Effects of Soybean Meal Substitution in Finishing Pig Diet on Carcass Traits, Meat Quality, and Muscle Antioxidant Capacity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Diets
2.2. Sample Collection and Measurements
2.3. Meat Quality Determination
2.4. Antioxidant Capacity Evaluation
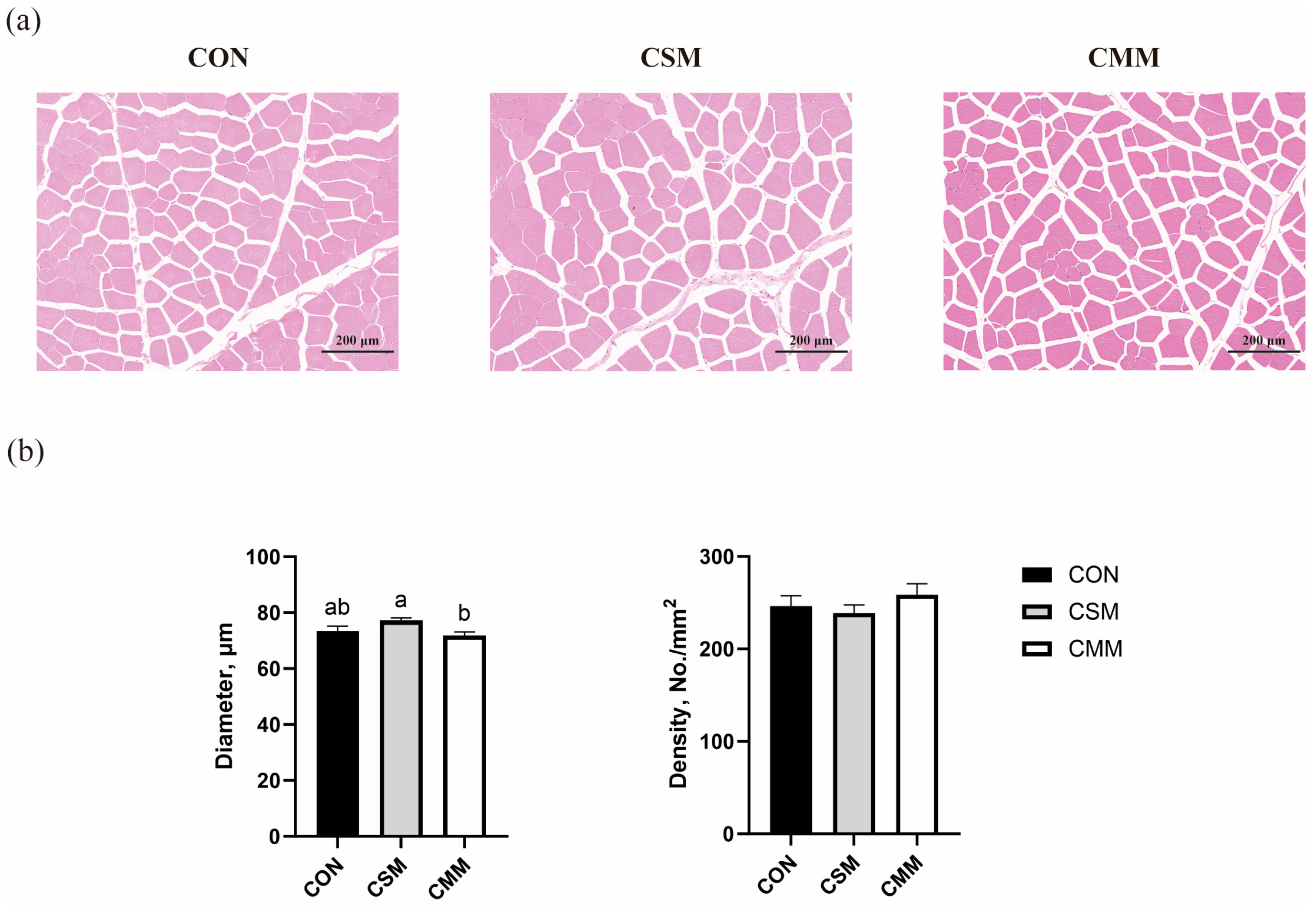
2.5. Histochemistry Staining
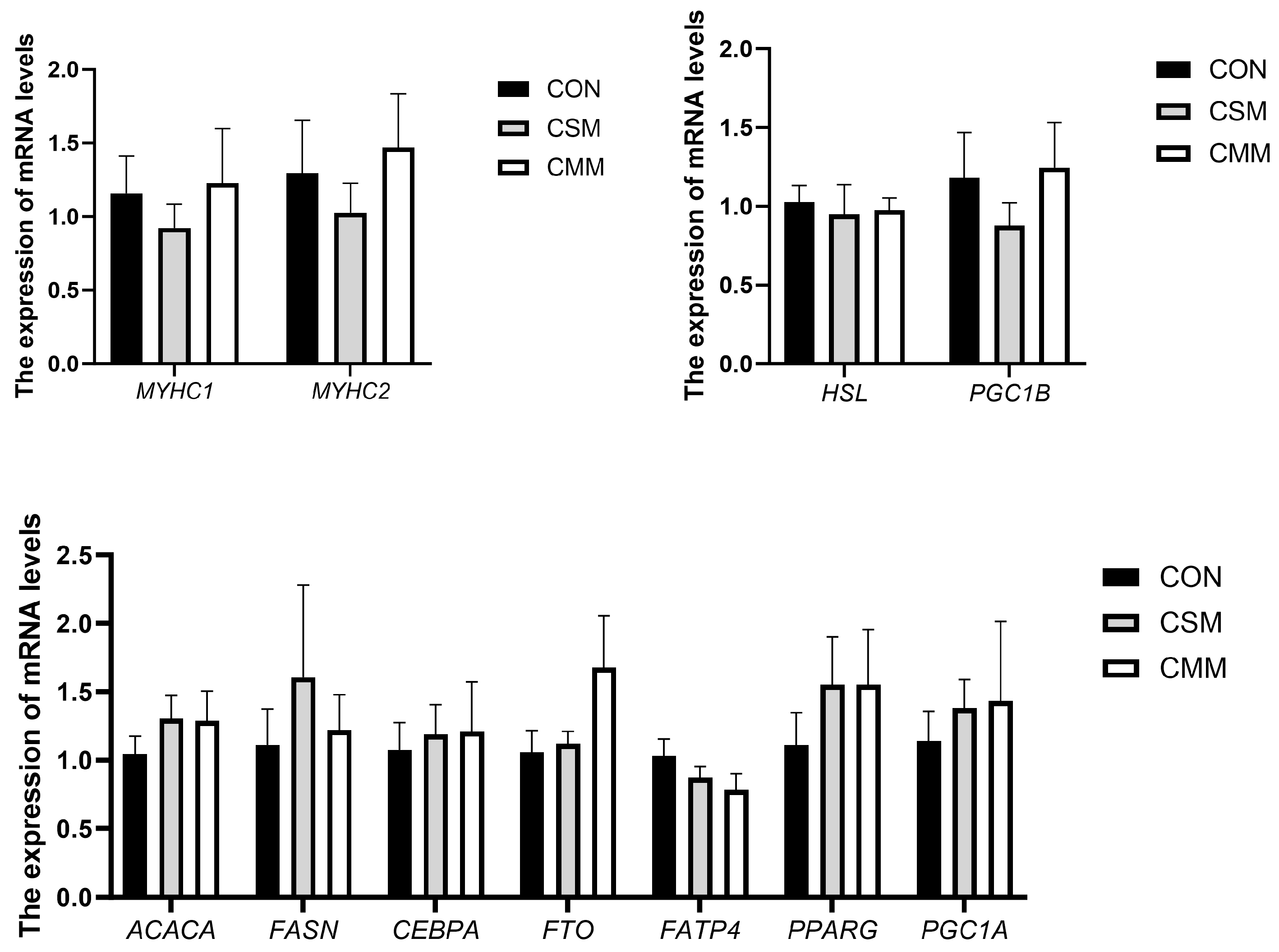
2.6. RNA Extraction, cDNA Synthesis, and Real-Time Quantitative PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
3.1. Growth Performance, Body Size, and Organ Indexes
3.2. Carcass Traits
3.3. Meat Quality
3.4. Muscle Fatty Acid Composition
3.5. Muscle Antioxidant Capacity
3.6. Muscle Fiber Density and Diameter
3.7. The mRNA Expression of Muscle Fiber Type and Lipid Deposition-Related Genes in the LT Muscles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erickson, D.R. Practical Handbook of Soybean Processing and Utilization; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Banaszkiewicz, T. Nutritional value of soybean meal. Soybean Nutr. 2011, 12, 1–20. [Google Scholar]
- Stein, H.H.; Berger, L.L.; Drackley, J.K.; Fahey, G.C., Jr.; Hernot, D.C.; Parsons, C.M. Nutritional properties and feeding values of soybeans and their coproducts. In Soybeans; Elsevier: Amsterdam, The Netherlands, 2008; pp. 613–660. [Google Scholar]
- Kim, E.; Utterback, P.; Parsons, C. Comparison of amino acid digestibility coefficients for soybean meal, canola meal, fish meal, and meat and bone meal among 3 different bioassays. Poult. Sci. 2012, 91, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Lagos, L.; Stein, H.-H. Chemical composition and amino acid digestibility of soybean meal produced in the United States, China, Argentina, Brazil, or India. J. Anim. Sci. 2017, 95, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Liener, I.E. Implications of antinutritional components in soybean foods. Crit. Rev. Food Sci. Nutr. 1994, 34, 31–67. [Google Scholar] [CrossRef]
- Benavides, P.T.; Cai, H.; Wang, M.; Bajjalieh, N. Life-cycle analysis of soybean meal, distiller-dried grains with solubles, and synthetic amino acid-based animal feeds for swine and poultry production. Anim. Feed. Sci. Technol. 2020, 268, 114607. [Google Scholar] [CrossRef]
- Aherne, F.; Kennelly, J. Oilseed Meals for Livestock Feeding; Butterworths: London, UK, 1985; Volume 278. [Google Scholar]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.-L.; Cabaraux, J.-F.; Chentouf, M. Growth performance, carcass characteristics, fatty acid profile, and meat quality of male goat kids supplemented by alternative feed resources: Bitter vetch and sorghum grains. Arch. Anim. Breed. 2024, 67, 481–492. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Olk, D.C. Chemical composition of defatted cottonseed and soy meal products. PLoS ONE 2015, 10, e0129933. [Google Scholar] [CrossRef]
