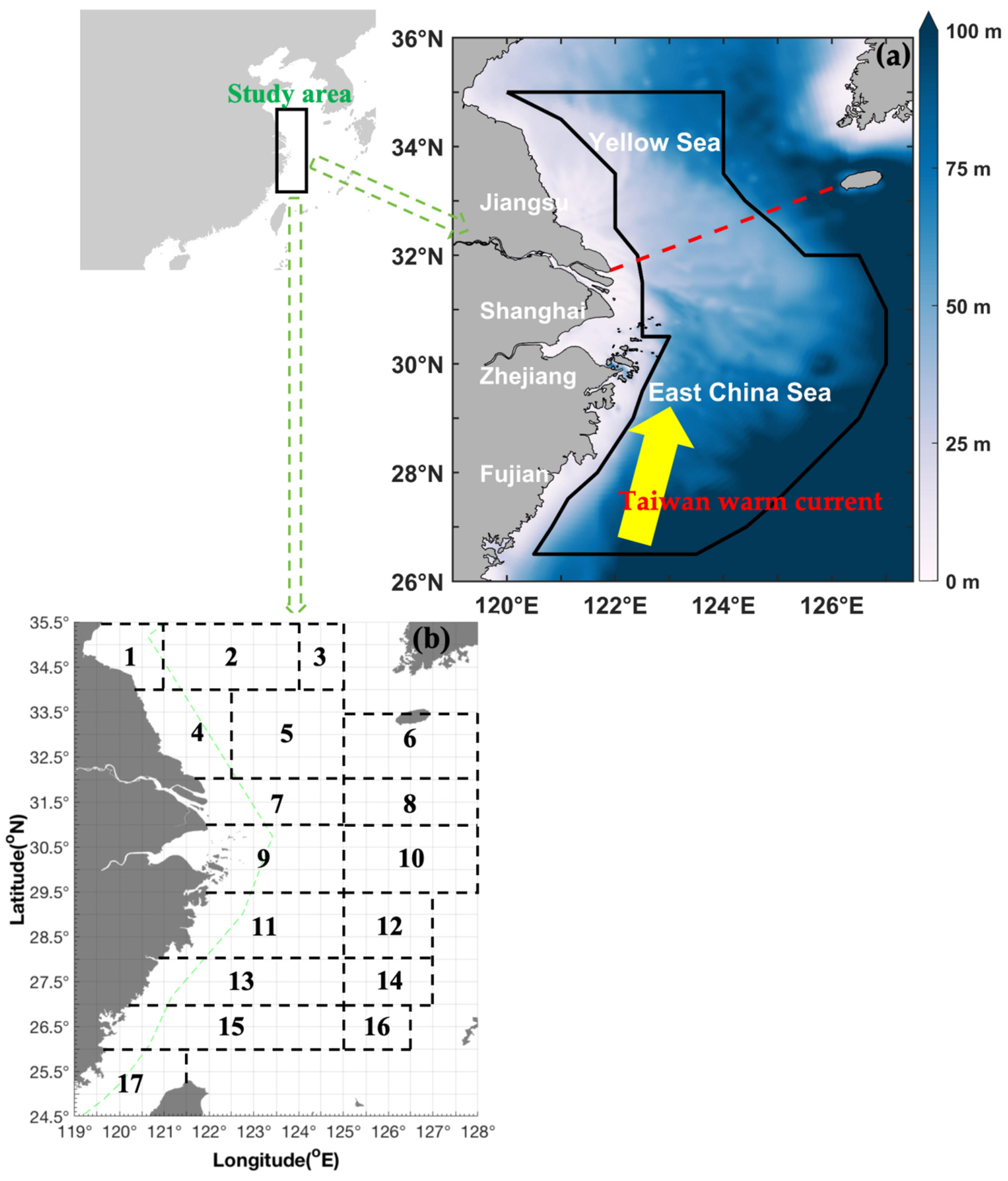
2.1. Geographical Information, Survey and Sampling Procedures
2.2. Ensemble Model, Selection of Environmental Variables, and Evaluations
3. Results and Discussion
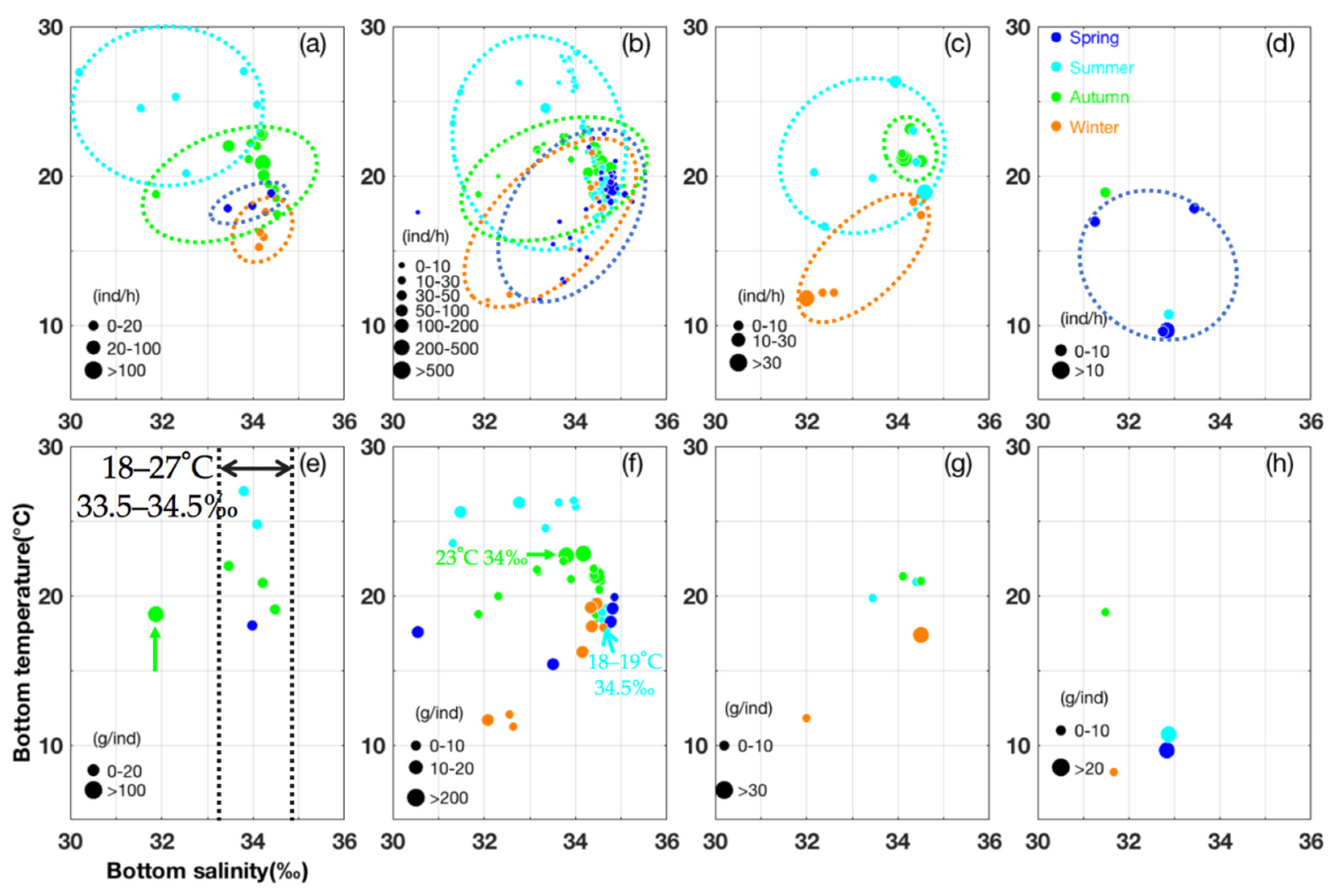
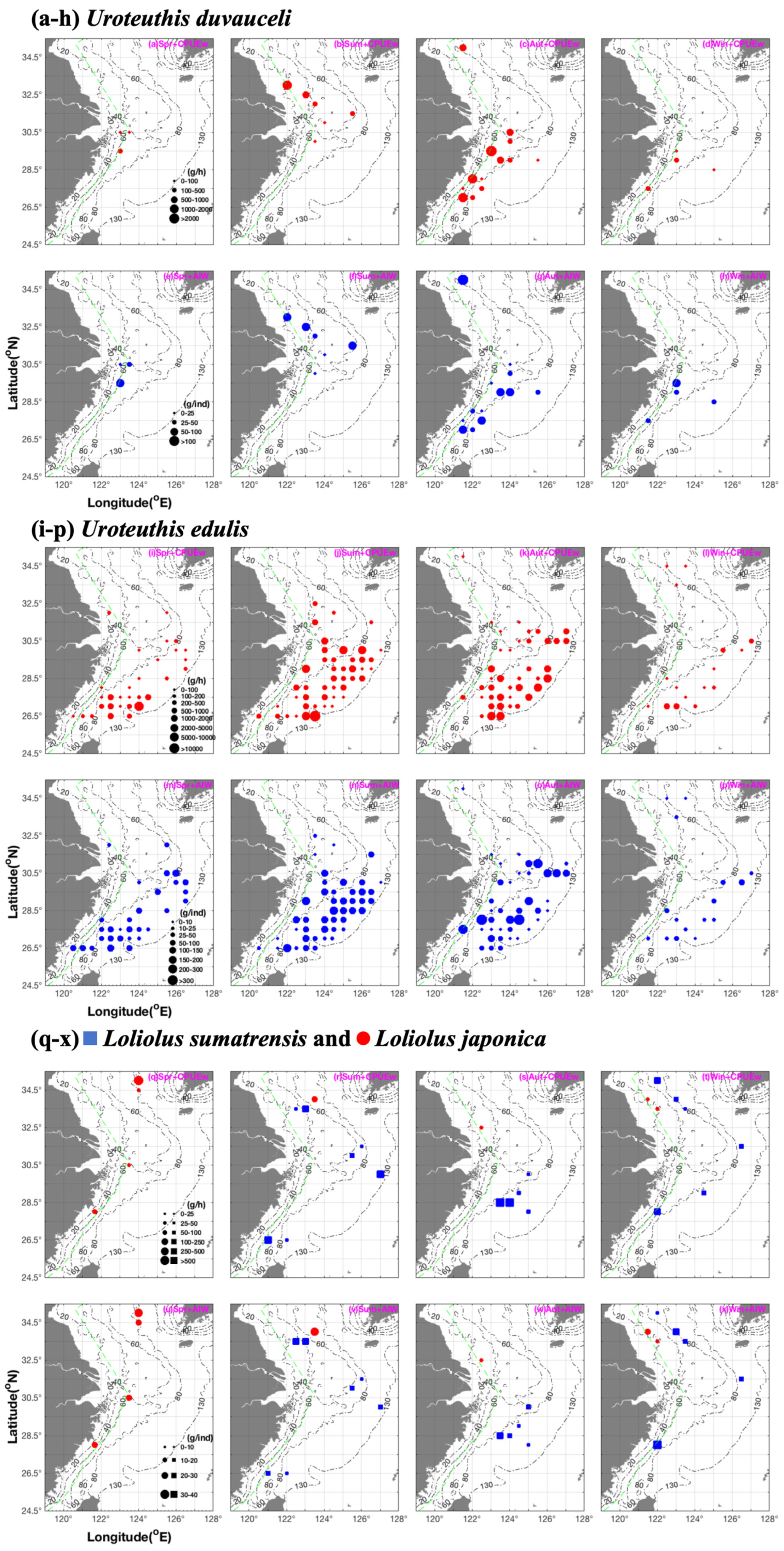
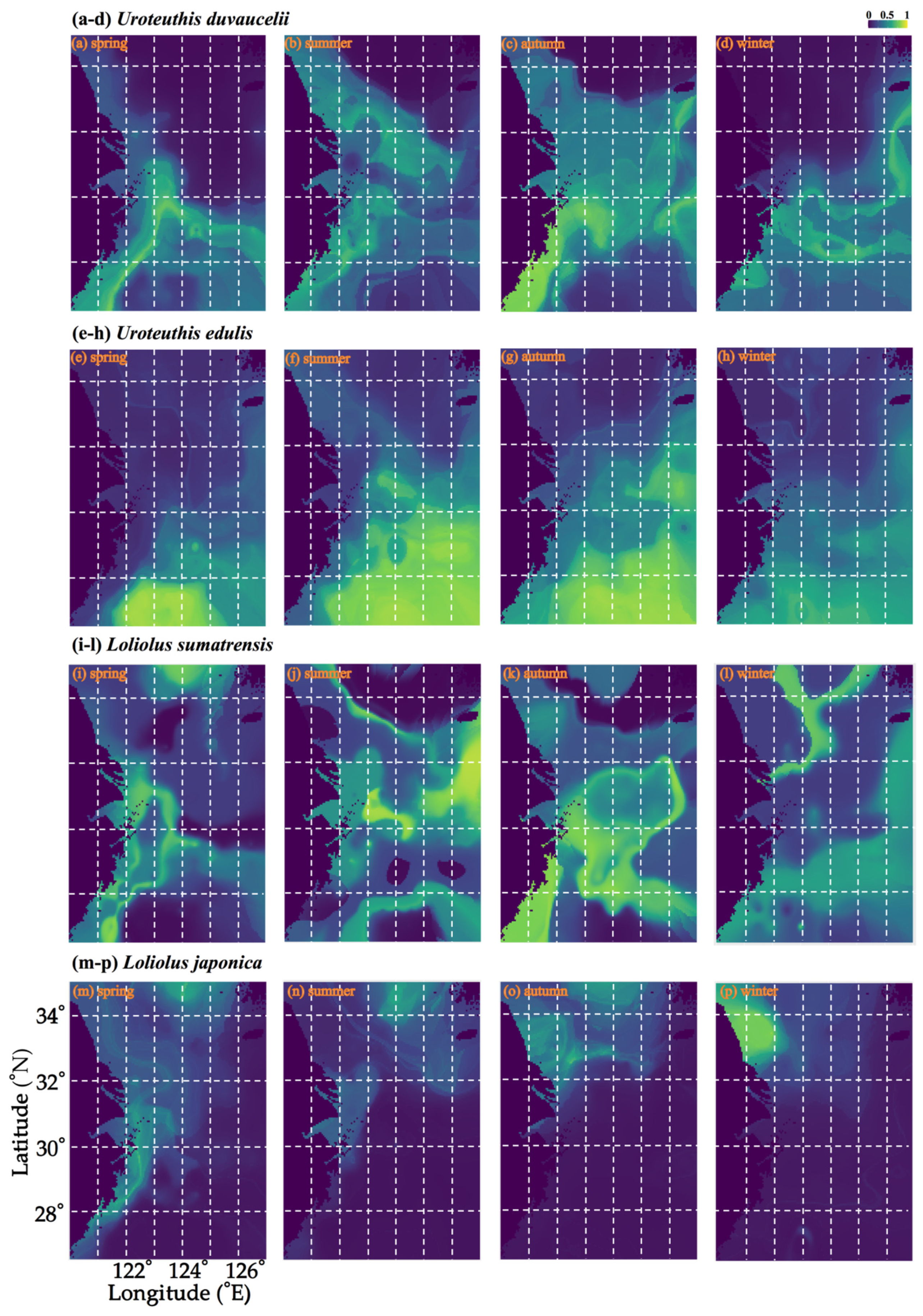
3.1. Seasonal Spatial Variation and Migration of U. duvauceli
3.2. Seasonal Spatial Variation and Migration of U. edulis
3.3. Seasonal Spatial Variation and Characteristics of L. sumatrensis and L. japonica
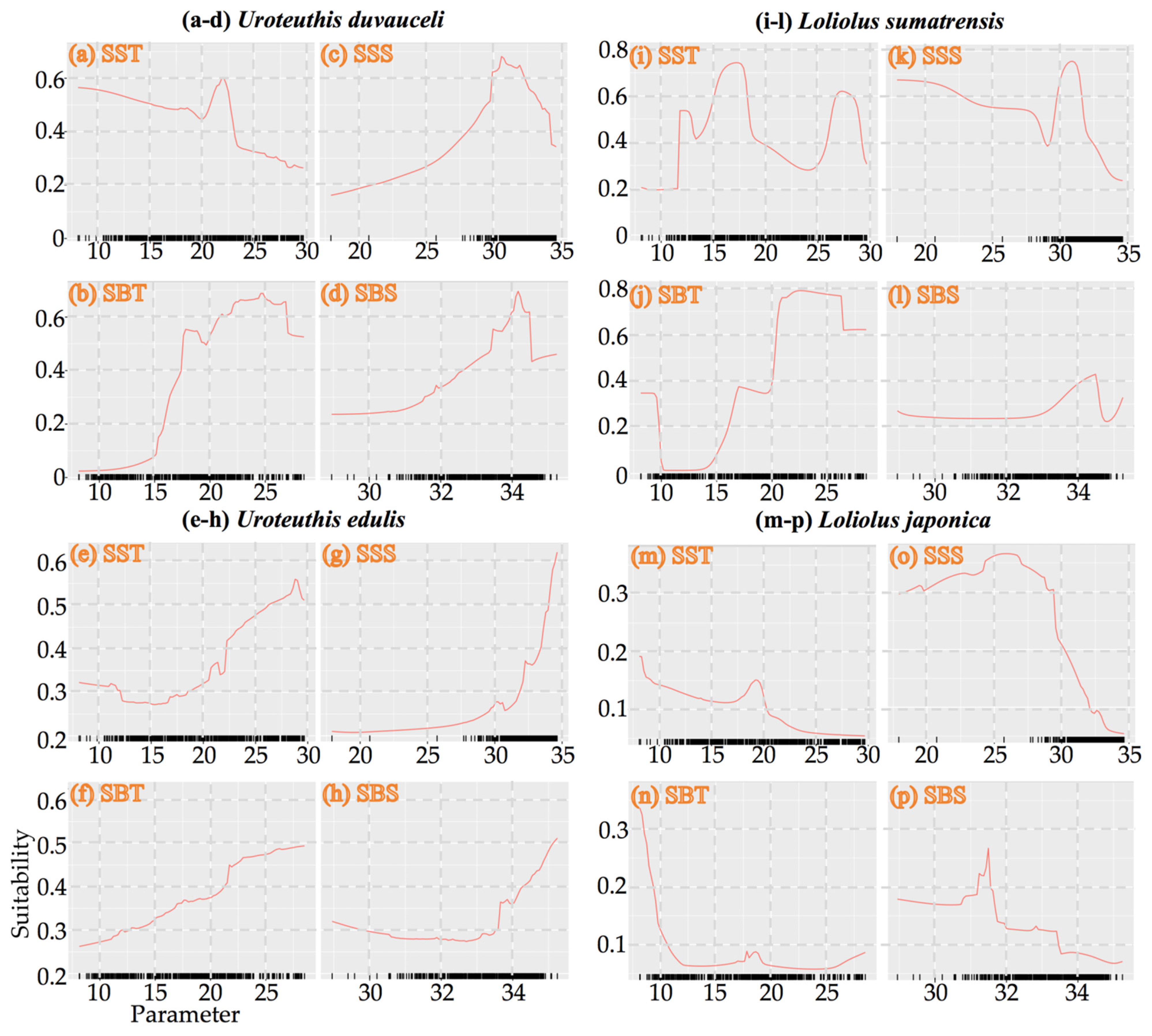
3.4. Model Evaluation and Suitable Environmental Factors and Habitat
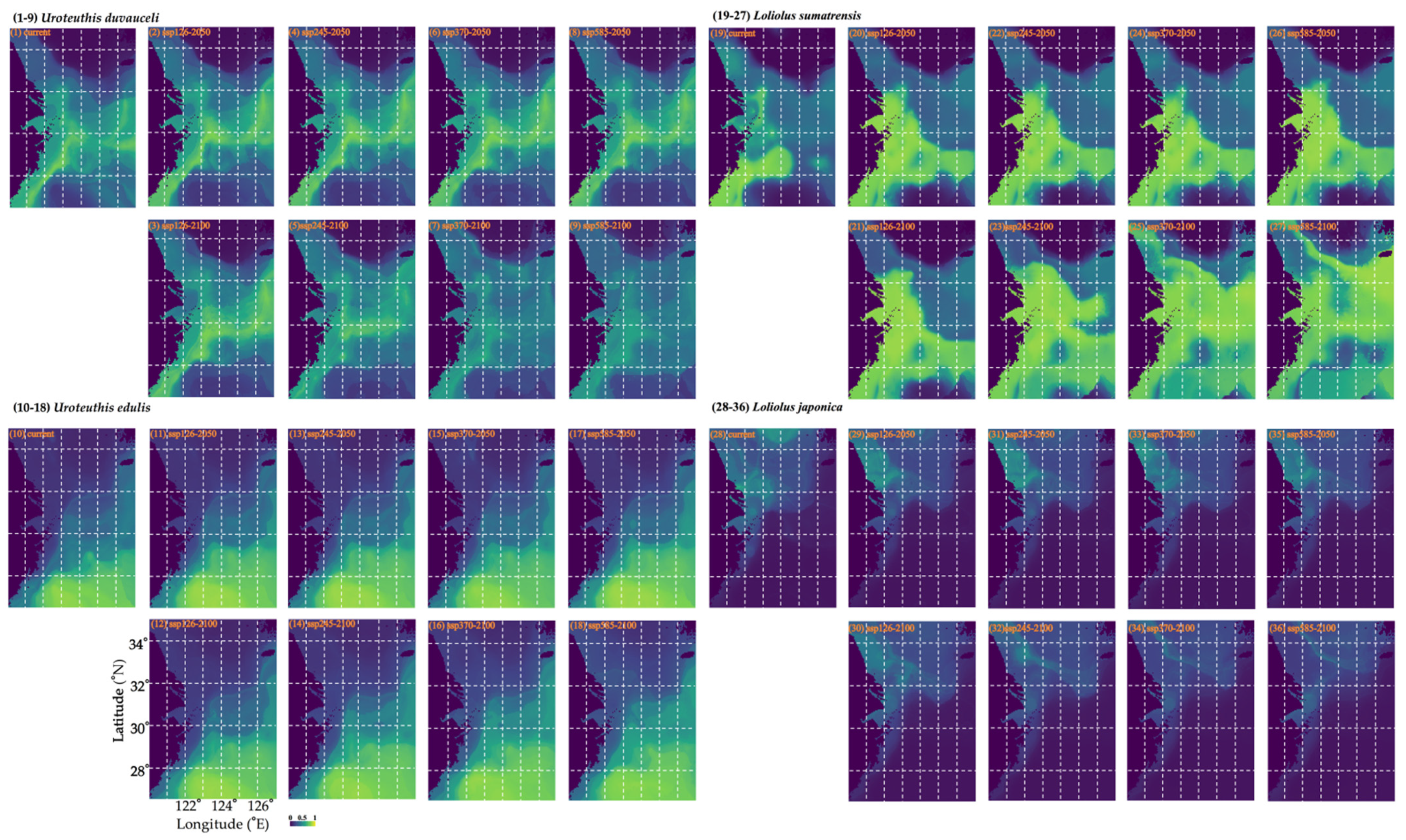
3.5. Habitat Predictions Under Different Climate Projections
3.6. Fishery Management Suggestions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). The Ocean and Cryosphere in a Changing Climate: Special Report of the Intergovernmental Panel on Climate Change, 1st ed.; Pörtner, H.-O., Ed.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Harley, C.D.G.; Hughes, A.R.; Hultgren, K.M.; Miner, B.G.; Sorte, C.J.B.; Thornber, C.S.; Rodriguez, L.F.; Tomanek, L.; Williams, S.L. The impacts of climate change in coastal marine systems: Climate change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, E.; Santos, C.; Rosa, I.C.; Ferreira, V.; Pörtner, H.-O.; Duarte, C.M.; Levin, L.A.; Rosa, R. Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat. Ecol. Evol. 2021, 5, 311–321. [Google Scholar] [CrossRef]
- Albouy, C.; Guilhaumon, F.; Leprieur, F.; Lasram, F.B.R.; Somot, S.; Aznar, R.; Velez, L.; Loc’h, F.; Mouillot, D. Projected climate change and the changing biogeography of coastal Mediterranean fishes. J. Biogeogr. 2013, 40, 534–547. [Google Scholar] [CrossRef]
- Jackson, G.D.; Domeier, M.L. The effects of an extraordinary El Niño/La Niña event on the size and growth of the squid Loligo opalescens off Southern California. Mar. Biol. 2003, 142, 925–935. [Google Scholar] [CrossRef]
- Guerreiro, M.F.; Borges, F.O.; Santos, C.P.; Rosa, R. Future distribution patterns of nine cuttlefish species under climate change. Mar. Biol. 2023, 170, 159. [Google Scholar]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Jin, Y.; Jin, X.; Gorfine, H.; Wu, Q.; Shan, X. Modeling the oceanographic impacts on the spatial distribution of common cephalopods during autumn in the Yellow Sea. Front. Mar. Sci. 2020, 7, 432. [Google Scholar] [CrossRef]
- Piatkowski, U.; Pierce, G.J.; Da Cunha, M.M. Impact of cephalopods in the food chain and their interaction with the environment and fisheries: An overview. Fish. Res. 2001, 52, 5–10. [Google Scholar] [CrossRef]
