Valorization of Carob and Brewer’s Spent Grain as Growth-Substrate Supplements in Tenebrio molitor Rearing
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Tenebrio Molitor Larvae Performance
2.3. Chemical Characterization of Rearing Substrate
2.4. Chemical Characterization of Tenebrio molitor Larvae Meal
2.5. Extraction of Tenebrio molitor Larvae Meal
2.6. Evaluation of Total Phenolic Content (TPC) of Tenebrio molitor Larvae Meal Extracts
2.7. Evaluation of Antioxidant Properties of Tenebrio molitor Larvae Meal Extracts
2.8. Statistical Analysis
3. Results and Discussion
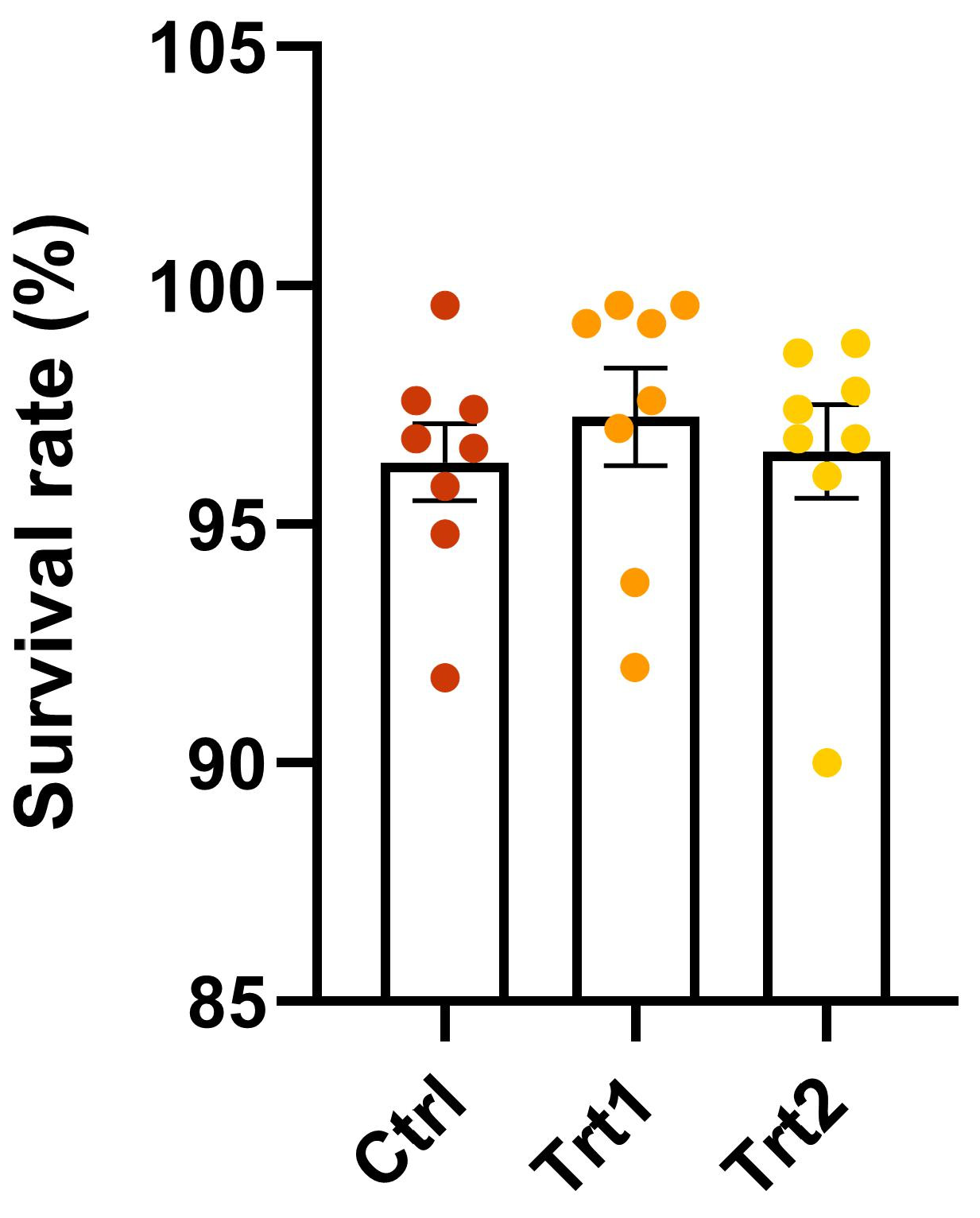
3.1. Growth Performance, Feed Conversion Efficiency, and Survival Rate of Insects
3.2. Chemical Composition of Rearing Substrates
3.3. Chemical Composition of Tenebrio molitor Meals
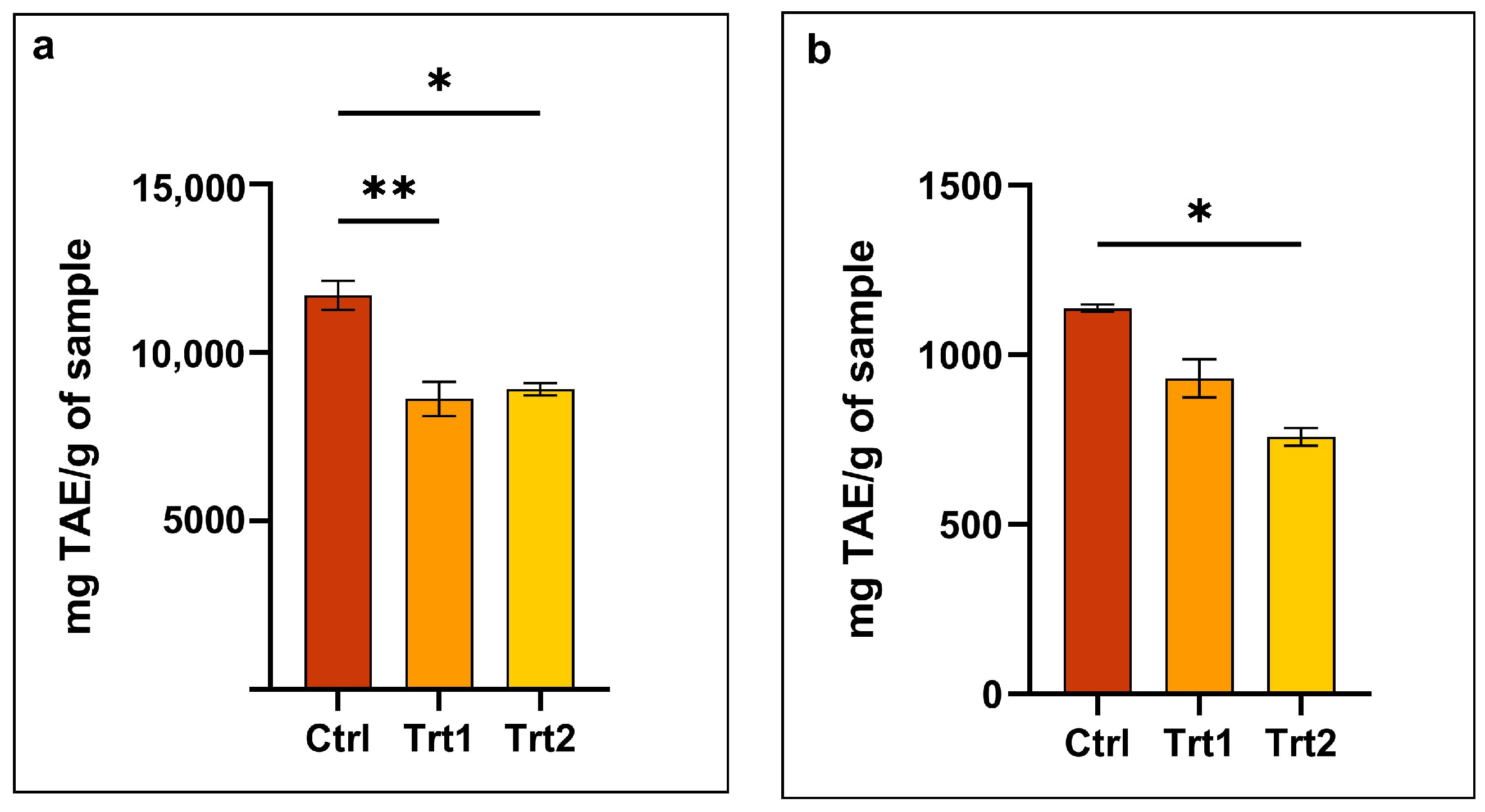
3.4. Total Phenol Content (TPC) of Tenebrio molitor Meals
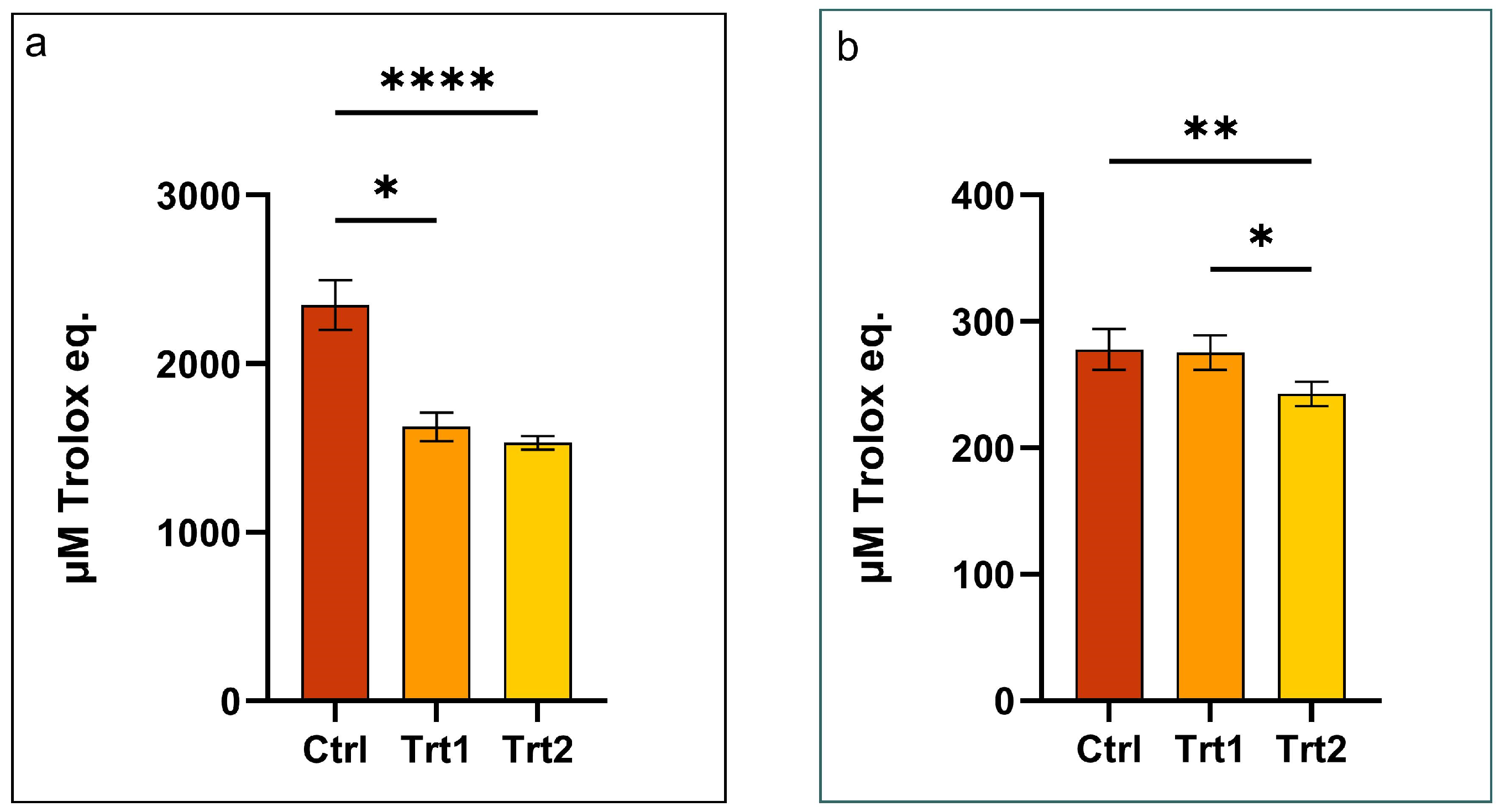
3.5. Antioxidant Properties of Tenebrio molitor Meals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grossmann, L.; Weiss, J. Alternative Protein Sources as Technofunctional Food Ingredients. Annu. Rev. Food Sci. Technol. 2021, 12, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). World Livestock: Transforming the Livestock Sector Through the Sustainable Development Goals; FAO: Rome, Italy, 2019; ISBN 978-92-5-130883-7. [Google Scholar]
- Hefferon, K.L.; De Steur, H.; Perez-Cueto, F.J.A.; Herring, R. Alternative Protein Innovations and Challenges for Industry and Consumer: An Initial Overview. Front. Sustain. Food Syst. 2023, 7, 1038286. [Google Scholar] [CrossRef]
- European Parliament Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (Text with EEA Relevance). Off. J. Eur. Union 2015. Available online: http://data.europa.eu/eli/reg/2015/2283/oj (accessed on 20 April 2025).
- European Parliament Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Reg (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Dir 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Dir 2002/67/EC and 2008/5/EC and Commission Reg (EC) No 608/2004. Off. J. Eur. Union 2011. Available online: http://data.europa.eu/eli/reg/2011/1169/oj (accessed on 20 April 2025).
