Correlation Analysis of Sperm Cryopreservation Quality with Serum Testosterone and Sperm gDNA Methylation Levels in Xiaoshan Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Semen Thawing and Sperm Quality Assessment
2.3. Serum Indicator Measurement
2.4. gDNA Extraction
2.5. Library Construction and Quality Control
2.6. Data Analysis
2.6.1. Analysis of Sperm Quality and Serum Indicators
2.6.2. Methylation Sequencing Data Analysis
2.7. Correlation Analysis of Key Gene Methylation Levels with Frozen Semen Quality and Serum Testosterone Levels
3. Results
3.1. Serum Testosterone Levels and Cryopreserved Sperm Qualities
3.2. Correlation Analysis Between Cryopreserved Sperm Qualities and Testosterone Level
3.3. Sperm and Semen Indexes in High- and Low-Quality Groups
3.4. Quality and Statistics of DNA Methylation Sequencing Data
3.5. Distribution of Genome-Wide Methylation Sites
3.6. Analysis of Differentially Methylated Regions
3.7. Enrichment and Analysis of Differentially Methylated Genes
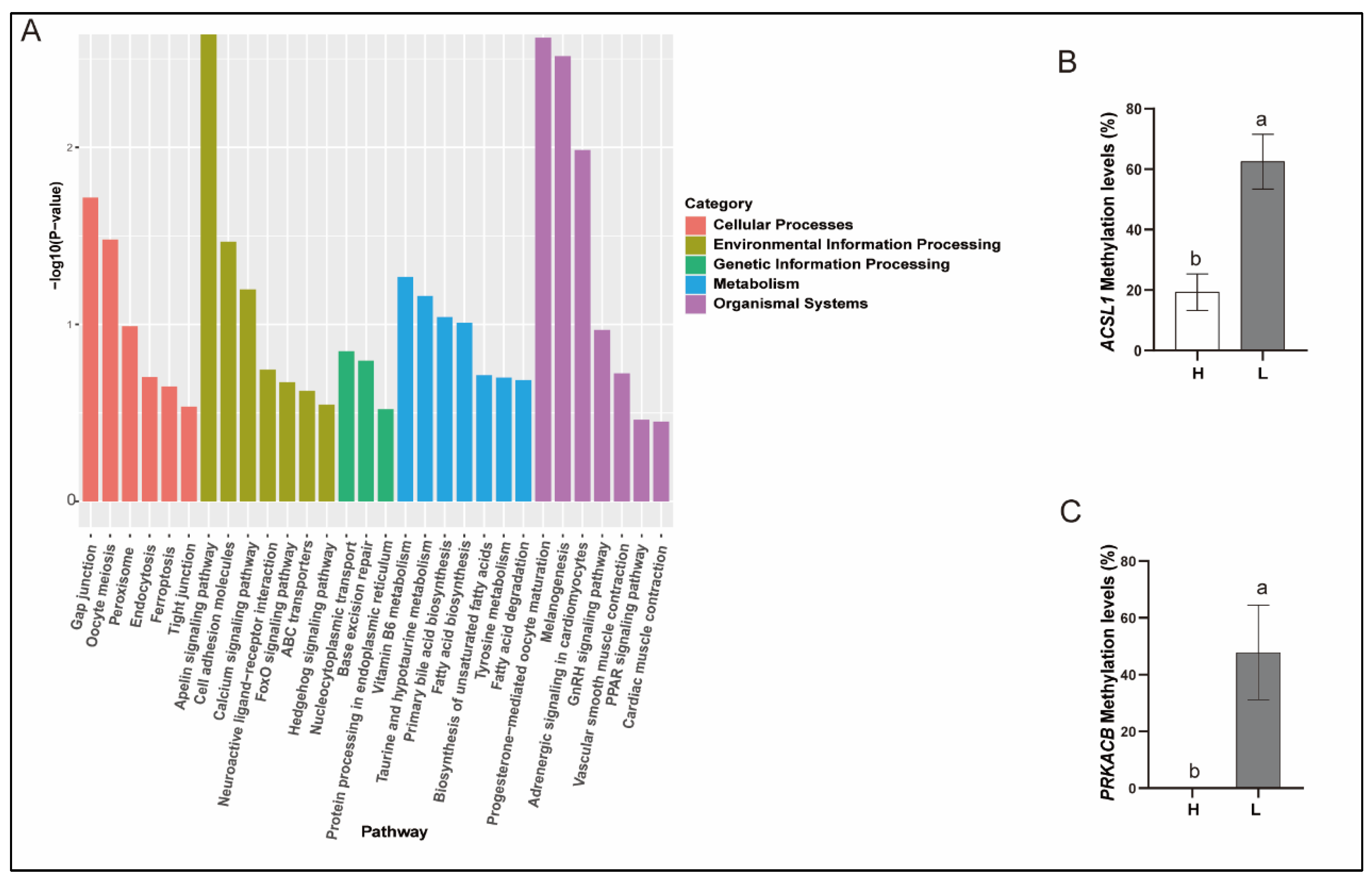
3.8. Analysis of the Correlation Between Key Genes’ Methylation Levels and Cryopreserved Sperm Qualities and Serum Testosterone Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Liu, J.; Liu, Y.; Li, X.; An, D.; Liu, X.; Zhang, L. Alpha-lipoic acid improves cryopreservation of rooster semen by reducing oxidative stress. Poult. Sci. 2024, 103, 103632. [Google Scholar] [CrossRef] [PubMed]
- Abouelezz, F.; Sayed, M.; Santiago-Moreno, J. Fertility disturbances of dimethylacetamide and glycerol in rooster sperm diluents: Discrimination among effects produced pre and post freezing-thawing process. Anim. Reprod. Sci. 2017, 184, 228–234. [Google Scholar] [CrossRef]
- Tang, M.; Cao, J.; Yu, Z.; Liu, H.; Yang, F.; Huang, S.; He, J.; Yan, H. New semen freezing method for chicken and drake using dimethylacetamide as the cryoprotectant. Poult. Sci. 2021, 100, 101091. [Google Scholar] [CrossRef] [PubMed]
- Mehaisen, G.M.K.; Elomda, A.M.; Hamad, S.K.; Ghaly, M.M.; Sun, Y.; Li, Y.; Zong, Y.; Chen, J.; Partyka, A.; Nazmi, A.; et al. Effect of Dimethylacetamide Concentration on Motility, Quality, Antioxidant Biomarkers, Anti-Freeze Gene Expression, and Fertilizing Ability of Frozen/Thawed Rooster Sperm. Animals 2022, 12, 2739. [Google Scholar] [CrossRef]
- Ratchamak, R.; Authaida, S.; Boonkum, W.; Chankitisakul, V. Improvement of rooster semen freezability and fertility rate after sericin supplementation in freezing semen extender. Anim. Biosci. 2023, 36, 1530–1535. [Google Scholar] [CrossRef]