- Adeniji, A.; Azeez, A. Effects of feeding growing pigs cotton seed cake with or without fish meal supplementation. J. Appl. Sci. Res. 2008, 4, 1253–1256. [Google Scholar]
- Villamide, M.; San Juan, L. Effect of chemical composition of sunflower seed meal on its true metabolizable energy and amino acid digestibility. Poult. Sci. 1998, 77, 1884–1892. [Google Scholar] [CrossRef]
- Von Rohr, P.; Hofer, A.; Künzi, N. Economic values for meat quality traits in pigs. J. Anim. Sci. 1999, 77, 2633–2640. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Protein (crude) determination in animal feed: Copper catalyst Kjeldahl method (984.13). In Official Methods of Analysis of AOAC International; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- Windham, W. AOAC official method 920.39, fat (crude) or ether extract in animal feed. In Official Methods of Analysis of AOAC International; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Biffin, T.E.; Smith, M.A.; Bush, R.D.; Morris, S.; Hopkins, D.L. The effect of whole carcase medium voltage electrical stimulation, tenderstretching and longissimus infusion with actinidin on alpaca meat quality. Meat Sci. 2020, 164, 108107. [Google Scholar] [CrossRef] [PubMed]
- NPPC. Procedures to Evaluate Market Hogs; National Pork Producers Council: Des Moines, IA, USA, 1991. [Google Scholar]
- Tian, Z.; Cui, Y.; Lu, H.; Wang, G.; Ma, X. Effect of long-term dietary probiotic Lactobacillus reuteri 1 or antibiotics on meat quality, muscular amino acids and fatty acids in pigs. Meat Sci. 2021, 171, 108234. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, S.; Wen, X.; Cao, S.; Zhan, X.; Hou, L.; Li, Y.; Chen, S.; Zheng, H.; Deng, D. Effect of mixed meal replacement of soybean meal on growth performance, nutrient apparent digestibility, and gut microbiota of finishing pigs. Front. Vet. Sci. 2024, 11, 1321486. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, X.; Qiao, S.; Zheng, C.; Chen, Y.; Piao, X.; Han, I.K.; Thacker, P. Growth performance of growing-finishing pigs fed diets supplemented with Chinese cottonseed meal based on amino acid digestibilities. Asian-Australas. J. Anim. Sci. 2000, 13, 521–527. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Xiao, Q.; Zhang, F.; Liu, N.; Tang, L.; Wang, J.; Ma, X.; Tan, B.; Chen, J. Rapeseed meal and its application in pig diet: A review. Agriculture 2022, 12, 849. [Google Scholar] [CrossRef]
