Development of a qPCR Tool for Detection, Quantification, and Molecular Characterization of Infectious Laryngotracheitis Virus Variants in Chile from 2019 to 2023
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Virus Detection
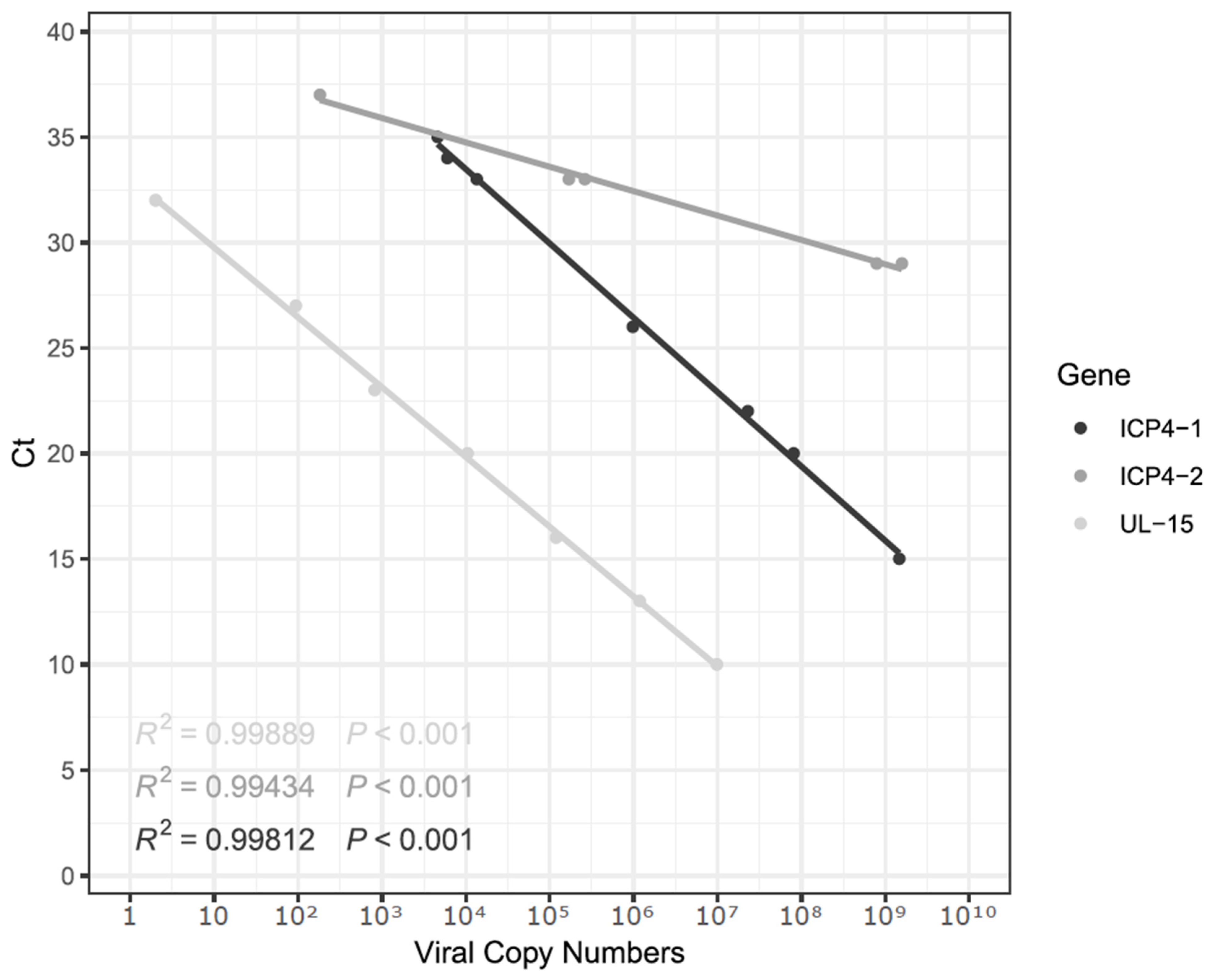
2.3. Cloning and Virus Quantification by Using qPCR
2.4. Sequence Analyses
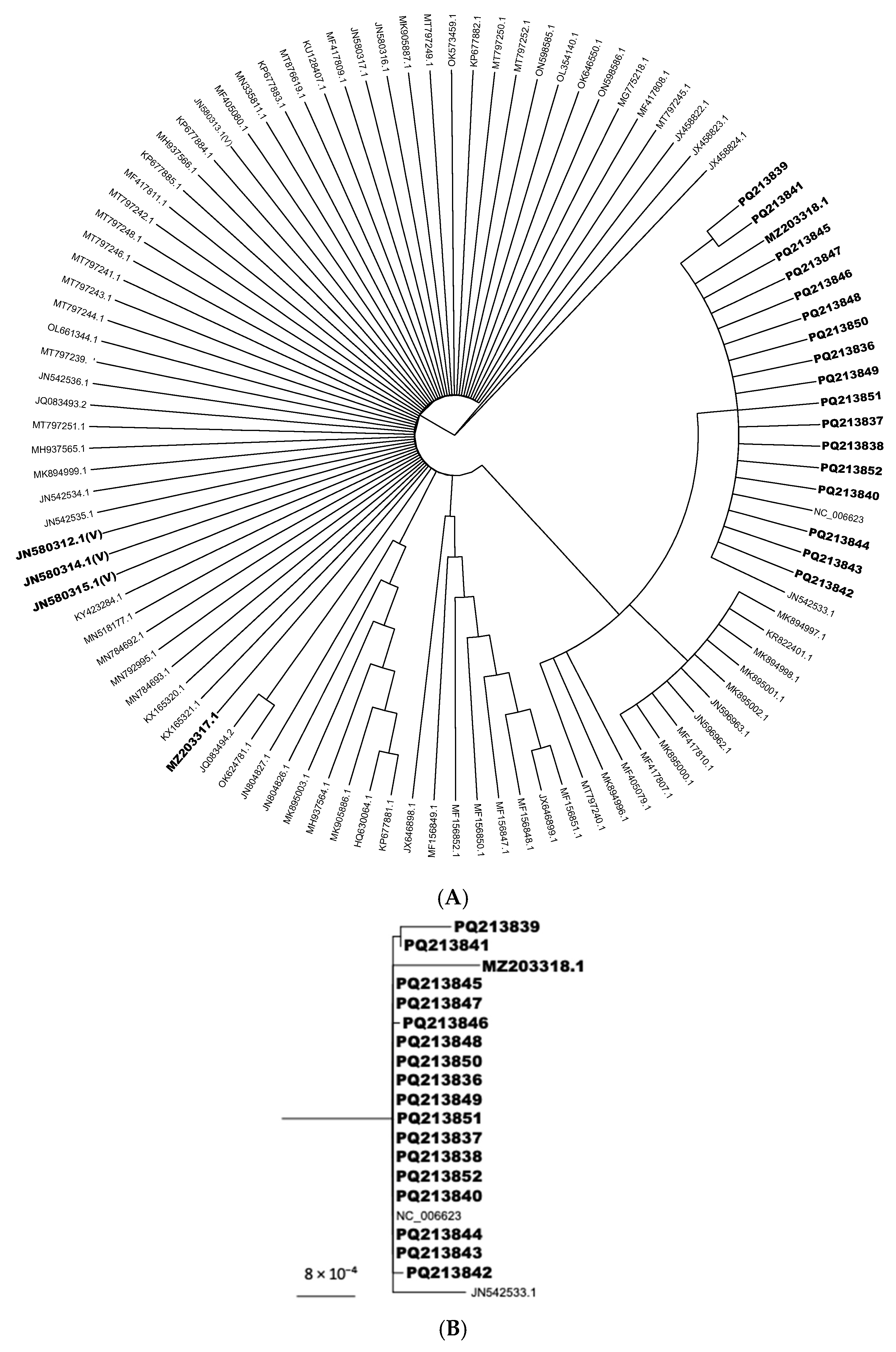
2.5. Phylogenetic Analyses
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirkpatrick, N.C.; Mahmoudian, A.; O’Rourke, D.; Noormohammadi, A.H. Differentiation of Infectious Laryngotracheitis Virus Isolates by Restriction Fragment Length Polymorphic Analysis of Polymerase Chain Reaction Products Amplified from Multiple Genes. Avian Dis. 2006, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Spatz, S. Infectious Laryngotracheitis. In Diseases of Poultry; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., et al., Eds.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 189–209. [Google Scholar]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Curtis Hendrickson, R.; et al. Recent Changes to Virus Taxonomy Ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef]
- Bagust, T.J. Laryngotracheitis (Gallid-1) Herpesvirus Infection in the Chicken. 4. Latency Establishment by Wild and Vaccine Strains of ILT Virus. Avian Pathol. 1986, 15, 581–595. [Google Scholar] [CrossRef]
- Fuchs, W.; Veits, J.; Helferich, D.; Granzow, H.; Teifke, J.P.; Mettenleiter, T.C. Molecular Biology of Avian Infectious Laryngotracheitis Virus. Vet. Res. 2007, 38, 261–279. [Google Scholar] [CrossRef]
- Loncoman, C.A.; Hartley, C.A.; Coppo, M.J.C.; Vaz, P.K.; Diaz-Méndez, A.; Browning, G.F.; García, M.; Spatz, S.; Devlin, J.M. Genetic Diversity of Infectious Laryngotracheitis Virus during In Vivo Coinfection Parallels Viral Replication and Arises from Recombination Hot Spots within the Genome. Appl. Environ. Microbiol. 2017, 83, e01532-17. [Google Scholar] [CrossRef]
- Coppo, M.J.C.; Noormohammadi, A.H.; Browning, G.F.; Devlin, J.M. Challenges and Recent Advancements in Infectious Laryngotracheitis Virus Vaccines. Avian Pathol. 2013, 42, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.S.; Barnes, H.J.; Smith, L. Increased Virulence of Modified-Live Infectious Laryngotracheitis Vaccine Virus Following Bird-to-Bird Passage. Avian Dis. 1991, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Williams, R.A.; Gaskell, R.M.; Jordan, F.T.; Bradbury, J.M.; Bennett, M.; Jones, R.C. Latency and Reactivation of Infectious Laryngotracheitis Vaccine Virus. Arch. Virol. 1991, 121, 213–218. [Google Scholar] [CrossRef]