- Janosikova, M.; Petricakova, K.; Ptacek, M.; Savvulidi, F.G.; Rychtarova, J.; Fulka, J.J. New approaches for long-term conservation of rooster spermatozoa. Poult. Sci. 2023, 102, 102386. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.; Sharafi, M.; Gourabi, H.; Yekta, A.A.; Esmaeili, V.; Sharbatoghli, M.; Janzamin, E.; Hajnasrollahi, M.; Mostafayi, F. Fertility and flow cytometric evaluations of frozen-thawed rooster semen in cryopreservation medium containing low-density lipoprotein. Theriogenology 2015, 83, 78–85. [Google Scholar] [CrossRef]
- Chuaychu-Noo, N.; Thananurak, P.; Chankitisakul, V.; Vongpralub, T. Supplementing rooster sperm with Cholesterol-Loaded-Cyclodextrin improves fertility after cryopreservation. Cryobiology 2017, 74, 8–12. [Google Scholar] [CrossRef]
- Partyka, A.; Nizanski, W. Supplementation of Avian Semen Extenders with Antioxidants to Improve Semen Quality-Is It an Effective Strategy? Antioxidants 2021, 10, 1927. [Google Scholar] [CrossRef]
- Khosravizadeh, Z.; Khodamoradi, K.; Rashidi, Z.; Jahromi, M.; Shiri, E.; Salehi, E.; Talebi, A. Sperm cryopreservation and DNA methylation: Possible implications for ART success and the health of offspring. J. Assist. Reprod. Genet. 2022, 39, 1815–1824. [Google Scholar] [CrossRef]
- Montjean, D.; Zini, A.; Ravel, C.; Belloc, S.; Dalleac, A.; Copin, H.; Boyer, P.; McElreavey, K.; Benkhalifa, M. Sperm global DNA methylation level: Association with semen parameters and genome integrity. Andrology 2015, 3, 235–240. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, Q.; Lu, Y.; Li, M.; Zhou, Y.; Wu, P.; Li, J.; Pan, F.; Han, X.; Chen, M.; et al. Semen quality and sperm DNA methylation in relation to long-term exposure to air pollution in fertile men: A cross-sectional study. Environ. Pollut. 2022, 300, 118994. [Google Scholar] [CrossRef]
- Olesen, I.A.; Andersson, A.M.; Aksglaede, L.; Skakkebaek, N.E.; Rajpert-de Meyts, E.; Joergensen, N.; Juul, A. Clinical, genetic, biochemical, and testicular biopsy findings among 1,213 men evaluated for infertility. Fertil. Steril. 2017, 107, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Albero, M.D.C.; Abril Sánchez, S.; Ros-Santaella, J.L.; Pintus, E.; Luongo, C.; Ruiz Díaz, S.; Barros García, C.; Sánchez Calabuig, M.J.; García Párraga, D.; García Vázquez, F.A. Characterization of Bottlenose Dolphin (Tursiops truncatus) Sperm Based on Morphometric Traits. Biology 2021, 10, 355. [Google Scholar] [CrossRef]
