Gradual Thickening of the Peritubular Lamina Propria in Healthy Boar Seminiferous Tubules Due to Cryptorchidism: Increased Immunoexpression of Diverse Proteins in Sertoli and Myoid Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Preparation
2.2. Histochemical Study and Immunohistochemical Identification of PLP-Related Proteins
2.3. Histochemical Semiquantitative Analysis of PLP in Seminiferous Tubule Atrophy Due to Cryptorchidism
2.4. Semiquantitative Analysis of HSP47, Vimentin, α-Actin, and Collagen IV Expression in Relation to Seminiferous Tubule Atrophy Due to Cryptorchidism
2.5. Statistical Analysis
3. Results
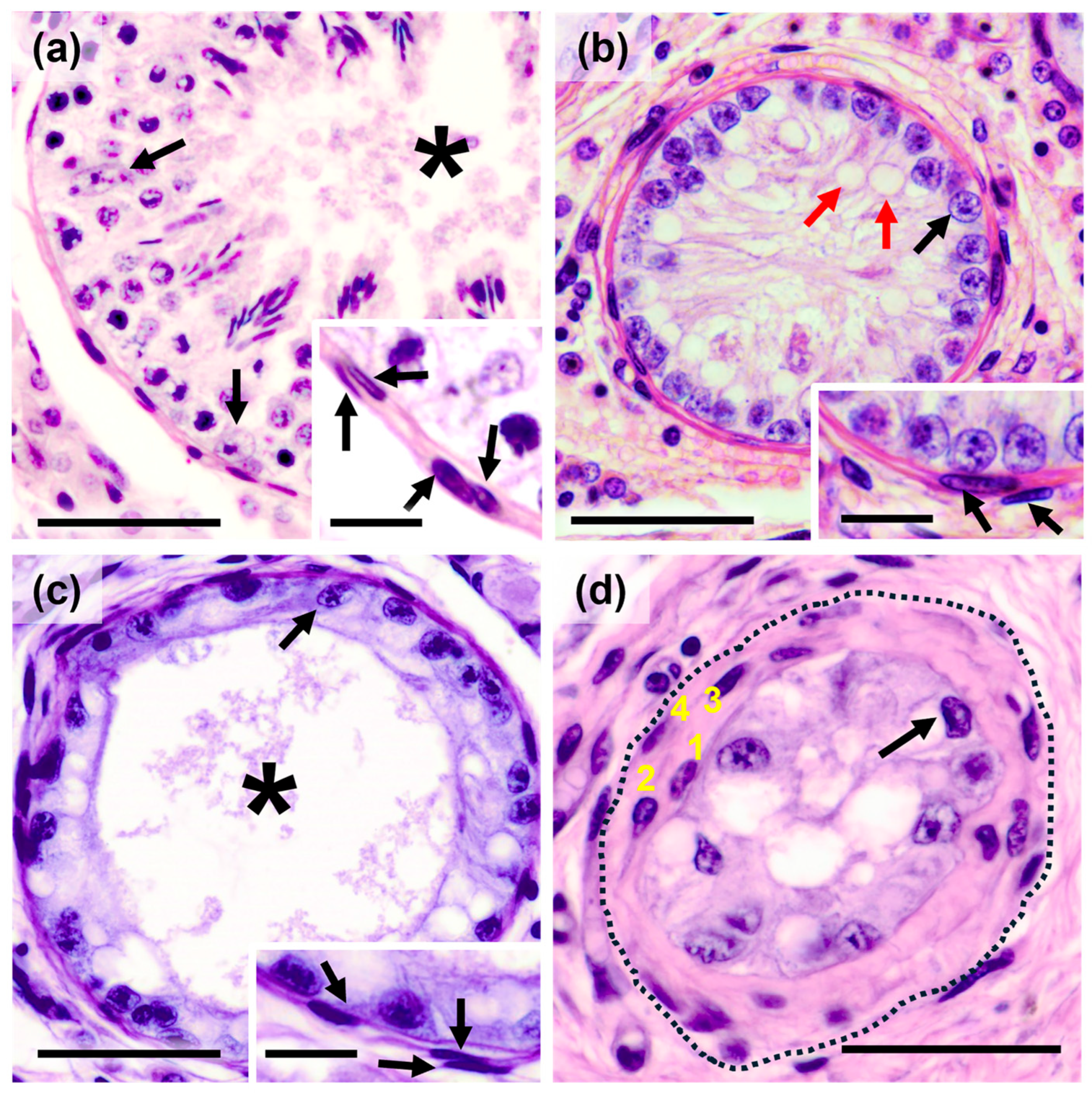
3.1. Histological Characterization of the Degree of Testicular Atrophy Due to Cryptorchidism
3.2. Semiquantitative Histochemical Evaluation of PLP Components During Seminiferous Tubule Atrophy in Cryptorchidism
3.3. Semiquantitative Immunohistochemical Evaluation of PLP in Seminiferous Tubules During Testicular Atrophy in Cryptorchidism
3.3.1. Heat Shock Protein 47
3.3.2. Vimentin
3.3.3. α-Actin in PLP Myoid Cells
3.3.4. Immunoexpression of Collagen IV in the Basement Membrane of Seminiferous Tubules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paniagua, R.; Nistal, M.; Sáez, F.J.; Fraile, B. Ultrastructure of the aging human testis. J. Electron. Microsc. Tech. 1991, 19, 241–260. [Google Scholar] [CrossRef]
- Haider, S.G.; Talati, J.; Servos, G. Ultrastructure of peritubular tissue in association with tubular hyalinization in human testis. Tissue Cell 1999, 31, 90–98. [Google Scholar] [CrossRef]
- Volkmann, J.; Müller, D.; Feuerstacke, C.; Kliesch, S.; Bergmann, M.; Mühlfeld, C.; Middendorff, R. Disturbed spermatogenesis associated with thickened lamina propria of seminiferous tubules is not caused by dedifferentiation of myofibroblasts. Hum. Reprod. 2011, 26, 1450–1461. [Google Scholar] [CrossRef]
- Santoro, G.; Romeo, C.; Impellizzeri, P.; Gentile, C.; Anastasi, G.; Santoro, A. Ultrastructural and immunohistochemical study of basal lamina of the testis in adolescent varicocele. Fertil. Steril. 2000, 73, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Santamaría, L.; Nistal, M.; Fraile, B.; Paniagua, R. The peritubular myofibroblasts in the testes from normal men and men with Klinefelter’s syndrome. A quantitative, ultrastructural, and immunohistochemical study. J. Pathol. 1992, 168, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, L.; Martín, R.; Nistal, M.; Paniagua, R. The peritubular myoid cells in the testes from men with varicocele: An ultrastructural, immunohistochemical and quantitative study. Histopathology 1992, 21, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C. Response of the human testis to long-term estrogen treatment: Morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell Tissue Res. 1988, 251, 31–43. [Google Scholar] [CrossRef]