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable Farming of the Mealworm Tenebrio molitor for the Production of Food and Feed. Z. Für Naturforschung C 2017, 72, 337–349. [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.; Stoner, G.D. Photochemoprevention of UVB-induced Skin Carcinogenesis in SKH-1 Mice by Brown Algae Polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Functional Food. Available online: https://www.fao.org/4/ae532e/ae532e00.htm#Contents (accessed on 20 April 2025).
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor Larva-Based Ingredients for the Food Industry: A Review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Exploiting Agri-Food Waste as Feed for Tenebrio molitor Larvae Rearing: A Review. Foods 2024, 13, 1027. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of Chemical Composition of Carob (Ceratonia siliqua): Protein, Fat, Carbohydrates, and Tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Grúz, J.; Strnad, M. Determination of Chemical Composition of Anatolian Carob Pod (Ceratonia siliqua L.): Sugars, Amino And Organic Acids, Minerals and Phenolic Compounds. J. Food Qual. 2007, 30, 1040–1055. [Google Scholar] [CrossRef]
- Kamal, M.; Youssef, E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Phenolic Compounds of Carob (Ceratonia siliqua L.). Food Public Health 2013, 6, 304–308. [Google Scholar]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Bottegal, D.N.; Latorre, M.Á.; Lobón, S.; Verdú, M.; Álvarez-Rodríguez, J. Fattening Pigs with Tannin-Rich Source (Ceratonia siliqua L.) and High Doses of Vitamin E: Effects on Growth Performance, Economics, Digestibility, Physiology, and Behaviour. Animals 2024, 14, 1855. [Google Scholar] [CrossRef] [PubMed]