- Aurich, C.; Schreiner, B.; Ille, N.; Alvarenga, M.; Scarlet, D. Cytosine methylation of sperm DNA in horse semen after cryopreservation. Theriogenology 2016, 86, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Raspa, M.; Paoletti, R.; Mahabir, E.; Scavizzi, F. d-aspartate treatment in vitro improves mouse sperm fertility in young B6N mice. Theriogenology 2020, 148, 60–67. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, F.; Li, Y.; Fu, L.; Bai, H.; Ma, H.; Xu, S.; Chen, J. Differences in semen quality, testicular histomorphology, fertility, reproductive hormone levels, and expression of candidate genes according to sperm motility in Beijing-You chickens. Poult. Sci. 2019, 98, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, Y.; Li, D.; Han, J.; Liu, Y.; Bai, L.; Huang, T.; Cui, M.; Wang, P.; Zheng, X.; et al. Metabolites assay offers potential solution to improve the rooster semen cryopreservation. Theriogenology 2024, 221, 9–17. [Google Scholar] [CrossRef]
- Krueger, F.; Andrews, S.R. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 2011, 27, 1571–1572. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, M.; Forouzanfar, M.; Eghbalsaied, S. Supplementation of Estradiol Into Semen Extender Improved Goat Sperm Cryopreservation. Biopreserv. Biobank. 2022, 20, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Asl, A.J.; Sharifi, M.; Dashti, A.; Dashti, G.R. Relationship between long non-coding RNA MALAT1 and HOTAIR expression with sperm parameters, DNA and malondialdehyde levels in male infertility. Tissue Cell 2023, 85, 102248. [Google Scholar] [CrossRef]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef]
- Depincé, A.; Gabory, A.; Dziewulska, K.; Le Bail, P.Y.; Jammes, H.; Labbé, C. DNA methylation stability in fish spermatozoa upon external constraint: Impact of fish hormonal stimulation and sperm cryopreservation. Mol. Reprod. Dev. 2020, 87, 124–134. [Google Scholar] [CrossRef]
- Liu, M.; Liu, P.; Chang, Y.; Xu, B.; Wang, N.; Qin, L.; Zheng, J.; Liu, Y.; Wu, L.; Yan, H. Genome-wide DNA methylation profiles and small noncoding RNA signatures in sperm with a high DNA fragmentation index. J. Assist. Reprod. Genet. 2022, 39, 2255–2274. [Google Scholar] [CrossRef]