- Pöllänen, P.P.; Kallajoki, M.; Risteli, L.; Risteli, J.; Suominen, J.J. Laminin and type iv collagen in the human testis. Int. J. Androl. 1985, 8, 337–347. [Google Scholar] [CrossRef]
- Bustos-Obregón, E.; Holstein, A.F. On structural patterns of the lamina propria of human seminiferous tubules. Z. Zellforsch. Mikrosk. Anat. 1973, 141, 413–425. [Google Scholar] [CrossRef]
- de Kretser, D.M.; Kerr, J.B.; Paulsen, C.A. The peritubular tissue in the normal and pathological human testis. An ultrastructural study. Biol. Reprod. 1975, 12, 317–324. [Google Scholar] [CrossRef]
- Dempsey, O.J.; Kerr, K.M.; Gomersall, L.; Remmen, H.; Currie, G.P. Idiopathic pulmonary fibrosis: An update. QJM 2006, 99, 643–654. [Google Scholar] [CrossRef]
- Arenas, M.I.; Bethencourt, F.R.; De Miguel, M.P.; Fraile, B.; Romo, E.; Paniagua, R. Immunocytochemical and quantitative study of actin, desmin and vimentin in the peritubular cells of the testes from elderly men. J. Reprod. Fertil. 1997, 110, 183–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, Y.; Nozawa, S.; Iwamoto, T. Study of spermatogenesis and thickening of lamina propria in the human seminiferous tubules. Fertil. Steril. 2008, 90, 1310–1312. [Google Scholar] [CrossRef]
- Santamaria, L.; Martinez-Onsurbe, P.; Paniagua, R.; Nistal, M. Laminin, type iv collagen, and fibronectin in normal and cryptorchid human testes. An immunohistochemical study. Int. J. Androl. 1990, 13, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Martinez-Onsurbe, P.; Santamaria, L.; Saez, F.J.; Amat, P.; Nistal, M. Quantitative and ultrastructural alterations in the lamina propria and Sertoli cells in human cryptorchid testes. Int. J. Androl. 1990, 13, 470–487. [Google Scholar] [CrossRef]
- Francavilla, S.; Santiemma, V.; Francavilla, F.; Bellocci, M.; Santucci, R.; Fabbrini, A. Human cryptorchidism: Changes in the basement membrane of the seminiferous tubules. Preliminary findings. Boll. Soc. Ital. Biol. Sper. 1978, 54, 154–160. [Google Scholar]
- Wang, Z.Q.; Watanabe, Y.; Toki, A.; Itano, T. Altered distribution of Sertoli cell vimentin and increased apoptosis in cryptorchid rats. J. Pediatr. Surg. 2002, 37, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Rune, G.M.; Mayr, J.; Neugebauer, H.; Anders, C.; Sauer, H. Pattern of Sertoli cell degeneration in cryptorchid prepubertal testes. Int. J. Androl. 1992, 15, 19–31. [Google Scholar] [CrossRef]
- Pinart, E.; Bonet, S.; Briz, M.; Pastor, L.M.; Sancho, S.; García, N.; Badia, E.; Bassols, J. Morphological and histochemical characteristics of the lamina propria in scrotal and abdominal testes from postpubertal boars: Correlation with the appearance of the seminiferous epithelium. J. Anat. 2001, 199, 435–448. [Google Scholar] [CrossRef]