- Liscovitch-Brauer, N.; Alon, S.; Porath, H.T.; Elstein, B.; Unger, R.; Ziv, T.; Levanon, E.Y.; Eisenberg, E. Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell 2017, 169, 191–202. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Song, X.; Yang, L. Changes in Seasonal Spatial Distribution Patterns of Euprymna berryi and Euprymna morsei: The Current and Predictions Under Climate Change Scenarios. Biology 2025, 14, 327. [Google Scholar] [CrossRef]
- Xu, M.; Liu, S.; Zhang, H.; Li, Z.; Song, X.; Yang, L.; Tang, B. Seasonal Analysis of Spatial Distribution Patterns and Characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region. Animals 2024, 14, 2716. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, L.; Liu, Z.; Zhang, Y.; Zhang, H. Seasonal and Spatial Distribution Characteristics of Sepia esculenta in the East China Sea Region: Transfer of the Central Distribution from 29° N to 28° N. Animals 2024, 14, 1412. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Liu, Z.; Zhang, Y.; Cui, Y.; Li, S. Seasonal and spatial distribution of Amphioctopus fangsiao and Octopus variabilis in the southern Yellow and East China Seas: Fisheries management implications based on climate scenario predictions. Reg. Stud. Mar. Sci. 2025, 83, 104072. [Google Scholar] [CrossRef]
- Xu, M.; Liu, S.; Yang, C.; Yang, L. Seasonal Spatial Distribution Patterns of Abralia multihamata in the East China Sea Region: Predictions Under Various Climate Scenarios. Animals 2025, 15, 903. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, M.; Cui, Y.; Liu, S. Seasonal spatial distribution patterns of AmphiOctopus ovulum in the East China Sea: Current status future projections under various climate change scenarios. Front. Mar. Sci. 2025, 12, 1573253. [Google Scholar] [CrossRef]
- Xu, M.; Feng, W.; Liu, Z.; Li, Z.; Song, X.; Zhang, H.; Zhang, C.; Yang, L. Seasonal-Spatial Distribution Variations and Predictions of Loliolus beka and Loliolus uyii in the East China Sea Region: Implications from Climate Change Scenarios. Animals 2024, 14, 2070. [Google Scholar] [CrossRef]
- Sasikumar, G.; Mohamed, K.S.; Mini, K.G.; Sajikumar, K.K. Effect of tropical monsoon on fishery abundance of Indian squid (Uroteuthis (Photololigo) duvaucelii). J. Nat. Hist. 2018, 52, 751–766. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Liu, Y. Quantity distribution and biological characteristics of Lolgio duvaucelii in the East China Sea. In Proceedings of the 26th Academic Exchange Conference of the Fisheries Academic Forum of the Aquatic Society of 16 Provinces (Cities and Districts) in Southern China (Volume II), Chongqing, China, 28–30 October 2010; pp. 77–81. (In Chinese). [Google Scholar]
- Wang, K.Y.; Chang, K.Y.; Chen, J.L.; Chen, R.G.; Liao, C.H. Biology of Uroteuthis duvauceli in the Southern East China Sea. J. Fish. Soc. Taiwan 2015, 42, 9–23. [Google Scholar]