- Li, P.; Wang, F.; Wu, F.; Wang, J.; Liu, L.; Lai, C. Chemical composition, energy and amino acid digestibility in double-low rapeseed meal fed to growing pigs. J. Anim. Sci. Biotechnol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Bell, J.; Keith, M.; Hutcheson, D. Nutritional evaluation of very low glucosinolate canola meal. Can. J. Anim. Sci. 1991, 71, 497–506. [Google Scholar] [CrossRef]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.-C.; Fine, F. Rapeseed and sunflower meal: A review on biotechnology status and challenges. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef]
- Poncet, C.; Remond, D.; Lepage, E.; Doreau, M. Comment mieux valoriser les protéagineux et oléagineux en alimentation des ruminants. Fourrages 2003, 174, 205–229. [Google Scholar]
- de Carvalho Carellos, D.; de Freitas Lima, J.A.; Fialho, E.T.; de Freitas, R.T.F.; Silva, H.O.; Branco, P.A.C.; de Souza, Z.A.; Vieira Neto, J. Evaluation of sunflower meal on growth and carcass traits of finishing pigs. Ciênc. Agrotecnol. 2005, 29, 208–215. [Google Scholar] [CrossRef]
- Zhan, X.; Hou, L.; He, Z.; Cao, S.; Wen, X.; Liu, S.; Li, Y.; Chen, S.; Zheng, H.; Deng, D. Effect of Miscellaneous Meals Replacing Soybean Meal in Feed on Growth Performance, Serum Biochemical Parameters, and Microbiota Composition of 25–50 kg Growing Pigs. Animals 2024, 14, 1354. [Google Scholar] [CrossRef]
- He, Z.; Zhan, X.; Cao, S.; Wen, X.; Hou, L.; Liu, S.; Zheng, H.; Gao, K.; Yang, X.; Jiang, Z. Effect of Miscellaneous Meal Replacements for Soybean Meal on Growth Performance, Serum Biochemical Parameters, and Gut Microbiota of 50–75 kg Growing Pigs. Animals 2023, 13, 3499. [Google Scholar] [CrossRef]
- Panda, S.; Gaur, G.K.; Chauhan, A.; Kar, J.; Mehrotra, A. Accurate assessment of body weights using morphometric measurements in Landlly pigs. Trop. Anim. Health Prod. 2021, 53, 362. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhu, J.; Liu, C.; Tian, X.; Zhang, S. Point cloud-based pig body size measurement featured by standard and non-standard postures. Comput. Electron. Agric. 2022, 199, 107135. [Google Scholar]
- Salter, D.; Montgomery, A.; Hudson, A.; Quelch, D.; Elliott, R.J. Lysine requirements and whole-body protein turnover in growing pigs. Br. J. Nutr. 1990, 63, 503–513. [Google Scholar] [CrossRef]
- Palma-Granados, P.; Haro, A.; Seiquer, I.; Lara, L.; Aguilera, J.; Nieto, R. Similar effects of lysine deficiency in muscle biochemical characteristics of fatty and lean piglets. J. Anim. Sci. 2017, 95, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Cortamira, O.; Gallego, A.; Kim, S. Evaluation of twice decorticated sunflower meal as a protein source compared with soybean meal in pig diets. Asian-Australas. J. Anim. Sci. 2000, 13, 1296–1303. [Google Scholar] [CrossRef]