- Kotrotsios, N.; Christaki, E.; Bonos, E.; Florou-Paneri, P. Dietary Carob Pods on Growth Performance and Meat Quality of Fattening Pigs. Asian-Australas J. Anim. Sci. 2012, 25, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Špoljarić, D.; Marenčić, D.; Benković, M.; Špoljarić, B.; Cvitanović, B.; Mršić, G.; Vlahović, K.; Popović, M.; Srečec, S.; Stolić, I. Effect of Dietary Carob Wholemeal on Blood Parameters in Weaned Pigs. Vet. Arh. 2019, 89, 351–366. [Google Scholar]
- Antonopoulou, E.; Panteli, N.; Feidantsis, K.; Mastoraki, M.; Koutsogeorgiou, E.; Grivaki, E.; Papagrigoriou, T.; Christias, S.; Chatzifotis, S.; Lazari, D.; et al. Carob (Ceratonia siliqua) as Functional Feed Is Beneficial in Yellow Mealworm (Tenebrio molitor) Rearing: Evidence from Growth, Antioxidant Status and Cellular Responses. Antioxidants 2022, 11, 1840. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s Spent Grain from Different Types of Malt: Evaluation of the Antioxidant Activity and Identification of the Major Phenolic Compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- Abeynayake, R.; Zhang, S.; Yang, W.; Chen, L. Development of Antioxidant Peptides from Brewers’ Spent Grain Proteins. LWT 2022, 158, 113162. [Google Scholar] [CrossRef]
- Ngodigha, E.M.; Sese, B.T.; Olaka, O.S.; Iyayi, E.A. Effect of Brewers Dried Grain on Growth Performance and Plasma Amino Acids of Young Pigs. J. Appl. Anim. Res. 1994, 6, 97–104. [Google Scholar] [CrossRef]
- Boontiam, W.; Hong, J.; Kim, Y.-Y. Dietary Brewer Grain Meal with Multienzymes Supplementation Affects Growth Performance, Gut Health, and Antioxidative Status of Weaning Pigs. Fermentation 2022, 8, 80. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Kong, X.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; et al. Influences of Wheat Bran Fiber on Growth Performance, Nutrient Digestibility, and Intestinal Epithelium Functions in Xiangcun Pigs. Heliyon 2023, 9, e17699. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.G.; Lee, K.Y.; Yoon, H.J.; Kim, N.J. Effects of Brewer’s Spent Grain (BSG) on Larval Growth of Mealworms, Tenebrio molitor (Coleoptera: Tenebrionidae). Int. J. Ind. Entomol. 2016, 32, 41–48. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Provera, I.; Dovicchi, J.; Tuccinardi, T.; Minieri, S.; Papini, R.A.; Forzan, M.; Paci, G. Growth Performances, Chemical Composition, and Microbiological Loads of Mealworm Reared with Brewery Spent Grains and Bread Leftovers. Ital. J. Anim. Sci. 2022, 21, 1419–1429. [Google Scholar] [CrossRef]
