1. Introduction
In Mexico, there are three main types of rabbit farmers, namely small, medium, and business farmers, with 50% of all rabbit farmers being medium-scale producers; these rabbit farmers use commercial feed and sometimes add a local plant [
1]. Over the past few years, commercial rabbit feed has shown considerable noncompliance with the nutrient levels indicated by the manufacturer; specifically, it does not comply with the minimum recommended levels of crude fiber and crude protein [
2]. In addition, the human population has grown significantly worldwide, and, therefore, there is a great demand for meat to satisfy the food needs of this expanding population. This has encouraged animal breeders to identify alternative sources to ensure the formulation of diets for their animals at a lower cost [
3] and provide a better supply of nutrients.
It has been proven that a wide range of fruits rich in different nutrients is being threatened by the lack of demand for fresh produce and crop damage suffered during the rainy season [
4]. It is for these reasons that the utilization of agro-industrial waste (fruit wastes, agricultural pulp wastes, crop residues, sun-dried brewers’ grains, and the pomace of some fruits) in animal feed has been proposed as a promising alternative for meat production. Also, it contributes to sustainable agriculture as well as improving meat quality [
5]. The feasibility of using fruit and vegetable residues for feeding different animals has been proven [
6].
Jackfruit (
Artocarpus heterophyllus Lam.) is considered one of the main indigenous fruits of India [
4]. This fruit is cultivated in subtropical zones, including in countries in Asia, Africa, and the Americas, where yields up to 26 ton·ha
−1 have been obtained [
7]. Likewise, it has an abundance of essential amino acids, minerals, vitamin C, and bioactive compounds that give it antioxidant, anti-inflammatory, anticancer, antidiabetic, and antiviral properties [
8]. The utilization of residues from this fruit as from many others contributes to the reduction in the impact of waste discharged into the environment [
9]. Different researchers have evaluated the addition of this fruit in the feed of different animals, such as tilapia [
10], goats [
11], broilers [
12,
13], and West African Dwarf Bucks [
14].
Rabbit farming is an activity that focuses on raising rabbits to obtain white meat, which is considered beneficial for the human body due to its supply of essential fatty acids, proteins, vitamins, and minerals [
15]. For this animal species, some agro-industrial residues and coproducts have been used [
9]. It would be expected that jackfruit could be a good source of nutrients and bioactive compounds to be used in feed for fattening rabbits.
The objective of this research was to evaluate the effects of the use of different parts of jackfruit (seed, pulp, and peel) as an agro-industrial waste feed additive on the productive parameters, carcass quality, and meat quality of rabbits.
4. Discussion
Rabbit meat production plays an important role in ensuring an adequate supply of sustainable meat around the world [
15]. Minimal initial investment is required to breed these animals, and their management is fairly straightforward. Therefore, optimizing the nutritional aspects would contribute to increasing their productivity [
26]. In general, the groups fed with jackfruit sections, especially PY and CY, increased productive performance compared to control group. The feed consumption of the rabbits fed with jackfruit peel (CY) was low consumption but high body weight, indicating that the assimilation of the feed components is possibly better in this diet. To the best of our knowledge, there is little information about the use of jackfruit sections influencing carcass traits. In one study, however, the use of jackfruit leaves to feed goats increased productivity, including feed efficiency and body weight [
27]. Muthukumar et al. [
28] reported that the use of jackfruit waste in dairy cows increased milk production and quality. In a further investigation, it was concluded that the use of processed jackfruit seed can be used to increase weight gain in Nile tilapia [
29]. The use other types of agro-industrial waste can affect the productivity of rabbits, as reported by Tavares et al. [
30], who evaluated acerola in the diet of growing rabbits and described improved weight and feed intake. Menchetti et al. [
26] incorporated goji berries into rabbit feed and obtained enhanced feed conversion and growth rates. It has been evidenced that carefully incorporating agro-industrial residues into rabbit diets can contribute to improved growth rates, feed conversion, and overall performance due to the richness of nutrients they provide [
5]. In this research, jackfruit could have contributed to animal nutrition, as the presence of antioxidants, minerals, essential amino acids has been confirmed [
31].