- Yamaguchi, T.; Kawakami, Y.; Matsuyama, M. Analysis of the hatching site and migratory behaviour of the swordtip squid (Uroteuthis edulis) caught in the Japan Sea and Tsushima Strait in autumn estimated by statolith analysis. Mar. Biol. Res. 2018, 14, 105–112. [Google Scholar] [CrossRef]
- Sun, D.R.; Li, Y.; Wang, X.H.; Wang, Y.Z.; Wu, Q.E. Biological characteristics and stock changes of Loligo edulis in Beibu Gulf, South China Sea. S. China Fish. Sci. 2011, 7, 8–13. (In Chinese) [Google Scholar]
- Song, H.T.; Ding, T.M.; Yu, K.J.; Shen, J.X.; Wang, X.Q.; Ruan, J. Species composition & quantitative distribution of cephalopod in the North of East China Sea. J. Zhejiang Ocean. Univ. (Nat. Sci.) 1999, 18, 99–106. (In Chinese) [Google Scholar]
- Li, N.; Fang, Z.; Chen, X.J. Fisheries biology characteristics of Uroteuthis edulis off the northern East China Sea. J. Shanghai Ocean Univ. 2020, 35, 637–644. (In Chinese) [Google Scholar]
- Wang, K.Y.; Chang, K.Y.; Liao, C.H.; Lee, M.A.; Lee, K.T. Growth strategies of the swordtip squid, Uroteuthis edulis, in response to environmental changes in the southern East China Sea—A cohort analysis. Bull. Mar. Sci. 2013, 89, 677–698. [Google Scholar] [CrossRef]
- Gao, X.D.; Jiang, Y.Z.; Yuan, X.W.; Yang, L.L.; Ling, J.Z.; Li, S.F. Modeling spatio-temporal variations in the habitat utilization of swordtip squid (Uroteuthis edulis) in the East China Sea and Southern Yellow Sea. Animals 2023, 13, 3492. [Google Scholar] [CrossRef]
- Ge, Y.; Qiu, S. A preliminary study on the prediction method of Japanese squid catch (Loliolus japonica) in the Yellow and Bohai Seas. Mar. Fish. 1991, 2, 56–60. (In Chinese) [Google Scholar]
- Ding, Y. A discussion about the periodic variation of Japanese squid in the Yellow and Bohai Seas. Fish. Sci. Technol. Inf. 1985, 6, 16–17. (In Chinese) [Google Scholar]
- Sun, D.R. A Study on Fishery Resources and Sustainable Fishery Development in Beibu Bay. Master’s Thesis, Ocean University of China, Qingdao, China, 2008. (In Chinese). [Google Scholar]
- Wang, K.Y.; Liao, C.H.; Lee, K.T. Population and maturation dynamics of the swordtip squid (Photololigo edulis) in the southern East China Sea. Fish. Res. 2008, 90, 178–186. [Google Scholar] [CrossRef]
- Wu, C.R.; Lu, H.F.; Chao, S.Y. A numerical study on the formation of upwelling off northeast Taiwan. J. Geo. Res. 2008, 113, C08025. [Google Scholar] [CrossRef]
- Yassien, M.H.; Hussein, M.S.; Gewida, A.G.A. Food and feeding of the Indian Squid Loligo duvauceli in the Gulf of Suez, Egypt. Egypt. J. Aquat. Biol. Fish. 2016, 20, 61–68. [Google Scholar] [CrossRef][Green Version]