- Ferri, I.; Dell’Anno, M.; Canala, B.; Magnaghi, S.; Petrali, B.; Rossi, L. Evaluation of Hydration with Lactoferrin on Late-Instar Tenebrio molitor Larvae Performance and Functional Properties of Obtained Meal. Ital. J. Anim. Sci. 2023, 22, 982–994. [Google Scholar] [CrossRef]
- Yu, X.; He, Q.; Wang, D. Dynamic Analysis of Major Components in the Different Developmental Stages of Tenebrio molitor. Front. Nutr. 2021, 8, 689746. [Google Scholar] [CrossRef]
- Ferri, I.; Dell’Anno, M.; Spano, M.; Canala, B.; Petrali, B.; Dametti, M.; Magnaghi, S.; Rossi, L. Characterisation of Tenebrio molitor Reared on Substrates Supplemented with Chestnut Shell. Insects 2024, 15, 512. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Self-Selection of Feeding Substrates by Tenebrio molitor Larvae of Different Ages to Determine Optimal Macronutrient Intake and the Influence on Larval Growth and Protein Content. Insects 2022, 13, 657. [Google Scholar] [CrossRef]
- Mancini, S.; Mattioli, S.; Paolucci, S.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Paci, G. Effect of Cooking Techniques on the in Vitro Protein Digestibility, Fatty Acid Profile, and Oxidative Status of Mealworms (Tenebrio molitor). Front. Vet. Sci. 2021, 8, 675572. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 22th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2023. [Google Scholar]
- McLoughlin, C.; McKie, V.A.; McCleary, B.V. Validation of the Test Method—Determination of Available Carbohydrates in Cereal and Cereal Products, Dairy Products, Vegetables, Fruit, and Related Food Products and Animal Feeds: Collaborative Study, Final Action 2020.07. J. AOAC Int. 2023, 106, 370–383. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant Activities in Vitro of Water and Liposoluble Extracts Obtained by Different Species of Edible Insects and Invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Attard, E. A Rapid Microtitre Plate Folin-Ciocalteu Method for the Assessment of Polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Rossi, L.; Canala, B.; Fifi, A.P.; Frazzini, S. In Vitro Evaluation of Functional Properties of Extracts of Fucus Vesiculosus Obtained with Different Conventional Solvents. Algal Res. 2024, 84, 103787. [Google Scholar] [CrossRef]
- Sacchetti, G.; Di Mattia, C.; Pittia, P.; Martino, G. Application of a Radical Scavenging Activity Test to Measure the Total Antioxidant Activity of Poultry Meat. Meat Sci. 2008, 80, 1081–1085. [Google Scholar] [CrossRef]
- Couto, A.; Barroso, C.; Guerreiro, I.; Pousão-Ferreira, P.; Matos, E.; Peres, H.; Oliva-Teles, A.; Enes, P. Carob Seed Germ Meal in Diets for Meagre (Argyrosomus regius) Juveniles: Growth, Digestive Enzymes, Intermediary Metabolism, Liver and Gut Histology. Aquaculture 2016, 451, 396–404. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional Value of the Black Soldier Fly (Hermetia illucens L.) and Its Suitability as Animal Feed—A Review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; del Nozal, M.J. Variability of Brewer’s Spent Grain within a Brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Vieira, E.; Rocha, M.A.M.; Coelho, E.; Pinho, O.; Saraiva, J.A.; Ferreira, I.M.P.L.V.O.; Coimbra, M.A. Valuation of Brewer’s Spent Grain Using a Fully Recyclable Integrated Process for Extraction of Proteins and Arabinoxylans. Ind. Crops. Prod. 2014, 52, 136–143. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Crofton, E.; Scannell, A.G.M.; Hannon, J.A.; Kilcawley, K.N.; Gallagher, E. Sensory Properties and Aromatic Composition of Baked Snacks Containing Brewer’s Spent Grain. J. Cereal Sci. 2013, 57, 384–390. [Google Scholar] [CrossRef]
- Yu, H.; Li, W.; Feng, S.; Loo, S.C.J. Impacts of Industrial Food Wastes on Nutritional Value of Mealworm (Tenebrio molitor) and Its Gut Microbiota Community Shift. Biomater. Adv. 2024, 165, 214022. [Google Scholar] [CrossRef]