- Menendez, K.R.; García, M.; Spatz, S.; Tablante, N.L. Molecular Epidemiology of Infectious Laryngotracheitis: A Review. Avian Pathol. 2014, 43, 108–117. [Google Scholar] [CrossRef]
- Lee, S.-W.; Markham, P.F.; Coppo, M.J.C.; Legione, A.R.; Markham, J.F.; Noormohammadi, A.H.; Browning, G.F.; Ficorilli, N.; Hartley, C.A.; Devlin, J.M. Attenuated Vaccines Can Recombine to Form Virulent Field Viruses. Science 2012, 337, 188. [Google Scholar] [CrossRef]
- Moreno, A.; Piccirillo, A.; Mondin, A.; Morandini, E.; Gavazzi, L.; Cordioli, P. Epidemic of Infectious Laryngotracheitis in Italy: Characterization of Virus Isolates by PCR-Restriction Fragment Length Polymorphism and Sequence Analysis. Avian Dis. 2010, 54, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.A.; McLaren, I.E.; Calvert, V.; Torrens, D.; Meehan, B.M. RFLP Analysis of Recent Northern Ireland Isolates of Infectious Laryngotracheitis Virus: Comparison with Vaccine Virus and Field Isolates from England, Scotland and the Republic of Ireland. Avian Pathol. 2000, 29, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zorman Rojs, O.; Dovč, A.; Krapež, U.; Žlabravec, Z.; Račnik, J.; Slavec, B. Detection of Laryngotracheitis Virus in Poultry Flocks with Respiratory Disorders in Slovenia. Viruses 2021, 13, 707. [Google Scholar] [CrossRef]
- La, T.-M.; Choi, E.-J.; Lee, J.-B.; Park, S.-Y.; Song, C.-S.; Choi, I.-S.; Lee, S.-W. Comparative Genome Analysis of Korean Field Strains of Infectious Laryngotracheitis Virus. PLoS ONE 2019, 14, e0211158. [Google Scholar] [CrossRef]
- Yan, Z.; Li, S.; Xie, Q.; Chen, F.; Bi, Y. Characterization of Field Strains of Infectious Laryngotracheitis Virus in China by Restriction Fragment Length Polymorphism and Sequence Analysis. J. Vet. Diagn. Investig. 2016, 28, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Lee, Y.-L.; Shien, J.-H.; Shieh, H.K. Rapid Differentiation of Vaccine Strains and Field Isolates of Infectious Laryngotracheitis Virus by Restriction Fragment Length Polymorphism of PCR Products. J. Virol. Methods 1997, 66, 179–186. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Sukumar, K.; Raja, A.; Saravanan, S.; Srinivasan, P.; Thangavelu, A. Characterization of Infectious Laryngotracheitis Virus Isolates from Laying Hens during 2019–2020 Outbreaks in Tamil Nadu, India. Arch. Virol. 2022, 167, 1819–1829. [Google Scholar] [CrossRef]
- Lee, S.-W.; Hartley, C.A.; Coppo, M.J.C.; Vaz, P.K.; Legione, A.R.; Quinteros, J.A.; Noormohammadi, A.H.; Markham, P.F.; Browning, G.F.; Devlin, J.M. Growth Kinetics and Transmission Potential of Existing and Emerging Field Strains of Infectious Laryngotracheitis Virus. PLoS ONE 2015, 10, e0120282. [Google Scholar] [CrossRef][Green Version]
- Elshafiee, E.A.; Hassan, M.S.H.; Provost, C.; Gagnon, C.A.; Ojkic, D.; Abdul-Careem, M.F. Comparative Full Genome Sequence Analysis of Wild-Type and Chicken Embryo Origin Vaccine-like Infectious Laryngotracheitis Virus Field Isolates from Canada. Infect. Genet. Evol. 2022, 104, 105350. [Google Scholar] [CrossRef]
- Craig, M.I.; Rojas, M.F.; van der Ploeg, C.A.; Olivera, V.; Vagnozzi, A.E.; Perez, A.M.; König, G.A. Molecular Characterization and Cluster Analysis of Field Isolates of Avian Infectious Laryngotracheitis Virus from Argentina. Front. Vet. Sci. 2017, 4, 212. [Google Scholar] [CrossRef]
- Morales Ruiz, S.; Bendezu, J.; Tataje-Lavanda, L.; Fernández-Díaz, M. Phylogenetic Evidence of a Close Relationship between the Peruvian Strain Vfar-043 and Two U.S. Origin Iltv Field Strains. Avian Dis. 2018, 62, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Santander-Parra, S.H.; Nuñez, L.F.N.; Buim, M.R.; Ferreira, C.S.A.; Loncoman, C.A.; Ferreira, A.J.P. Detection and Molecular Characterization of Infectious Laryngotracheitis Virus (ILTV) in Chicken with Respiratory Signs in Brazil during 2015 and 2016. Braz. J. Microbiol. 2022, 53, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Parra, S.; Nuñez, L.; Ferreira, A. Epidemiology of Avian Infectious Laryngotracheitis with Special Focus to South America: An Update. Braz. J. Poult. Sci. 2016, 18, 551–562. [Google Scholar] [CrossRef]