Blood analysis is an essential procedure for evaluating animal health [
32]. According to Brandão et al. [
33], all the values from this study are within the normal values reported for the species. High values of total alkaline phosphatase indicate the presence of a type of abnormal organic function. It is clear that the jackfruit could have contributed to reducing the risk of developing a pathology, although further studies would be needed to determine the true origin of these high values since this enzyme can be found in different areas of the body, such as the liver, bone, kidneys, and intestines [
34]. However, it has been reported that processed jackfruit seed can induce low red blood cell counts in fish [
29]. Jackfruit sections maintain animal health, leading to improved animal productivity. The use of jackfruit leaves to feed goats does not have an effect on blood biochemistry parameters [
27]. Moreover, in other investigations involving the incorporation of agricultural waste such as passion fruit seed [
35], acerola [
30], and herbal mixtures [
36] to the diet of rabbits, no alterations in the blood parameters were observed.
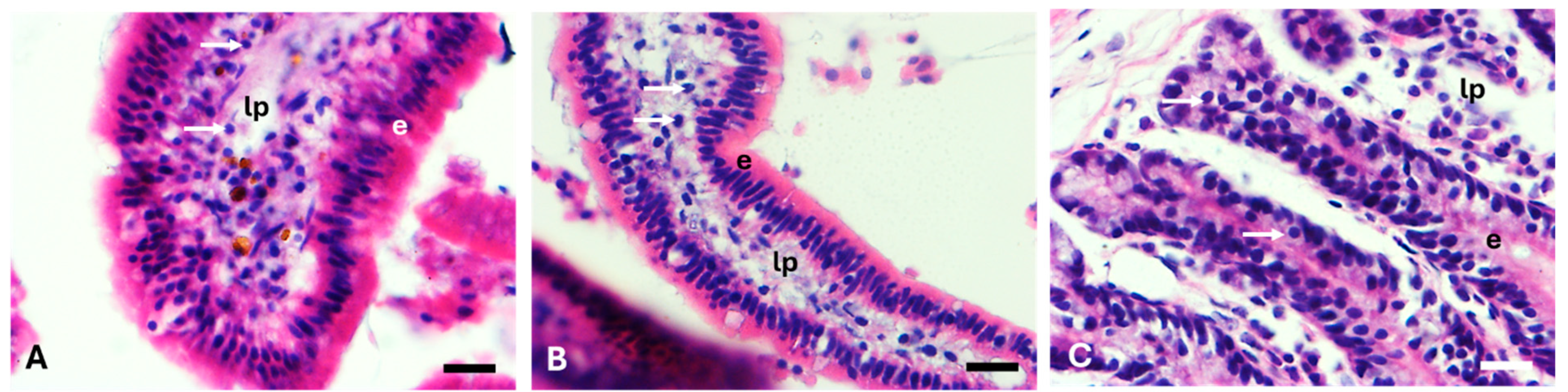
The structure and variety of cells in the small intestine create a complex environment, where digestion is facilitated by the absorption of nutrients [
37]. The efficiency of jackfruit sections in productive performance could be associated with intestinal epithelial cell integrity, since Fitrya et al. [
38] demonstrated that an ethanolic extract of jackfruit is effective in lowering the presence of peptic ulcers. Similarly, other studies have shown that the addition of agro-industry waste, such as pomegranate extract in rabbit feed, provided evidence of an apparent positive effect on the histological structure of the animals’ small intestine [
39]. It has been shown that jackfruit peel presents polysaccharides, which promote the growth of beneficial flora when degraded in the intestine [
40].