- Pinart, E.; Sancho, S.; Briz, M.; Bonet, S. Morphologic study of the testes from spontaneous unilateral and bilateral abdominal cryptorchid boars. J. Morphol. 1999, 239, 225–243. [Google Scholar] [CrossRef]
- Pinart, E.; Sancho, S.; Briz, M.D.; Bonet, S.; Badia, E. Efficiency of the process of meiosis in scrotal testes of healthy boars and unilateral abdominal cryptorchid boars. Teratology 1999, 60, 209–214. [Google Scholar] [CrossRef]
- Pinart, E.; Sancho, S.; Briz, M.D.; Bonet, S.; Garcia, N.; Badia, E. Ultrastructural study of the boar seminiferous epithelium: Changes in cryptorchidism. J. Morphol. 2000, 244, 190–202. [Google Scholar] [CrossRef]
- Pinart, E.; Bonet, S.; Briz, M.; Sancho, S.; García, N.; Badia, E. Cytology of the interstitial tissue in scrotal and abdominal testes of post-puberal boars. Tissue Cell 2001, 33, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mañas, C.M.; Morales, E.; Pastor, L.M.; Pinart, E.; Bonet, S.; de la Rosa, P.; Briz, M.D.; Zuasti, A.; Ferrer, C.; Canteras, M. Proliferation and apoptosis of spermatogonia in postpuberal boar (Sus. domesticus) testes with spontaneous unilateral and bilateral abdominal cryptorchidism. Acta Histochem. 2005, 107, 365–372. [Google Scholar] [CrossRef]
- Nagata, K. Hsp47: A collagen-specific molecular chaperone. Trends Biochem. Sci. 1996, 21, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Horn, R.; Pastor, L.M.; Santamaría, L.; Pallarés, J.; Zuasti, A.; Ferrer, C.; Canteras, M. Involution of seminiferous tubules in aged hamsters: An ultrastructural, immunohistochemical and quantitative morphological study. Histol. Histopathol. 2004, 19, 445–455. [Google Scholar] [CrossRef]
- Beltrán-Frutos, E.; Seco-Rovira, V.; Ferrer, C.; Martínez-Hernández, J.; Madrid, J.F.; Sáez, F.J.; Canteras, M.; Pastor, L.M. Changes in testicular interstitial connective tissue of hamsters (Mesocricetus auratus) during ageing and after exposure to short photoperiod. Reprod. Domest. Anim. 2016, 51, 47–53. [Google Scholar] [CrossRef]
- Taguchi, T.; Razzaque, M.S. The collagen-specific molecular chaperone hsp47: Is there a role in fibrosis? Trends Mol. Med. 2007, 13, 45–53. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Le, V.T.; Taguchi, T. Heat shock protein 47 and renal fibrogenesis. Contrib. Nephrol. 2005, 148, 57–69. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Burgy, O.; Bonniaud, P.; Kolb, M. Hsp47: A potential target for fibrotic diseases and implications for therapy. Expert. Opin. Ther. Targets 2021, 25, 49–62. [Google Scholar] [CrossRef]