- Jakeman, A.J.; Elsawah, S.; Wang, H.H.; Hamilton, S.H.; Melsen, L.; Grimm, V. Towards normalizing good practice across the whole modeling cycle: Its instrumentation and future research topics. Socio-Environ. Syst. Model. 2024, 6, 18755. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Alabia, I.D.; Tian, Y.; Ye, Z.; Yu, H.; Li, J.; Cheng, J. Impact of climate change on wintering ground of Japanese Anchovy (Engraulis japonicus) using marine geospatial statistics. Front. Mar. Sci. 2020, 7, 604. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Teschke, K.; Hindell, M.A.; Downey, R.; Woods, B.; Kang, B.; Ma, S.; Zhang, C.; Li, J.; et al. Incorporating mesopelagic fish into the evaluation of conservation areas for marine living resources under climate change scenarios. Mar. Life Sci. Technol. 2024, 6, 68–83. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Liu, Y.; Alabia, I.D.; Cheng, J.; Ito, S. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackerel Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep Sea Res. Part II 2023, 207, 105227. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Xing, Q.; Li, Y.; Tian, H.; Luo, Y.; Ito, S.; Tian, Y. Climate change drives fish communities: Changing multiple facets of fish biodiversity in the Northwest Pacific Ocean. Sci. Total Environ. 2024, 955, 176854. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Yáñez, E.; Barbieri, M.A.; Bernal, C.; Aranis, A. Forecasts of swordfish (Xiphias gladius) and common sardine (Strangomera bentincki) off Chile under the A2 IPCC climate change scenario. Prog. Oceanogr. 2015, 134, 343–355. [Google Scholar] [CrossRef]
- Magnusson, A.; Punt, A.E.; Hilborn, R. Measuring uncertainty in fisheries stock assessment: The delta method, bootstrap, and MCMC. Fish Fish. 2013, 14, 325–342. [Google Scholar] [CrossRef]
- Beyer, R.; Krapp, M.; Manica, A. An empirical evaluation of bias correction methods for palaeoclimate simulations. Clim. Past 2020, 16, 1493–1508. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Brodeur, R.D.; Okey, T.A.; Pauly, D. Projecting future changes in distributions of pelagic fish species of Northeast Pacific shelf seas. Prog. Oceanogr. 2015, 130, 19–31. [Google Scholar] [CrossRef]
- Nitin, P.; Nirmale, V.H.; Metar, S.Y.; Bhosale, B.P.; Sawant, M.S.; Naik, S.D. Age, growth and mortality studies of Indian squid, Uroteuthis (Photololigo) duvauceli (d’Orbigny) along Ratnagiri coast of Maharashtra, India. Indian J. Geo-Mar. Sci. 2015, 1, 93–96. [Google Scholar]
- Worms, J. Loligo vulgaris. In Cephalopod Life Cycles Vol. I: Species Accounts; Boyle, P.R., Ed.; Academic Press: London, UK, 1983; pp. 143–157. [Google Scholar]
- Mohamed, K.S. Spawning congregations of Indian squid Loligo duvauceli (Cephalopoda Loliginidae) in the Arabian Sea off Mangalore and Malpe. Indian J. Mar. Sci. 1993, 22, 172–175. [Google Scholar]