- McDonnell, P.; O’Shea, C.; Figat, S.; O’Doherty, J.V. Influence of incrementally substituting dietary soya bean meal for rapeseed meal on nutrient digestibility, nitrogen excretion, growth performance and ammonia emissions from growing-finishing pigs. Arch. Anim. Nutr. 2010, 64, 412–424. [Google Scholar] [CrossRef]
- Seneviratne, R.; Beltranena, E.; Goonewardene, L.; Zijlstra, R. Effect of crude glycerol combined with solvent-extracted or expeller-pressed canola meal on growth performance and diet nutrient digestibility of weaned pigs. Anim. Feed. Sci. Technol. 2011, 170, 105–110. [Google Scholar] [CrossRef]
- Shim, Y.; Chae, B.; Lee, J. Effects of phytase and carbohydrases supplementation to diet with a partial replacement of soybean meal with rapeseed meal and cottonseed meal on growth performance and nutrient digestibility of growing pigs. Asian-Australas. J. Anim. Sci. 2003, 16, 1339–1347. [Google Scholar] [CrossRef]
- da Silva, C.A.; Pinheiro, J.W.; Fonseca, N.A.N.; Cabrera, L.; Novo, V.C.C.; da Silva, M.A.A.; Canteri, R.C.; Hoshi, E.H. Sunflower meal to swine on growing and finishing phase: Digestibility, performance and carcass quality. Rev. Bras. Zootec. 2002, 31, 982–990. [Google Scholar]
- Jakobsen, K.; Thorbekt, G. The respiratory quotient in relation to fat deposition in fattening–growing pigs. Br. J. Nutr. 1993, 69, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Van Milgen, J.; Noblet, J.; Dubois, S. Energetic efficiency of starch, protein and lipid utilization in growing pigs. J. Nutr. 2001, 131, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Kil, D.Y.; Ji, F.; Stewart, L.; Hinson, R.; Beaulieu, A.; Allee, G.; Patience, J.; Pettigrew, J.; Stein, H. Effects of dietary soybean oil on pig growth performance, retention of protein, lipids, and energy, and the net energy of corn in diets fed to growing or finishing pigs. J. Anim. Sci. 2013, 91, 3283–3290. [Google Scholar] [CrossRef]
- Skugor, A.; Kjos, N.P.; Sundaram, A.Y.; Mydland, L.T.; Ånestad, R.; Tauson, A.-H.; Øverland, M. Effects of long-term feeding of rapeseed meal on skeletal muscle transcriptome, production efficiency and meat quality traits in Norwegian Landrace growing-finishing pigs. PLoS ONE 2019, 14, e0220441. [Google Scholar] [CrossRef]
- Grabež, V.; Egelandsdal, B.; Kjos, N.P.; Håkenåsen, I.M.; Mydland, L.T.; Vik, J.O.; Hallenstvedt, E.; Devle, H.; Øverland, M. Replacing soybean meal with rapeseed meal and faba beans in a growing-finishing pig diet: Effect on growth performance, meat quality and metabolite changes. Meat Sci. 2020, 166, 108134. [Google Scholar] [CrossRef]
