Antitumor Effect of Curcumin, D6 Turmeric, and Hydrochloride Mitoxantrone on Canine and Human Urothelial Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Cell Culture
2.3. Curcumin, D6 Turmeric, and Mitoxantrone Hydrochloride Preparations
2.4. MTT Cell Viability Assay
2.5. Annexin V/Propidium Iodide Assay
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. In Vitro Migration Assay
2.8. Statistical Analysis
3. Results
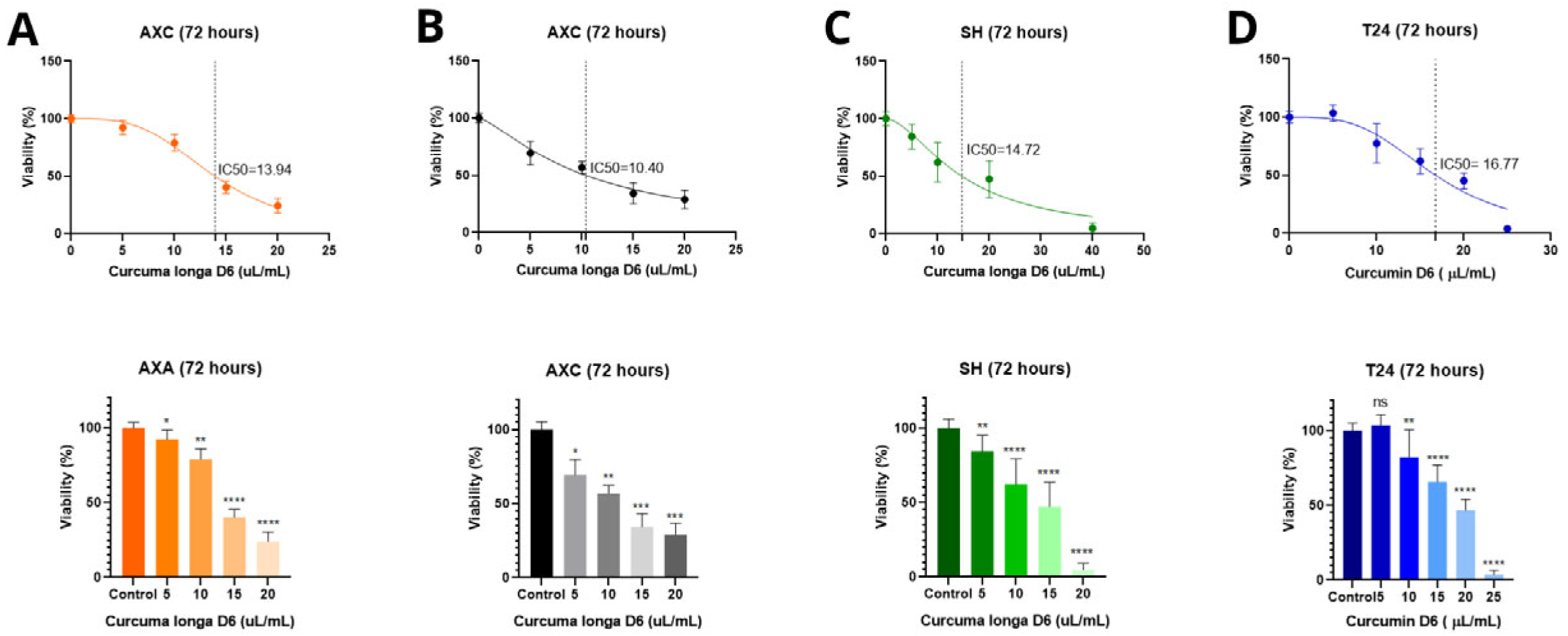
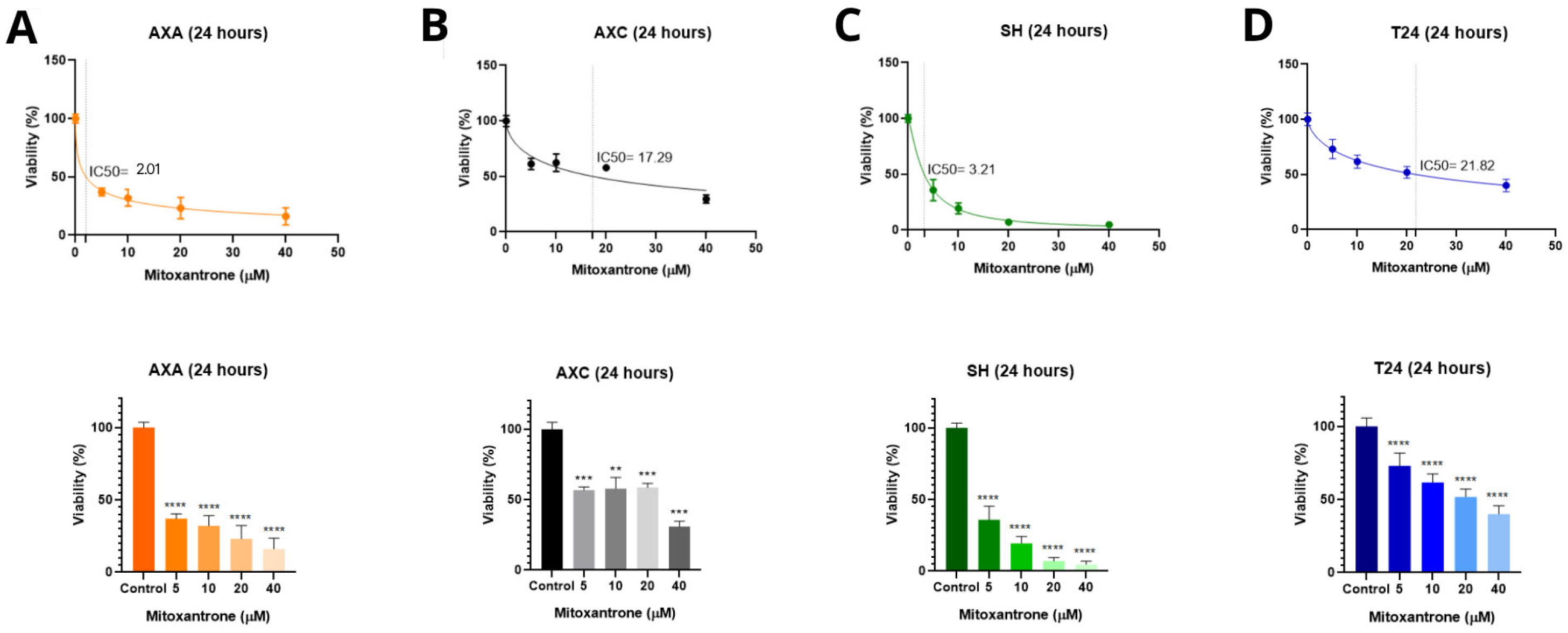
3.1. MTT Cell Viability Assay and Determination of IC50 After Treatments