- Park, Y.C.; Yoda, M.; Hiyama, Y. Stock assessment for swordtip squid, Loligo edulis, in the East China Sea and the southwest sea of Japan. Fish. Sci. 2002, 68 (Suppl. 1), 89–92. [Google Scholar] [CrossRef]
- Li, N. Study on Population Structure, Age, Growth and Migration of Uroteuthis edulis Based on Statolith Information in the Northern Waters of the East China Sea. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2024. (In Chinese). [Google Scholar]
- Chen, F.; Li, N.; Fang, Z.; Liang, J.; Zhang, H.L.; Zhou, Y.D.; Jiang, R.J. Habitat distribution change pattern of Uroteuthis edulis during spring and summer in the coastal waters of Zhejiang Province. J. Shanghai Ocean Univ. 2021, 30, 847–855. (In Chinese) [Google Scholar]
- Liao, C.H.; Lan, K.W.; Ho, H.Y.; Wang, K.Y.; Wu, Y.L. Variation in the catch rate and distribution of swordtip squid Uroteuthis edulis associated with factors of the oceanic environment in the southern East China Sea. Mar. Coast. Fish. 2018, 10, 452–464. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takayama, K.; Hirose, N.; Matsuyama, M. The Sea of Amakusa playing the role of a distributor of swordtip squid (Uroteuthis edulis) migrating from the East China Sea to the east and west sides of Japan. Fish. Res. 2020, 225, 105475. [Google Scholar] [CrossRef]
- Du, Y.; Xu, B.; Xue, Y.; Ji, Y.; Ren, Y.; Zhang, C. Seasonal variations of spatial structure of Japanese squid (Loligo japonica) and octopus (Octopus ochellatus) in Haizhou Bay. J. Fish. China 2017, 41, 1888–1895. (In Chinese) [Google Scholar]
- Wei, Z.B. Preliminary observation on AmphiOctopus fangsiao. Chin. J. Zool. 1966, 1, 26–28. (In Chinese) [Google Scholar]
- Rodhouse, P.G.; Pierce, G.J.; Nichols, O.C.; Sauer, W.H.; Arkhipkin, A.I.; Laptikhovsky, V.V.; Lipiński, M.R.; Ramos, J.E.; Gras, M.; Kidokoro, H.; et al. Environmental effects on cephalopod population dynamics: Implications for management of fisheries. Adv. Mar. Biol. 2014, 67, 99–233. [Google Scholar]
- Rowell, T.W.; Young, J.H.; Poulard, J.C.; Robin, J.P. Changes in the distribution and biological characteristics of Illex illecebrosus on the Scotian shelf, 1980–1983. NAFO Sci. Coun Stud. 1985, 9, 11–26. [Google Scholar]
- Martins, R.S.; Perez, J.A.A. The ecology of loliginid squid in shallow waters around Santa Catarina Island, Southern Brazil. Bull. Mar. Sci. 2007, 80, 125–146. [Google Scholar]
- Cabanellas-Reboredo, M.; Alós, J.; Palmer, M.; Morales-Nin, B. Environmental effects on recreational squid jigging fishery catches. ICES J. Mar. Sci. 2012, 69, 1823–1830. [Google Scholar] [CrossRef]
- Jackson, D.G. Advances in defining the life histories of myopsid squid. Mar. Freshwat. Res. 2004, 55, 357–365. [Google Scholar] [CrossRef]