- Blair, R. Nutrition and Feeding of Organic Pigs; CABI: Wallingford, UK, 2017. [Google Scholar]
- Cameron, N.; Warriss, P.; Porter, S.; Enser, M. Comparison of Duroc and British landrace pigs for meat a and eating quality. Meat Sci. 1990, 27, 227–247. [Google Scholar] [CrossRef]
- Zmudzińska, A.; Bigorowski, B.; Banaszak, M.; Roślewska, A.; Adamski, M.; Hejdysz, M. The Effect of diet based on legume seeds and rapeseed meal on pig performance and meat quality. Animals 2020, 10, 1084. [Google Scholar] [CrossRef]
- Roura, E.; Fu, M. Taste, nutrient sensing and feed intake in pigs (130 years of research: Then, now and future). Anim. Feed. Sci. Technol. 2017, 233, 3–12. [Google Scholar] [CrossRef]
- Nishimura, T.; Ra Rhue, M.; Okitani, A.; Kato, H. Components contributing to the improvement of meat taste during storage. Agric. Biol. Chem. 1988, 52, 2323–2330. [Google Scholar]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. In Natural Antioxidants, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 21–58. [Google Scholar]
- Králová, M. The effect of lipid oxidation on the quality of meat and meat products. Maso Int. J. Food Sci. Technol. 2015, 2, 125–132. [Google Scholar]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Jang, A.; Liu, X.-D.; Shin, M.-H.; Lee, B.-D.; Lee, S.-K.; Lee, J.-H.; Jo, C. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult. Sci. 2008, 87, 2382–2389. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Dai, J.-H.; Cai, M.-L.; Cheng, K.-M.; Hu, Y.; Luo, Z. Effects of dietary replacement of fishmeal by cottonseed meal on the growth performance, immune and antioxidant responses, and muscle quality of juvenile crayfish Procambarus clarkii. Aquac. Rep. 2023, 31, 101639. [Google Scholar] [CrossRef]
- Zheng, Q.; Wen, X.; Han, C.; Li, H.; Xie, X. Effect of replacing soybean meal with cottonseed meal on growth, hematology, antioxidant enzymes activity and expression for juvenile grass carp, Ctenopharyngodon idellus. Fish Physiol. Biochem. 2012, 38, 1059–1069. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Hornick, J.-L.; Chentouf, M.; Cabaraux, J.-F. Effects of sulla flexuosa hay as alternative feed resource on goat’s milk production and quality. Animals 2023, 13, 709. [Google Scholar] [CrossRef]
- Ryu, Y.; Kim, B. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005, 71, 351–357. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Wang, Y.; Jia, J.; Li, M.; Hei, W.; He, Z.; Zhao, Y.; Cai, C.; Gao, P. MyHC s developmental expression patterns and its effect on muscle fibre characteristics in pig. J. Appl. Anim. Res. 2020, 48, 176–183. [Google Scholar] [CrossRef]