- Sato, K.; Yomogida, K.; Wada, T.; Yorihuzi, T.; Nishimune, Y.; Hosokawa, N.; Nagata, K. Type XXVI collagen, a new member of the collagen family, is specifically expressed in the testis and ovary. J. Biol. Chem. 2002, 277, 37678–37684. [Google Scholar] [CrossRef] [PubMed]
- Syntin, P.; Chen, H.; Zirkin, B.R.; Robaire, B. Gene expression in brown Norway rat Leydig cells: Effects of age and of age-related germ cell loss. Endocrinology 2001, 142, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Takihara, H.; Naito, K. Quantitative analysis of testicular interstitial fibrosis after vasectomy in humans. Aktuelle Urol. 2003, 34, 262–264. [Google Scholar] [CrossRef]
- Seco-Rovira, V.; Serrano-Sánchez, M.I.; Beltrán-Frutos, E.; Martínez-Hernández, J.; Ferrer, C.; Pastor, L.M. Hsp47 expression in the hamster Sertoli cell: An immunohistochemical study. Histol. Histopathol. 2024, 39, 1295–1302. [Google Scholar] [CrossRef]
- López De Padilla, C.M.; Coenen, M.J.; Tovar, A.; De la Vega, R.E.; Evans, C.H.; Müller, S.A. Picrosirius red staining: Revisiting its application to the qualitative and quantitative assessment of collagen type I and type iii in tendon. J. Histochem. Cytochem. 2021, 69, 633–643. [Google Scholar] [CrossRef]
- Calvo, A.; Pastor, L.M.; Horn, R.; Pallares, J. Histochemical study of glycoconjugates in the epididymis of the hamster (Mesocricetus auratus). Histochem. J. 1995, 27, 670–680. [Google Scholar] [CrossRef]
- Nagata, K. Hsp47 as a collagen-specific molecular chaperone: Function and expression in normal mouse development. Semin. Cell Dev. Biol. 2003, 14, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Inoue, A. Senescence-associated alterations of cytoskeleton: Extraordinary production of vimentin that anchors cytoplasmic p53 in senescent human fibroblasts. Histochem. Cell Biol. 2005, 123, 263–273. [Google Scholar] [CrossRef]
- Nishio, K.; Inoue, A.; Qiao, S.; Kondo, H.; Mimura, A. Senescence and cytoskeleton: Overproduction of vimentin induces senescent-like morphology in human fibroblasts. Histochem. Cell Biol. 2001, 116, 321–327. [Google Scholar] [CrossRef]
- de Miguel, M.P.; Bethencourt, F.R.; Arenas, M.I.; Fraile, B.; Paniagua, R. Intermediate filaments in the Sertoli cells of the ageing human testis. Virchows Arch. 1997, 431, 131–138. [Google Scholar] [CrossRef]
- Saito, H.; Yokota, S.; Kitajima, S. Immunohistochemical analysis of the vimentin filaments in Sertoli cells is a powerful tool for the prediction of spermatogenic dysfunction. Acta Histochem. 2023, 125, 152046. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Song, H.; Woo, J.-S.; Chung, H.-J.; Park, J.-K.; Cho, K.-H.; Yeo, J.M.; Lee, W.-Y. Expression patterns of male germ cell markers in cryptorchid pig testes. Acta Histochem. 