- Gaitskell-Phillips, G.; Martín-Cano, F.E.; da Silva-Álvarez, E.; Tapia, J.A.; Silva, A.; Gil, M.C.; Ortega-Ferrusola, C.; Peña, F.J. Phosphoproteomics for the identification of new mechanisms of cryodamage: The role of SPATA18 in the control of stallion sperm functiondagger. Biol. Reprod. 2023, 108, 324–337. [Google Scholar] [CrossRef]
- Sekar, B.; Arangasamy, A.; Naidu, S.J.; Reddy, I.J.; Bhatta, R. Organic mineral supplementation on differential protein profile of Osmanabadi bucks (Capra hircus). Reprod. Biol. 2021, 21, 100533. [Google Scholar] [CrossRef]
- Fan, Q.; He, R.; Li, Y.; Gao, P.; Huang, R.; Li, R.; Zhang, J.; Li, H.; Liang, X. Studying the effect of hyperoside on recovery from cyclophosphamide induced oligoasthenozoospermia. Syst. Biol. Reprod. Med. 2023, 69, 333–346. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, C.; Wang, J.; Li, X.; Wang, P.; Fang, Y.; Zhang, Y.; Ding, N.; Jiang, L. Proteomic Analysis of Sperm with Differernt Freezability in Chinese Holstein Bulls. Acta Vet. Et Zootech. Sin. 2024, 55, 1052–1061. [Google Scholar]



| T (pmol/L) | LIN/% | TM/% | SM/% | Dmr/% | DD/% | PD/% | NM/% |
|---|---|---|---|---|---|---|---|
| 66.76 ± 17.36 | 11.47 ± 7.77 | 48.77 ± 20.25 | 8.05 ± 4.40 | 2.78 ± 2.35 | 4.35 ± 3.93 | 2.61 ± 2.31 | 82.40 ± 12.75 |
| Indexes | T | TM | LIN | SM | DD | PD | NM | CT |
|---|---|---|---|---|---|---|---|---|
| T | 1 | |||||||
| TM | 0.825 ** | 1 | ||||||
| LIN | 0.539 ** | 0.697 ** | 1 | |||||
| SM | 0.15 | 0.283 ** | −0.026 | 1 | ||||
| DD | −0.429 ** | −0.473 ** | −0.245 * | −0.309 ** | 1 | |||
| PD | −0.285 ** | −0.321 ** | −0.239 * | −0.321 ** | 0.353 ** | 1 | ||
| NM | 0.481 ** | 0.591 ** | 0.345 ** | 0.380 ** | −0.493 ** | −0.375 ** | 1 | |
| CT | −0.385 ** | −0.180 | −0.121 | 0.014 | 0.128 | 0.086 | −0.228 * | 1 |
| Groups | LIN/% | TM/% | SM/% | CT/% | NM/% |
|---|---|---|---|---|---|
| H | 34.25 ± 10.15 a | 73.93 ± 12.57 a | 4.30 ± 1.20 | 0.00 ± 0.00 a | 89.80 ± 1.94 a |
| L | 3.65 ± 0.39 b | 18.28 ± 2.92 b | 4.88 ± 3.35 | 0.25 ± 0.50 b | 67.85 ± 16.77 b |
| Groups | T (pmol/L) | E2 (pmol/L) | SOD (U/mL) | GSH-px (U/mL) |
|---|---|---|---|---|
| H | 68.01 ± 6.24 a | 94.50 ± 16.32 | 70.68 ± 6.28 | 2400.00 ± 96.21 |
| L | 79.36 ± 3.31 b | 89.07 ± 13.66 | 73.44 ± 10.71 | 2363.64 ± 218.18 |
| Sample | Reads Num | Raw Bases (bp) | Clean Bases (bp) | GC Content/% | Q20/% | Q30/% | BS Conversion Rate/% |
|---|---|---|---|---|---|---|---|
| H1 | 222,315,222 | 33,569,598,522 | 30,221,998,491 | 23.19 | 97.49 | 93.89 | 99.49 |