- Qiu, Y.-Q.; Yang, X.-F.; Ma, X.-Y.; Xiong, Y.-X.; Tian, Z.-M.; Fan, Q.-L.; Wang, L.; Jiang, Z.-Y. CIDE gene expression in adipose tissue, liver, and skeletal muscle from obese and lean pigs. J. Zhejiang Univ. Sci. B 2017, 18, 492–500. [Google Scholar] [CrossRef]
- Soares, M.H.; de Amorim Rodrigues, G.; Júnior, D.T.V.; da Silva, C.B.; Costa, T.C.; de Souza Duarte, M.; Saraiva, A. Performance, carcass traits, pork quality and expression of genes related to intramuscular fat metabolism of two diverse genetic lines of pigs. Foods 2022, 11, 2280. [Google Scholar] [CrossRef]
| Ingredients, % | Treatments 1 | ||
|---|---|---|---|
| CON | CSM | CMM | |
| Corn | 56.05 | 52.94 | 50.15 |
| Cassava | 25.00 | 25.00 | 25.00 |
| Soybean meal | 16.46 | 7.40 | |
| Double-low rapeseed meal | 3.52 | 6.46 | |
| Cottonseed meal | 3.52 | 6.46 | |
| Sunflower meal | 3.52 | 6.46 | |
| Soybean oil | 1.50 | 2.75 | |
| Calcium carbonate | 0.60 | 0.60 | 0.58 |
| Calcium phosphate | 0.09 | 0.04 | 0.03 |
| NaCl | 0.40 | 0.40 | 0.40 |
| L-lysine hydrochloride | 0.08 | 0.21 | 0.33 |
| L-threonine | 0.01 | 0.04 | 0.06 |
| L-tryptophan | 0.01 | ||
| Choline chloride | 0.10 | 0.10 | 0.10 |
| Premix 2 | 1.21 | 1.21 | 1.21 |
| total | 100.00 | 100.00 | 100.00 |
| Calculated composition 5 3 | |||
| Metabolic energy, MJ/kg | 13.86 | 13.80 | 13.75 |
| Crude protein | 12.50 | 12.50 | 12.50 |
| Ca | 0.49 | 0.49 | 0.49 |
| STTD P | 0.22 | 0.22 | 0.22 |
| SID Lys | 0.61 | 0.61 | 0.61 |
| SID Met | 0.18 | 0.19 | 0.20 |
| SID Met + Cys | 0.38 | 0.39 | 0.40 |
| SID Thr | 0.40 | 0.40 | 0.40 |
| SID Trp | 0.13 | 0.11 | 0.11 |
| SID Val | 0.48 | 0.47 | 0.46 |
| SID Ile | 0.45 | 0.40 | 0.35 |
| Analyzed value | |||
| Gross energy, MJ/kg | 15.65 | 16.08 | 16.51 |
| Crude protein | 12.18 | 13.02 | 12.63 |
| Ether extract | 2.87 | 3.30 | 3.39 |
| Antinutritional factors | |||
| Glycinin, mg/g | 19.03 | 8.87 | <2.00 |
| β-conglycinin, mg/g | 18.87 | 9.18 | <2.00 |
| Trypsin inhibitor, mg/g | <0.40 | <0.40 | <0.40 |
| Agglutinin, mg/g | <0.20 | <0.20 | <0.20 |
| Item | Treatments | p-Value | ||
|---|---|---|---|---|
| CON | CSM | CMM | ||
| Live weight, kg | 126.16 ± 1.13 | 125.65 ± 1.36 | 125.14 ± 1.31 | 0.85 |
| Body length, cm | 146.83 ± 1.83 | 143.83 ± 0.91 | 142.83 ± 1.45 | 0.16 |
| Body height, cm | 74.83 ± 1.68 | 74.50 ± 1.73 | 75.83 ± 0.87 | 0.81 |
| Chest circumference, cm | 121.00 ± 2.35 | 116.67 ± 0.95 | 115.33 ± 2.03 | 0.12 |
| Abdomen circumference, cm | 119.83 ± 0.87 | 122.50 ± 2.22 | 126.00 ± 1.93 | 0.08 |
| Heart index, % | 0.35 ± 0.01 | 0.37 ± 0.02 | 0.36 ± 0.01 | 0.88 |
| Liver index, % | 1.59 ± 0.08 | 1.56 ± 0.05 | 1.46 ± 0.06 | 0.33 |