- Postuma, F.A.; Gasalla, M.A. On the relationship between squids and environmental conditions: The artisanal jigging for Loligo plei at São Sebastião Island 24° (S), Southeastern Brazil. ICES J. Mar. Sci. 2010, 67, 1353–1362. [Google Scholar] [CrossRef][Green Version]
- Sauer, W.H.H.; Goschen, W.S.; Koorts, A.S. A preliminary investigation of the effect of sea temperature fluctuations and wind direction on catches of Chokka squid Loligo vulgaris reynaudii off the Eastern Cape, South Africa. Afr. J. Mar. Sci. 1991, 11, 467–473. [Google Scholar] [CrossRef][Green Version]
- Sims, D.W.; Genner, M.J.; Southward, A.J.; Hawkins, S.J. Timing of squid migration reflects North Atlantic climate variability. Proc. R. Soc. Lond. Ser. B Biol. Scis. 2001, 268, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Kornder, N.A.; Riegl, B.M.; Figueiredo, J. Thresholds and drivers of coral calcification responses to climate change. Glob. Change Biol. 2018, 24, 5084–5095. [Google Scholar] [CrossRef]
- Cai, R.; Tan, H.; Qi, Q. Impacts of and adaptation to inter-decadal marine climate change in coastal China seas. Int. J. Climatol. 2016, 36, 3770–3780. [Google Scholar] [CrossRef]
- Payne, N.L.; Smith, J.A.; van der Meulen, D.E.; Taylor, M.D.; Watanabe, Y.Y.; Takahashi, A.; Cadiou, G.; Payne, N.L.; Watanabe, Y.Y.; van der Meulen, D.E. Temperature dependence of fish performance in the wild: Links with species biogeography and physiological thermal tolerance. Funct. Ecol. 2016, 30, 903–912. [Google Scholar] [CrossRef]
- Pierce, G.J.; Guerra, A. Stock assessment methods used for cephalopod fisheries. Fish. Res. 1994, 21, 255–285. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Kirkwood, G.P.; Crombie, J.A.; Beddington, J.R. The assessment of annual stocks of squid species. Fish. Res. 1990, 8, 335–350. [Google Scholar] [CrossRef]
| Season | U. duvauceli | U. edulis | L. sumatrensis | L. japonica | ||||
|---|---|---|---|---|---|---|---|---|
| CPUEw | CPUEn | CPUEw | CPUEn | CPUEw | CPUEn | CPUEw | CPUEn | |
| Spring | 205.54 | 9 | 12,201.04 | 494 | / | / | 459.16 | 19 |
| Summer | 3202.84 | 52 | 47,658.02 | 1041 | 1137.32 | 92 | 26 | 2 |
| Autumn | 9495.05 | 525 | 29,493.32 | 2532 | 1543.66 | 114 | 2.688 | 5 |
| Winter | 590.2 | 14 | 2606.87 | 145 | 701.9 | 53 | 28.3 | 3 |
| Total | 13,493.63 | 600 | 91,959.25 | 4212 | 3382.88 | 259 | 516.148 | 29 |
| Variable | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Uroteuthis duvauceli | ||||
| Depth (m) | 49–61 | 19–68 | 36–97 | 46–105 |
| SST (°C) | 17.17–18.58 | 25.97–29.09 | 18.66–23.13 | 15.03–17.54 |
| SBT (°C) | 17.83–18.85 | 20.14–26.97 | 17.45–22.83 | 15.24–17.57 |