| H2 | 182,670,788 | 27,583,288,988 | 24,848,377,670 | 23.31 | 97.49 | 93.83 | 99.51 |
| H3 | 182,225,238 | 27,516,010,938 | 24,812,522,476 | 23.64 | 97.54 | 93.96 | 99.5 |
| H4 | 193,707,308 | 29,249,803,508 | 26,349,086,697 | 22.96 | 97.48 | 93.83 | 99.5 |
| L1 | 207,922,812 | 31,396,344,612 | 28,295,6320,93 | 23.93 | 97.51 | 93.91 | 99.51 |
| L2 | 208,033,018 | 31,412,985,718 | 28,334,726,009 | 23.47 | 97.57 | 94.08 | 99.51 |
| L3 | 206,532,318 | 31,186,380,018 | 28,066,514,129 | 23.79 | 97.41 | 93.7 | 99.5 |
| L4 | 210,131,334 | 31,729,831,434 | 28,622,210,423 | 23.21 | 97.55 | 94.01 | 99.51 |
| Samples | mCG/CG/% | mCHG/CHG/% | mCHH/CHH/% | mC/C/% |
|---|---|---|---|---|
| H1 | 47.30 | 0.30 | 0.40 | 2.52 |
| H2 | 45.80 | 0.30 | 0.30 | 2.51 |
| H3 | 47.90 | 0.30 | 0.40 | 2.69 |
| H4 | 46.70 | 0.30 | 0.40 | 2.46 |
| Mean (H) | 46.93 | 0.30 | 0.38 | 2.54 |
| L1 | 48.90 | 0.30 | 0.40 | 2.79 |
| L2 | 46.90 | 0.30 | 0.30 | 2.57 |
| L3 | 47.80 | 0.30 | 0.40 | 2.71 |
| L4 | 46.40 | 0.30 | 0.30 | 2.54 |
| Mean (L) | 47.50 | 0.30 | 0.35 | 2.65 |
| Mean (H + L) | 47.21 | 0.30 | 0.36 | 2.60 |
| DMR | Num | mCG | Length/bp |
|---|---|---|---|
| Total | 217 | 1365 | 51~915 |
| Gene | 125 | 799 | 51~915 |
| Promoter | 16 | 128 | 59~135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Li, D.; Jia, L.; Tong, X.; Huang, Z.; Liu, Y.; Wang, P.; Zhao, A. Correlation Analysis of Sperm Cryopreservation Quality with Serum Testosterone and Sperm gDNA Methylation Levels in Xiaoshan Chickens. Animals 2025, 15, 1745. https://doi.org/10.3390/ani15121745
Du X, Li D, Jia L, Tong X, Huang Z, Liu Y, Wang P, Zhao A. Correlation Analysis of Sperm Cryopreservation Quality with Serum Testosterone and Sperm gDNA Methylation Levels in Xiaoshan Chickens. Animals. 2025; 15(12):1745. https://doi.org/10.3390/ani15121745
Chicago/Turabian StyleDu, Xue, Duoxi Li, Luya Jia, Xiaopeng Tong, Zhiyuan Huang, Yali Liu, Panlin Wang, and Ayong Zhao. 2025. "Correlation Analysis of Sperm Cryopreservation Quality with Serum Testosterone and Sperm gDNA Methylation Levels in Xiaoshan Chickens" Animals 15, no. 12: 1745. https://doi.org/10.3390/ani15121745
APA StyleDu, X., Li, D., Jia, L., Tong, X., Huang, Z., Liu, Y., Wang, P., & Zhao, A. (2025). Correlation Analysis of Sperm Cryopreservation Quality with Serum Testosterone and Sperm gDNA Methylation Levels in Xiaoshan Chickens. Animals, 15(12), 1745. https://doi.org/10.3390/ani15121745