- Verdugo, C.; Pinto, A.; Ariyama, N.; Moroni, M.; Hernandez, C. Molecular identification of avian viruses in Neotropic cormorants (Phalacrocorax brasilianus) in Chile. J. Wildl. Dis. 2019, 55, 105–112. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, C.; Cui, X.; Cui, H.; Shi, X.; Zhang, X.; Hu, S.; Hao, L.; Wang, Y. Detection of Infectious Laryngotracheitis Virus by Real-Time PCR in Naturally and Experimentally Infected Chickens. PLoS ONE 2013, 8, e67598. [Google Scholar] [CrossRef]
- Davison, S.; Gingerich, E.N.; Casavant, S.; Eckroade, R.J. Evaluation of the Efficacy of a Live Fowlpox-Vectored Infectious Laryngotracheitis/Avian Encephalomyelitis Vaccine against ILT Viral Challenge. Avian Dis. 2006, 50, 50–54. [Google Scholar] [CrossRef]
- Esaki, M.; Godoy, A.; Rosenberger, J.K.; Rosenberger, S.C.; Gardin, Y.; Yasuda, A.; Dorsey, K.M. Protection and Antibody Response Caused by Turkey Herpesvirus Vector Newcastle Disease Vaccine. Avian Dis. 2013, 57, 750–755. [Google Scholar] [CrossRef]
- Bublot, M.; Pritchard, N.; Swayne, D.E.; Selleck, P.; Karaca, K.; Suarez, D.L.; Audonnet, J.-C.; Mickle, T.R. Development and Use of Fowlpox Vectored Vaccines for Avian Influenza. Ann. N. Y. Acad. Sci. 2006, 1081, 193–201. [Google Scholar] [CrossRef]
- Vagnozzi, A.; Zavala, G.; Riblet, S.M.; Mundt, A.; García, M. Protection Induced by Commercially Available Live-Attenuated and Recombinant Viral Vector Vaccines against Infectious Laryngotracheitis Virus in Broiler Chickens. Avian Pathol. 2012, 41, 21–31. [Google Scholar] [CrossRef]
- Nguyen, V.-G.; Cao, T.-B.-P.; Le, V.-T.; Truong, H.-T.; Chu, T.-T.-H.; Dang, H.-A.; Nguyen, T.-H.; Le, T.-L.; Huynh, T.-M.-L. A Multiplex PCR Method for Simultaneous Detection of Infectious Laryngotracheitis Virus and Ornithobacterium Rhinotracheale. Vet. Sci. 2023, 10, 272. [Google Scholar] [CrossRef]
- Mahmoudian, A.; Kirkpatrick, N.C.; Coppo, M.; Lee, S.-W.; Devlin, J.M.; Markham, P.F.; Browning, G.F.; Noormohammadi, A.H. Development of a SYBR Green Quantitative Polymerase Chain Reaction Assay for Rapid Detection and Quantification of Infectious Laryngotracheitis Virus. Avian Pathol. 2011, 40, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Chacón, J.L.; Ferreira, A.J.P. Differentiation of Field Isolates and Vaccine Strains of Infectious Laryngotracheitis Virus by DNA Sequencing. Vaccine 2009, 27, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Li, J.; Mei, X.; Liu, Y.; Huang, H. qPCRtools: An R Package for qPCR Data Processing and Visualization. Front. Genet. 