| SSS (‰) | 30.46–32.02 | 29.81–32.82 | 31.86–34.38 | 34.06–34.18 |
| SBS (‰) | 33.45–34.4 | 30.2–34.08 | 31.88–34.53 | 34.12–34.27 |
| Uroteuthis edulis | ||||
| Depth (m) | 22–140 | 10–133 | 35–135 | 40–126 |
| SST (°C) | 13.28–25.99 | 25.25–29.5 | 18.66–26.29 | 11.21–22.34 |
| SBT (°C) | 11.73–22.79 | 17.23–28.19 | 17.88–23.15 | 11.24–21.55 |
| SSS (‰) | 30.08–34.63 | 27.69–34.3 | 31.86–34.45 | 32.16–34.52 |
| SBS (‰) | 30.55–35.25 | 31.31–34.68 | 31.88–35.07 | 32.08–34.61 |
| Loliolus sumatrensis | ||||
| Depth (m) | / | 31–97 | 54–100 | 49–90 |
| SST (°C) | / | 25.11–29.67 | 21.46–23.66 | 11.75–18.06 |
| SBT (°C) | / | 16.62–26.28 | 20.99–23.15 | 11.78–18.29 |
| SSS (‰) | / | 29.59–33.7 | 33.65–34.23 | 32.09–34.39 |
| SBS (‰) | / | 32.17–34.59 | 34.09–34.5 | 32–34.51 |
| Loliolus japonica | ||||
| Depth (m) | 29–83 | 67 | 33 | 15–16 |
| SST (°C) | 13.37–17.91 | 27.21 | 18.89 | 8.09–8.21 |
| SBT (°C) | 9.6–17.83 | 10.74 | 18.9 | 8.14–8.17 |
| SSS (‰) | 29.38–32.73 | 28.76 | 31.53 | 31.86–31.95 |
| SBS (‰) | 31.26–33.45 | 32.87 | 31.49 | 31.67–31.98 |
| Measurement | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Uroteuthis duvauceli | ||||
| Mean CPUEw at collection stations | 68.51 | 533.81 | 730.39 | 147.55 |
| Value range of CPUEw | 41.68–114.2 | 33–1752.54 | 38.8–3768.33 | 34.8–392 |
| Mean CPUEn at collection stations | 3 | 8.67 | 40.38 | 3.5 |
| Value range of CPUEn | 1–6 | 3–19 | 2–370 | 1–8 |
| Mean AIW | 37.9 | 45.57 | 61.19 | 40.8 |
| Value range of AIW | 6.95–57.1 | 9.28–92.24 | 10.18–321.3 | 28–51.4 |
| Uroteuthis edulis | ||||
| Mean CPUEw at collection stations | 406.7 | 1059.07 | 737.33 | 162.93 |
| Value range of CPUEw | 12.3–7018.4 | 23.4–20,684.4 | 5.14–3742 | 17.5–747 |
| Mean CPUEn at collection stations | 16.47 | 23.13 | 63.3 | 9.06 |
| Value range of CPUEn | 1–356 | 1–549 | 1–488 | 1–33 |
| Mean AIW | 50.04 | 67 | 71.87 | 22.74 |
| Value range of AIW | 6.26–123.1 | 5.6–175.41 | 0.8–384 | 2.95–59 |
| Loliolus sumatrensis | ||||
| Mean CPUEw at collection stations | / | 162.47 | 308.73 | 116.98 |
| Value range of CPUEw | / | 4.1–481 | 23.66–858 | 17.4–249.6 |
| Mean CPUEn at collection stations | / | 13.14 | 22.8 | 8.83 |
| Value range of CPUEn | / | 1–47 | 2–60 | 1–36 |
| Mean AIW | / | 12.9 | 11.17 | 23.12 |
| Value range of AIW | / | 2–27 | 2.3–24.5 | 6.63–43.35 |
| Loliolus japonica | ||||
| Mean CPUEw at collection stations | 114.79 | 52 | 13.44 | 14.15 |
| Value range of CPUEw | 23.28–372.63 | 52 | 13.44 | 3.9–24.4 |
| Mean CPUEn at collection stations | 4.75 | 2 | 5 | 1.5 |
| Value range of CPUEn | 2–13 | 2 | 5 | 1–2 |
| Mean AIW | 17.98 | 26 | 2.69 | 8.05 |
| Value range of AIW | 11.64–28.66 | 26 | 2.69 | 3.9–12.2 |
| Scenario | Uroteuthis duvauceli | Uroteuthis edulis | ||||