2019, 121, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Devkota, B.; Sasaki, M.; Matsui, M.; Montoya, C.A.; Miyake, Y.-I. Alterations in the immunohistochemical localization patterns of alpha-smooth muscle actin (SMA) and vimentin in the postnatally developing bovine cryptorchid testis. J. Reprod. Dev. 2006, 52, 329–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.; Hu, Z.; Song, X.; Xiao, L.; Zou, R.; Han, C.; Liu, Y. Disrupted expression of intermediate filaments in the testis of rhesus monkey after experimental cryptorchidism. Int. J. Androl. 2004, 27, 234–239. [Google Scholar] [CrossRef]
- Himelreich-Perić, M.; Katušić-Bojanac, A.; Hohšteter, M.; Sinčić, N.; Mužić-Radović, V.; Ježek, D. Mast Cells in the Mammalian Testis and Epididymis—Animal Models and Detection Methods. Int. J. Mol. Sci. 2022, 23, 2547. [Google Scholar] [CrossRef]





| Normal | Stage I | Stage II | Stage III | |
|---|---|---|---|---|
| Tubular diameter (µm) | 214.89 ± 8.89 a | 111.29 ± 1.38 b | 148.80 ± 3.69 c | 77.03 ± 2.55 d |
| Lumen diameter (µm) | 85.44 ± 9.15 a | 30.43 ± 6.69 b | 107.73 ± 5.26 a | 16.21 ± 1.69 b |
| Epithelial height (µm) | 64.72 ± 3.13 a | 51.90 ± 0.99 a | 20.52 ± 2.06 b | 23.83 ± 1.91 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seco-Rovira, V.; Martínez-Hernández, J.; Freire-Brito, L.; Beltrán-Frutos, E.; Madrid, J.F.; Pastor, L.M. Gradual Thickening of the Peritubular Lamina Propria in Healthy Boar Seminiferous Tubules Due to Cryptorchidism: Increased Immunoexpression of Diverse Proteins in Sertoli and Myoid Cells. Animals 2025, 15, 1696. https://doi.org/10.3390/ani15121696
Seco-Rovira V, Martínez-Hernández J, Freire-Brito L, Beltrán-Frutos E, Madrid JF, Pastor LM. Gradual Thickening of the Peritubular Lamina Propria in Healthy Boar Seminiferous Tubules Due to Cryptorchidism: Increased Immunoexpression of Diverse Proteins in Sertoli and Myoid Cells. Animals. 2025; 15(12):1696. https://doi.org/10.3390/ani15121696
Chicago/Turabian StyleSeco-Rovira, Vicente, Jesús Martínez-Hernández, Laís Freire-Brito, Ester Beltrán-Frutos, Juan Francisco Madrid, and Luis Miguel Pastor. 2025. "Gradual Thickening of the Peritubular Lamina Propria in Healthy Boar Seminiferous Tubules Due to Cryptorchidism: Increased Immunoexpression of Diverse Proteins in Sertoli and Myoid Cells" Animals 15, no. 12: 1696. https://doi.org/10.3390/ani15121696
APA StyleSeco-Rovira, V., Martínez-Hernández, J., Freire-Brito, L., Beltrán-Frutos, E., Madrid, J. F., & Pastor, L. M. (2025). Gradual Thickening of the Peritubular Lamina Propria in Healthy Boar Seminiferous Tubules Due to Cryptorchidism: Increased Immunoexpression of Diverse Proteins in Sertoli and Myoid Cells. Animals, 15(12), 1696. https://doi.org/10.3390/ani15121696