| Spleen index, % | 0.21 ± 0.01 | 0.19 ± 0.02 | 0.20 ± 0.01 | 0.77 |
| Lung index, % | 0.79 ± 0.08 | 0.75 ± 0.09 | 0.84 ± 0.12 | 0.83 |
| Kidney index, % | 0.29 ± 0.01 | 0.27 ± 0.01 | 0.78 ± 0.48 | 0.37 |
| Item | Treatments | p-Value | ||
|---|---|---|---|---|
| CON | CSM | CMM | ||
| Carcass straight length, cm | 113.33 ± 1.67 | 113.50 ± 1.75 | 112.17 ± 0.83 | 0.79 |
| Carcass oblique length, cm | 102.67 ± 1.63 | 101.58 ± 1.45 | 102.50 ± 0.99 | 0.84 |
| Carcass weight, kg | 94.65 ± 1.59 | 92.64 ± 1.63 | 93.17 ± 0.83 | 0.59 |
| Carcass yield, % | 74.54 ± 1.02 | 74.08 ± 0.96 | 74.77 ± 0.65 | 0.86 |
| Leaf fat weight, kg | 1.49 ± 0.14 b | 1.51 ± 0.17 ab | 2.00 ± 0.09 a | 0.03 |
| Average backfat thickness, mm | 23.65 ± 0.79 | 27.73 ± 1.41 | 24.96 ± 1.43 | 0.09 |
| Loin–eye area, cm2 | 67.83 ± 4.99 | 68.40 ± 5.92 | 63.33 ± 4.32 | 0.74 |
| Item | Treatments | p-Value | ||
|---|---|---|---|---|
| CON | CSM | CMM | ||
| pH45min | 6.43 ± 0.08 | 6.56 ± 0.15 | 6.39 ± 0.11 | 0.54 |
| pH24h | 5.53 ± 0.04 | 5.52 ± 0.03 | 5.52 ± 0.03 | 0.99 |
| pH48h | 5.60 ± 0.03 | 5.58 ± 0.02 | 5.57 ± 0.03 | 0.74 |
| L*45min | 16.86 ± 0.39 | 17.62 ± 0.87 | 16.76 ± 0.32 | 0.56 |
| a*45min | 6.69 ± 0.29 | 7.48 ± 0.53 | 6.46 ± 0.31 | 0.17 |
| b*45min | 61.77 ± 1.10 | 64.25 ± 0.86 | 61.70 ± 1.23 | 0.18 |
| L*24h | 16.83 ± 0.53 | 16.05 ± 0.58 | 16.72 ± 0.36 | 0.51 |
| a*24h | 8.69 ± 0.33 | 9.28 ± 0.17 | 8.61 ± 0.35 | 0.23 |
| b*24h | 62.26 ± 0.84 | 64.13 ± 1.10 | 61.54 ± 0.90 | 0.18 |
| L*48h | 18.60 ± 2.12 | 15.81 ± 0.43 | 16.84 ± 0.20 | 0.34 |
| a*48h | 9.20 ± 0.54 | 9.62 ± 0.44 | 8.55 ± 0.43 | 0.33 |
| b*48h | 16.86 ± 0.39 | 17.62 ± 0.87 | 16.76 ± 0.32 | 0.56 |
| Drip loss24h, % | 2.45 ± 0.27 | 1.92 ± 0.33 | 2.33 ± 0.10 | 0.32 |
| Drip loss48h, % | 2.91 ± 0.21 | 3.76 ± 0.63 | 3.06 ± 0.18 | 0.31 |
| Shear force, N | 54.83 ± 3.61 | 53.13 ± 4.63 | 49.78 ± 5.14 | 0.73 |
| Marbling scores | 3.59 ± 0.29 | 3.69 ± 0.35 | 3.48 ± 0.22 | 0.88 |
| Item | Treatments | p-Value | ||
|---|---|---|---|---|
| CON | CSM | CMM | ||
| TFA | 4716.72 ± 1345.29 | 3953.28 ± 678.62 | 3514.16 ± 659.01 | 0.67 |
| SFA | 2020.34 ± 590.67 | 1637.54 ± 303.22 | 1272.22 ± 205.30 | 0.44 |
| C10:0 | 5.42 ± 1.56 | 4.76 ± 0.57 | 5.51 ± 0.69 | 0.86 |
| C12:0 | 4.38 ± 1.32 | 4.35 ± 0.67 | 4.58 ± 0.72 | 0.98 |
| C14:0 | 64.23 ± 18.40 | 59.11 ± 9.67 | 57.82 ± 10.61 | 0.94 |
| C16:0 | 1224.87 ± 357.07 | 973.29 ± 189.84 | 587.33 ± 102.18 | 0.20 |
| C17:0 | 11.40 ± 3.04 | 8.93 ± 1.88 | 8.59 ± 2.09 | 0.67 |
| C18:0 | 697.55 ± 205.99 | 576.48 ± 105.35 | 597.33 ± 101.15 | 0.82 |
| C20:0 | 10.80 ± 3.08 | 9.57 ± 1.49 | 10.11 ± 1.96 | 0.93 |
| TUFA | 2698.17 ± 754.71 | 2315.20 ± 384.16 | 2241.94 ± 460.71 | 0.83 |