- Deruytter, D.; Coudron, C.L.; Claeys, J. The Influence of Wet Feed Distribution on the Density, Growth Rate and Growth Variability of Tenebrio molitor. J. Insects Food Feed 2021, 7, 141–149. [Google Scholar] [CrossRef]
- Li, L.; Stasiak, M.; Li, L.; Xie, B.; Fu, Y.; Gidzinski, D.; Dixon, M.; Liu, H. Rearing Tenebrio molitor in BLSS: Dietary Fiber Affects Larval Growth, Development, and Respiration Characteristics. Acta Astronaut. 2016, 118, 130–136. [Google Scholar] [CrossRef]
- Belperio, S.; Cattaneo, A.; Nannoni, E.; Sardi, L.; Martelli, G.; Dabbou, S.; Meneguz, M. Assessing Substrate Utilization and Bioconversion Efficiency of Black Soldier Fly (Hermetia illucens) Larvae: Effect of Diet Composition on Growth and Development Temperature. Animals 2024, 14, 1340. [Google Scholar] [CrossRef]
- Jankauskienė, A.; Aleknavičius, D.; Andrulevičiūtė, V.; Mockus, E.; Bartkienė, E.; Juknienė, I.; Kiseliovienė, S.; Zavistanavičiūtė, P.; Zaborskienė, G.; Kabašinskienė, A. Nutritional Composition and Safety Parameters of Mealworms (Tenebrio molitor) Reared on Substrates Derived from By-Products. Appl. Sci. 2024, 14, 2744. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Sam, O.; Niringiyimana, E. Assessment of Brewery Spent Grains as a Source of Protein in Food Applications. IAA J. Appl. Sci. 2024, 11, 22–31. [Google Scholar] [CrossRef]
- Syahrulawal, L.; Torske, M.O.; Sapkota, R.; Næss, G.; Khanal, P. Improving the Nutritional Values of Yellow Mealworm Tenebrio molitor (Coleoptera: Tenebrionidae) Larvae as an Animal Feed Ingredient: A Review. J. Anim. Sci. Biotechnol. 2023, 14, 146. [Google Scholar] [CrossRef]
- Jankauskienė, A.; Aleknavičius, D.; Kiseliovienė, S.; Antanaitis, Š.; Falkauskas, R.; Šumskienė, M.; Juknienė, I.; Kabašinskienė, A. The Influence of Different Sustainable Substrates on the Nutritional Value of Tenebrio molitor Larvae. Foods 2024, 13, 365. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected Species of Edible Insects as a Source of Nutrient Composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Jali, B.R.; Kuang, Y.; Neamati, N.; Baruah, J.B. Selective Binding of Naphthoquinone Derivatives to Serum Albumin Proteins and Their Effects on Cytotoxicity. Chem. Biol. Interact. 2014, 214, 10–17. [Google Scholar] [CrossRef] [PubMed]