2022, 13, 1002704. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.-Y. Ggtree: An r Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Piccirillo, A.; Lavezzo, E.; Niero, G.; Moreno, A.; Massi, P.; Franchin, E.; Toppo, S.; Salata, C.; Palù, G. Full Genome Sequence-Based Comparative Study of Wild-Type and Vaccine Strains of Infectious Laryngotracheitis Virus from Italy. PLoS ONE 2016, 11, e0149529. [Google Scholar] [CrossRef]
- Rodríguez-Avila, A.; Oldoni, I.; Riblet, S.; García, M. Replication and Transmission of Live Attenuated Infectious Laryngotracheitis Virus (ILTV) Vaccines. Avian Dis. 2007, 51, 905–911. [Google Scholar] [CrossRef]
| Primer | Sequence (5′-3′) | Amplicon |
|---|---|---|
| ICP4-1-Fw | ACTGATAGCTTTTCGTACAGCACG | 604 bp |
| ICP4-1-Rv | CATCGGGACATTCTCCAGGTAGCA | |
| ICP4-2-Fw | CTTCAGACTCCAGCTCATCTG | 662 bp |
| ICP4-2-Rv | AGTCATGCGTCTATGGCGTTGAC | |
| UL15a-Fw | TTGCTGTGCTATTTCGCGTG | 113 bp |
| UL15a-Rv | GTAAATCGTTTAGTGCGGCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatica, T.; Salgado, S.; Reyes, H.; Loncoman, C. Development of a qPCR Tool for Detection, Quantification, and Molecular Characterization of Infectious Laryngotracheitis Virus Variants in Chile from 2019 to 2023. Animals 2025, 15, 1623. https://doi.org/10.3390/ani15111623
Gatica T, Salgado S, Reyes H, Loncoman C. Development of a qPCR Tool for Detection, Quantification, and Molecular Characterization of Infectious Laryngotracheitis Virus Variants in Chile from 2019 to 2023. Animals. 2025; 15(11):1623. https://doi.org/10.3390/ani15111623
Chicago/Turabian StyleGatica, Tomás, Sebastián Salgado, Humberto Reyes, and Carlos Loncoman. 2025. "Development of a qPCR Tool for Detection, Quantification, and Molecular Characterization of Infectious Laryngotracheitis Virus Variants in Chile from 2019 to 2023" Animals 15, no. 11: 1623. https://doi.org/10.3390/ani15111623
APA StyleGatica, T., Salgado, S., Reyes, H., & Loncoman, C. (2025). Development of a qPCR Tool for Detection, Quantification, and Molecular Characterization of Infectious Laryngotracheitis Virus Variants in Chile from 2019 to 2023. Animals, 15(11), 1623. https://doi.org/10.3390/ani15111623