| MUFA | 2045.12 ± 601.67 | 1689.16 ± 233.00 | 1615.64 ± 309.28 | 0.74 |
| C16:1 (cis-9) | 110.26 ± 32.02 | 104.14 ± 11.64 | 87.66 ± 14.03 | 0.74 |
| C18:1 (trans-9) | 3.66 ± 1.26 | 4.35 ± 0.48 | 4.60 ± 0.96 | 0.78 |
| C18:1 (cis-9) | 1890.90 ± 556.76 | 1547.68 ± 216.64 | 1492.28 ± 287.66 | 0.73 |
| C20:1 (cis-11) | 40.06 ± 12.53 | 32.99 ± 6.14 | 31.1 ± 7.09 | 0.77 |
| PUFA | 653.04 ± 155.63 | 626.04 ± 159.64 | 626.3 ± 156.58 | 0.99 |
| C18:2 (all-cis-9,12) | 553.40 ± 134.88 | 527.89 ± 138.99 | 526.39 ± 135.18 | 0.99 |
| C18:3 (all-cis-9,12,15) | 24.65 ± 5.97 | 27.42 ± 8.57 | 29.63 ± 8.41 | 0.90 |
| C20:2 (all-cis-11,14) | 26.51 ± 6.86 | 24.35 ± 7.26 | 23.5 ± 6.63 | 0.95 |
| C20:3 (all-cis-8,11,14) | 7.15 ± 1.28 | 6.44 ± 1.11 | 6.88 ± 1.26 | 0.92 |
| C20:4 (all-cis-5,8,11,14) | 33.18 ± 4.48 | 32.26 ± 2.32 | 31.85 ± 2.76 | 0.96 |
| C20:3 (all-cis-11,14,17) | 4.11 ± 1.51 | 5.06 ± 1.63 | 5.35 ± 1.63 | 0.85 |
| C22:6 (all-cis-4,7,10,13,16,19) | 1.72 ± 0.78 | 1.88 ± 0.63 | 1.65 ± 0.79 | 0.98 |
| Item | Treatments | p-Value | ||
|---|---|---|---|---|
| CON | CSM | CMM | ||
| MDA, mmol/g port | 0.21 ± 0.01 | 0.27 ± 0.04 | 0.20 ± 0.04 | 0.34 |
| CAT, U/mg port | 0.61 ± 0.05 | 0.40 ± 0.08 | 0.61 ± 0.10 | 0.11 |
| T-SOD, U/mg port | 50.22 ± 1.47 | 49.62 ± 1.03 | 48.19 ± 2.11 | 0.66 |
| T-AOC, mmol/g port | 57.44 ± 4.32 | 55.28 ± 5.00 | 50.29 ± 2.53 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; He, Z.; Wen, X.; Zhan, X.; Hou, L.; Deng, D.; Gao, K.; Yang, X.; Cao, S.; Jiang, Z.; et al. Effects of Soybean Meal Substitution in Finishing Pig Diet on Carcass Traits, Meat Quality, and Muscle Antioxidant Capacity. Animals 2025, 15, 1611. https://doi.org/10.3390/ani15111611
Liu S, He Z, Wen X, Zhan X, Hou L, Deng D, Gao K, Yang X, Cao S, Jiang Z, et al. Effects of Soybean Meal Substitution in Finishing Pig Diet on Carcass Traits, Meat Quality, and Muscle Antioxidant Capacity. Animals. 2025; 15(11):1611. https://doi.org/10.3390/ani15111611
Chicago/Turabian StyleLiu, Shuai, Zhentao He, Xiaolu Wen, Xianliang Zhan, Lei Hou, Dongyan Deng, Kaiguo Gao, Xuefen Yang, Shuting Cao, Zongyong Jiang, and et al. 2025. "Effects of Soybean Meal Substitution in Finishing Pig Diet on Carcass Traits, Meat Quality, and Muscle Antioxidant Capacity" Animals 15, no. 11: 1611. https://doi.org/10.3390/ani15111611
APA StyleLiu, S., He, Z., Wen, X., Zhan, X., Hou, L., Deng, D., Gao, K., Yang, X., Cao, S., Jiang, Z., & Wang, L. (2025). Effects of Soybean Meal Substitution in Finishing Pig Diet on Carcass Traits, Meat Quality, and Muscle Antioxidant Capacity. Animals, 15(11), 1611. https://doi.org/10.3390/ani15111611