- van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth Performance and Feed Conversion Efficiency of Three Edible Mealworm Species (Coleoptera: Tenebrionidae) on Diets Composed of Organic by-Products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adolfi, A.; Pondeville, E.; Lynd, A.; Bourgouin, C.; Lycett, G.J. Multi-Tissue GAL4-Mediated Gene Expression in All Anopheles Gambiae Life Stages Using an Endogenous Polyubiquitin Promoter. Insect Biochem. Mol. Biol. 2018, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Granadolorencio, F.; Olmedillaalonso, B.; Herrerobarbudo, C.; Blanconavarro, I.; Perezsacristan, B.; Blazquezgarcia, S. In vitro Bioaccessibility of Carotenoids and Tocopherols from Fruits and Vegetables. Food Chem. 2007, 102, 641–648. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.-N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and Biotechnological Processes To Enhance the Bioavailability of Dietary (Poly)Phenols in Humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
| p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Item | Ctrl | Trt1 | Trt2 | Trt | Time | Trt x Time | F-Value | DF (n, d) |
| Substrate consumed (%) | 0.0487 | 0.0897 | 0.7405 | 0.30 | 2, 21 | |||
| d 0–7 | 69.82 ± 1.23 | 70.65 ± 0.36 | 71.61 ± 0.44 | |||||
| d 7–14 | 68.72 ± 0.27 | 70.29 ± 0.15 | 70.56 ± 0.09 | |||||
| Average weight (g) | 0.0006 | <0.0001 | <0.0001 | 9.44 | 4, 42 | |||
| d 0–7 | 104.5 ± 1.44 | 107.7 ± 0.85 | 107.3 ± 0.67 | |||||
| d 7–14 | 104.9 ± 2.69 a | 114.8 ± 1.26 b | 116.9 ± 1.07 b | |||||
| Growth rate (%) | 0.0005 | 0.0014 | 0.0395 | 3.84 | 2, 19 | |||
| d 0–7 | 4.46 ± 1.45 | 7.72 ± 0.86 | 7.13 ± 0.66 | |||||
| d 7–14 | 4.05 ± 0.17 a | 12.11 ± 1.44 b | 12.10 ± 1.42 b | |||||
| FCR | 0.0068 | 0.0193 | 0.752 | 0.34 | 2, 18 | |||