|---|---|---|---|---|---|---|
| Loss% | Gain% | Gain–Loss% | Loss% | Gain% | Gain–Loss% | |
| SSP126-2050 | −13.504 | 10.955 | −2.549 | 0 | 26.674 | 26.674 |
| SSP126-2100 | −16.812 | 14.65 | −2.162 | −0.087 | 20.174 | 20.087 |
| SSP245-2050 | −13.705 | 12.101 | −1.604 | 0 | 25.583 | 25.583 |
| SSP245-2100 | −25.09 | 20.564 | −4.525 | 0 | 24.34 | 24.34 |
| SSP370-2050 | −10.683 | 12.072 | 1.389 | −0.065 | 20.065 | 20 |
| SSP370-2100 | −61.378 | 2.764 | −58.614 | 0 | 36.445 | 36.445 |
| SSP585-2050 | −16.855 | 15.208 | −1.647 | 0 | 29.706 | 29.706 |
| SSP585-2100 | −55.521 | 1.575 | −53.945 | 0 | 51.32 | 51.32 |
| Scenario | Loliolus sumatrensis | Loliolus japonica | ||||
| Loss% | Gain% | Gain–Loss% | Loss% | Gain% | Gain–Loss% | |
| SSP126-2050 | –0.491 | 71.333 | 70.842 | –100 | 0 | 100 |
| SSP126-2100 | –0.697 | 111.88 | 111.183 | –100 | 0 | 100 |
| SSP245-2050 | –0.62 | 76.705 | 76.085 | –100 | 0 | 100 |
| SSP245-2100 | –5.656 | 193.233 | 187.577 | –100 | 0 | 100 |
| SSP370-2050 | –2.04 | 76.756 | 74.716 | –100 | 0 | 100 |
| SSP370-2100 | –14.618 | 270.713 | 256.095 | –100 | 0 | 100 |
| SSP585-2050 | –0.517 | 90.393 | 89.876 | –100 | 0 | 100 |
| SSP585-2100 | –25.413 | 274.897 | 249.483 | –100 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhang, H.; Xu, B.; Liu, Y.; Yang, L. Seasonal Spatial Distribution Characteristics and Patterns of the Squid Uroteuthis duvauceli, Uroteuthis edulis, Loliolus sumatrensis, and Loliolus japonica in the Southern Yellow and East China Seas: Predictions Under Different Climate Scenarios. Animals 2025, 15, 1744. https://doi.org/10.3390/ani15121744
Xu M, Zhang H, Xu B, Liu Y, Yang L. Seasonal Spatial Distribution Characteristics and Patterns of the Squid Uroteuthis duvauceli, Uroteuthis edulis, Loliolus sumatrensis, and Loliolus japonica in the Southern Yellow and East China Seas: Predictions Under Different Climate Scenarios. Animals. 2025; 15(12):1744. https://doi.org/10.3390/ani15121744
Chicago/Turabian StyleXu, Min, Hui Zhang, Bingqing Xu, Yong Liu, and Linlin Yang. 2025. "Seasonal Spatial Distribution Characteristics and Patterns of the Squid Uroteuthis duvauceli, Uroteuthis edulis, Loliolus sumatrensis, and Loliolus japonica in the Southern Yellow and East China Seas: Predictions Under Different Climate Scenarios" Animals 15, no. 12: 1744. https://doi.org/10.3390/ani15121744
APA StyleXu, M., Zhang, H., Xu, B., Liu, Y., & Yang, L. (2025). Seasonal Spatial Distribution Characteristics and Patterns of the Squid Uroteuthis duvauceli, Uroteuthis edulis, Loliolus sumatrensis, and Loliolus japonica in the Southern Yellow and East China Seas: Predictions Under Different Climate Scenarios. Animals, 15(12), 1744. https://doi.org/10.3390/ani15121744