Carcass traits are influenced by the feed and its components, as well as the increase in feed consumption and body weight. However, raw jackfruit seed meal fed to Guinea fowl keets had no effect on cut parts and internal organs [
41]. However, the use of agro-industrial waste or other plants in rabbits modified carcass traits, such as with the research described by Volek et al. [
42], who evaluated white lupine seed in rabbit diets, reporting higher cold carcass yield and greater weight in the posterior paste from the carcass. In addition, the study suggested that the rapidly degradable proteins and the energy provided by lupine are efficiently utilized for the synthesis of tissues such as muscles, which in turn contributes to the higher weight achieved. Similarly, this phenomenon could have occurred in this research, since it has been proven that jackfruit provides proteins and essential amino acids [
43], with the animals in group CY obtaining a higher body weight.
Meat color is an indicator of quality, as it is associated with a pleasing appearance by the consumer. The color of rabbit muscles is pale pink, although natural pigments present in fruits have been found to contribute to the intensification of redness and yellowness of the flesh [
44]. It has been stated that jackfruit has different contents of natural pigments such as beta-carotene all-trans and lutein all-trans, which may have contributed to the intensification of the color. Rabbits that consumed jackfruit tended to have lower L* values and higher chroma values, which is related to higher feed consumption. This indicates that jackfruit sections contain molecules that provide color, as mentioned above.
During the rigor mortis process, hardening and acidification occur due to glycogenolysis, which are changes that influence meat quality. According to Menchetti et al. [
45], the pH of the meat may have been influenced by the jackfruit, which affected glycogen storage and enzyme activity in the muscle. In one study that evaluated meat from rabbits fed with different residues such as passion fruit seed [
35] and acerola [
30], the researchers found pH values lower than those from this research.
The water-holding capacity of meat is an important indicator that determines visual acceptability, yield, and sensory traits at the time of consumption. The water lost during cooking is probably due to heat-induced protein denaturation during this process, which results in less water being trapped within the protein structures held by capillary forces [
46]. The animals in the groups that consumed jackfruit sections had the lowest WHC values, indicating that the meat from the animals fed with this waste would be able to lose moisture. There are some factors, such as pH, ion availability, and degree of the myofibrillar proteins, that affect WHC. The jackfruit sections seem to modify the pH of the meat, which makes it possible to increase the ions available for water trapping. Similar results were found by Sosnówka-Czajka et al. [
47], who fed dried fruit pomace to broilers. However, the use of goji berries to supplement the diet of fattening rabbits did not affect the WHC [
26]. In another study, color, which is related to pH and WHC, was influenced by the use of citrus for feeding rabbits [
48]. Lower cooking loss results in better meat quality, because during cooking, nutrient loss may occur [
49]. However, in this study, cooking loss parameters were similar among the C, SY, and CY groups, which is related to the WHC of these groups. The cooking method employed for rabbit meat has an influence on WHC and cooking loss [
50].
Meat texture is a multidimensional property describing structural, mechanical, and surface properties, which are all directly related to sensory appreciation by the consumer [
51]. In works evaluating brown algae in rabbits, an improvement in meat texture and flavor has been reported, while the addition of polyphenol-rich sources probably protects proteolytic enzymes (calpain and m-calpain) from the oxidative process, increasing their functionality and consequently the tenderness of the meat [
52]. In contrast to the results in this study, other works have reported that the use of herbs or other vegetal compounds do not have an effect on TPA parameters [
53]. However, it is possible that some bioactive compounds present in jackfruit sections have an effect on meat hardness by modifying the action of the endogenous meat enzymes.
In a sensory analysis of rabbit meat carried out by Tavares et al. [
30], it was stated that the use of substances with abundant phenolic compounds can provide greater integrity of the myofibrillar membranes and consequently an improvement in the texture of the meat. On the other hand, Kuang et al. [
54] mentioned that a low fat content in muscle will lead to a loss of qualities such as texture and flavor. The addition of different plant sources resulted in the improvement of meat texture, juiciness, flavor, and acceptability, such as wine grape pomace [
55], tomato pomace [
56], plant extracts [
57], and
Saccharina latissima and
Himanthalia elongata [
58].