| d 0–7 | 9.16 ± 2.25 | 6.16 ± 0.60 | 6.48 ± 0.58 | |||||
| d 7–14 | 8.36 ± 0.40 a | 3.97 ± 0.56 b | 3.97 ± 0.64 b | |||||
| Components (%) | Ctrl | Trt1 | Trt2 | p-Values |
|---|---|---|---|---|
| Dry Matter | 90.62 ± 1.26 | 89.10 ± 0.15 | 89.42 ± 0.18 | 0.0997 |
| Ash | 6.09 ± 1.86 | 7.40 ± 0.32 | 7.15 ± 0.29 | 0.3625 |
| Ether Extract | 2.93 ± 0.94 | 2.57 ± 0.32 | 2.17 ± 0.30 | 0.3602 |
| Crude Fiber | 11.87 ± 4.30 a | 18.70 ± 1.02 b | 16.31 ± 0.48 ab | 0.0446 |
| Crude Protein | 17.00 ± 0.42 ab | 15.92 ± 0.24 a | 18.14 ± 0.90 b | 0.0107 |
| Non-Structural Carbohydrates | 62.11 ± 3.77 a | 55.40 ± 1.11 b | 56.22 ± 0.96 b | 0.0242 |
| Components (%) | Ctrl | Trt1 | Trt2 | p-Value |
|---|---|---|---|---|
| Dry matter | 94.23 ± 1.84 a | 83.42 ± 1.75 b | 85.13 ± 3.44 b | 0.0036 |
| Crude protein | 56.84 ± 1.68 | 58.57 ± 1.56 | 56.99 ± 2.33 | 0.7734 |
| Ether Extract | 27.60 ± 2.30 | 25.23 ± 1.62 | 25.33 ± 3.30 | 0.4383 |
| Ash | 3.63 ± 0.47 | 3.81 ± 0.48 | 3.98 ± 0.32 | 0.6326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferri, I.; Dametti, M.R.; Frazzini, S.; Dell’Anno, M.; Rossi, L. Valorization of Carob and Brewer’s Spent Grain as Growth-Substrate Supplements in Tenebrio molitor Rearing. Animals 2025, 15, 1697. https://doi.org/10.3390/ani15121697
Ferri I, Dametti MR, Frazzini S, Dell’Anno M, Rossi L. Valorization of Carob and Brewer’s Spent Grain as Growth-Substrate Supplements in Tenebrio molitor Rearing. Animals. 2025; 15(12):1697. https://doi.org/10.3390/ani15121697
Chicago/Turabian StyleFerri, Irene, Matilda Rachele Dametti, Sara Frazzini, Matteo Dell’Anno, and Luciana Rossi. 2025. "Valorization of Carob and Brewer’s Spent Grain as Growth-Substrate Supplements in Tenebrio molitor Rearing" Animals 15, no. 12: 1697. https://doi.org/10.3390/ani15121697
APA StyleFerri, I., Dametti, M. R., Frazzini, S., Dell’Anno, M., & Rossi, L. (2025). Valorization of Carob and Brewer’s Spent Grain as Growth-Substrate Supplements in Tenebrio molitor Rearing. Animals, 15(12), 1697. https://doi.org/10.3390/ani15121697