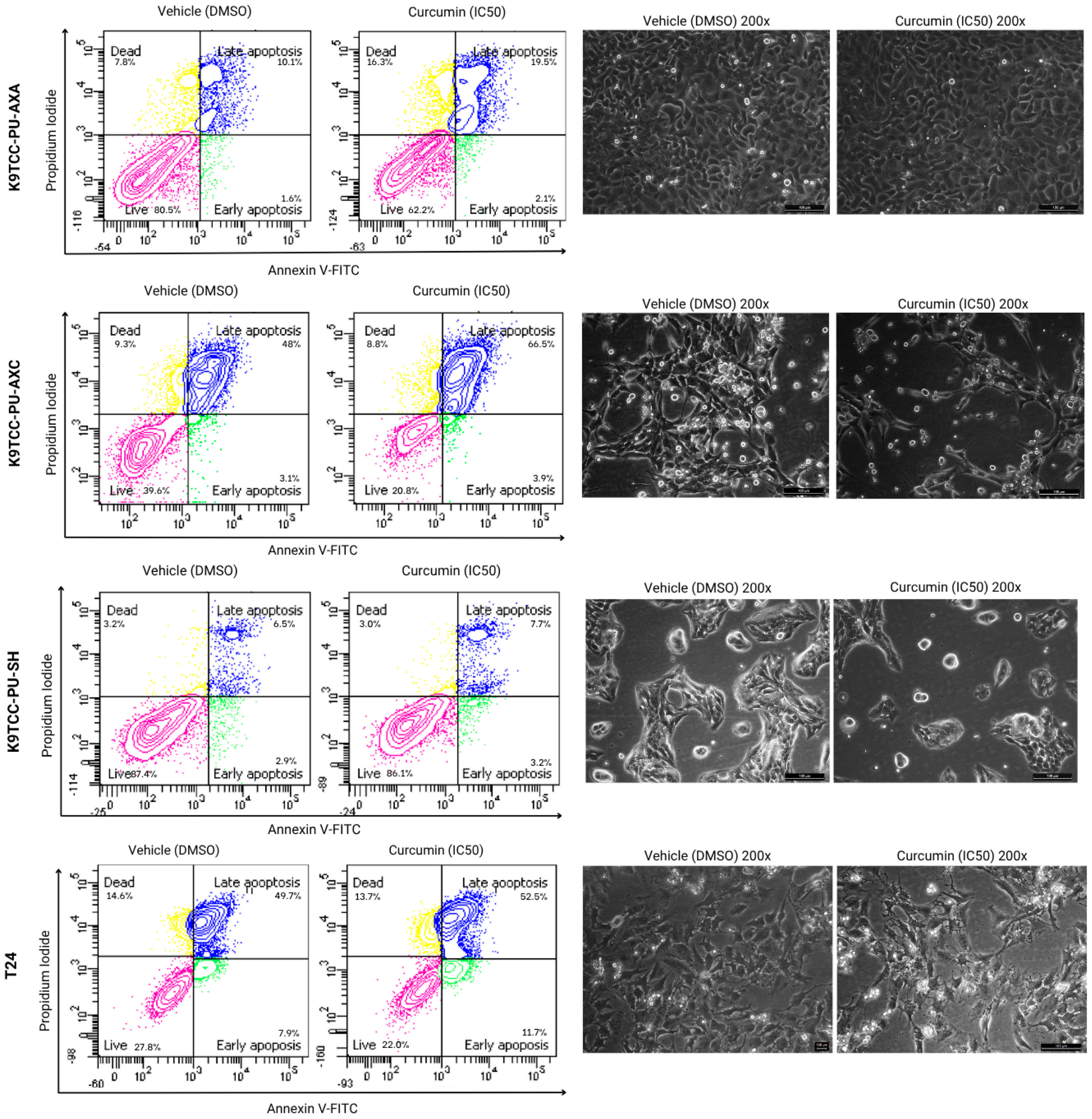
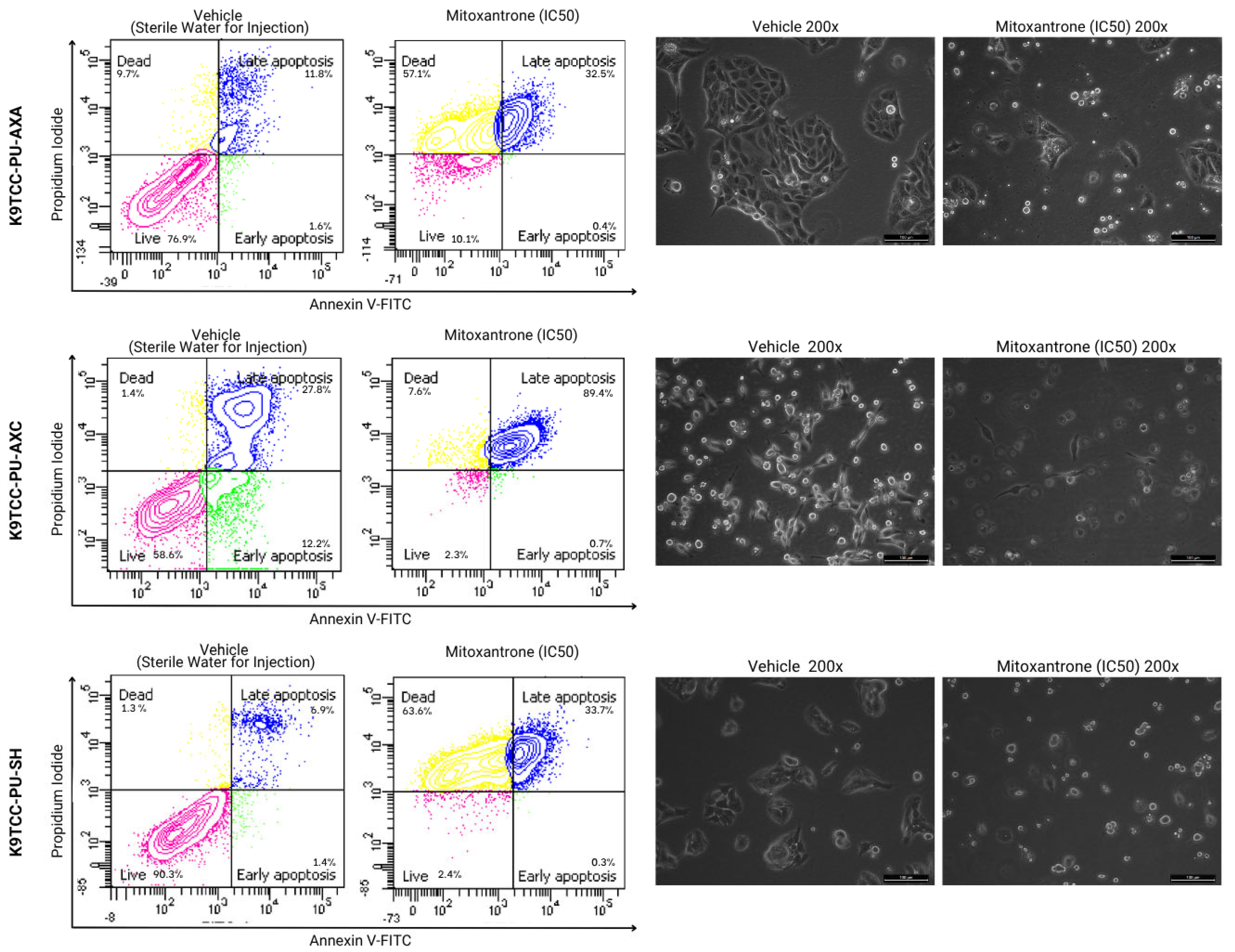
3.2. Cell Death Analysis by Annexin V/PI Staining
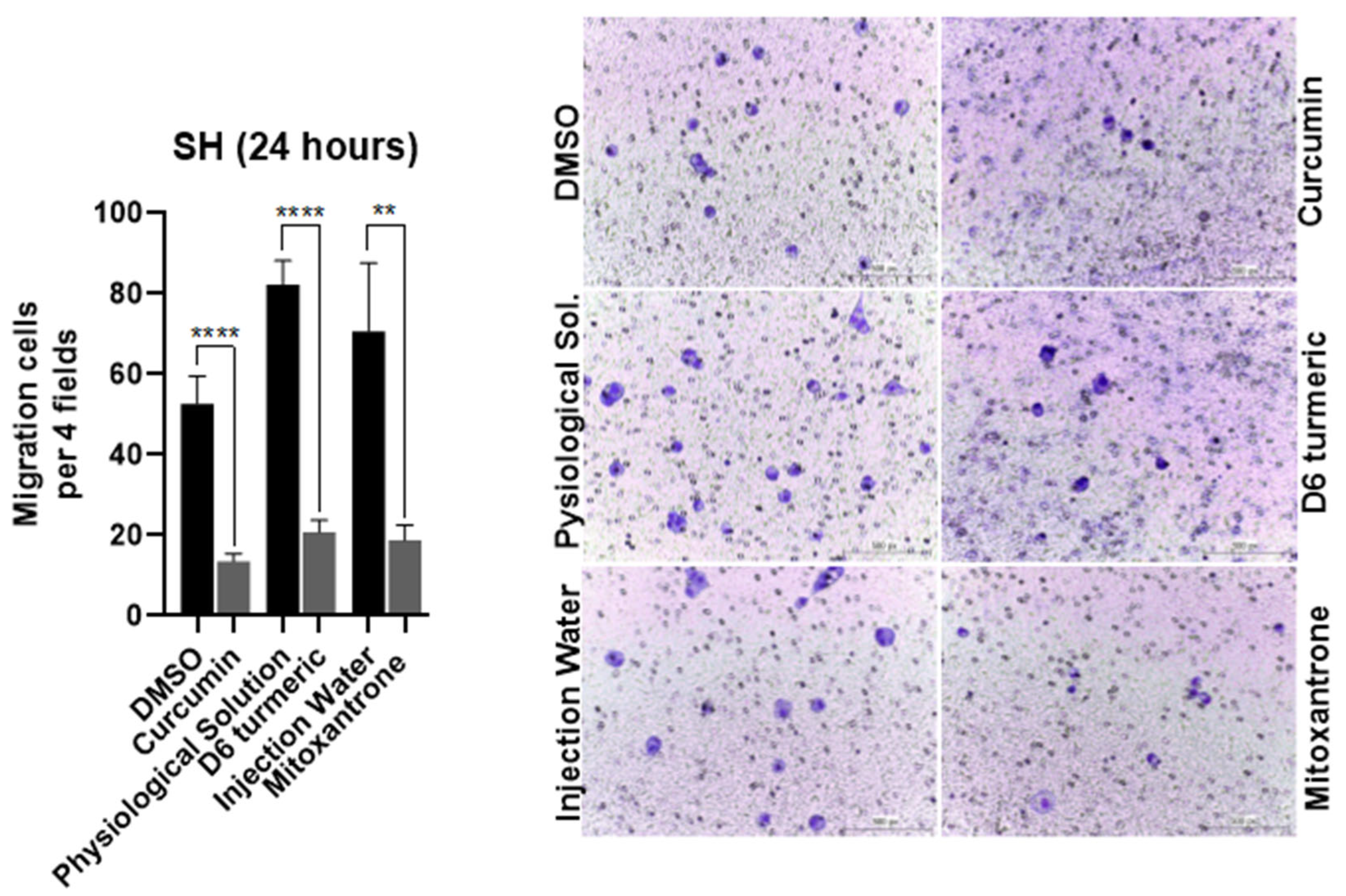
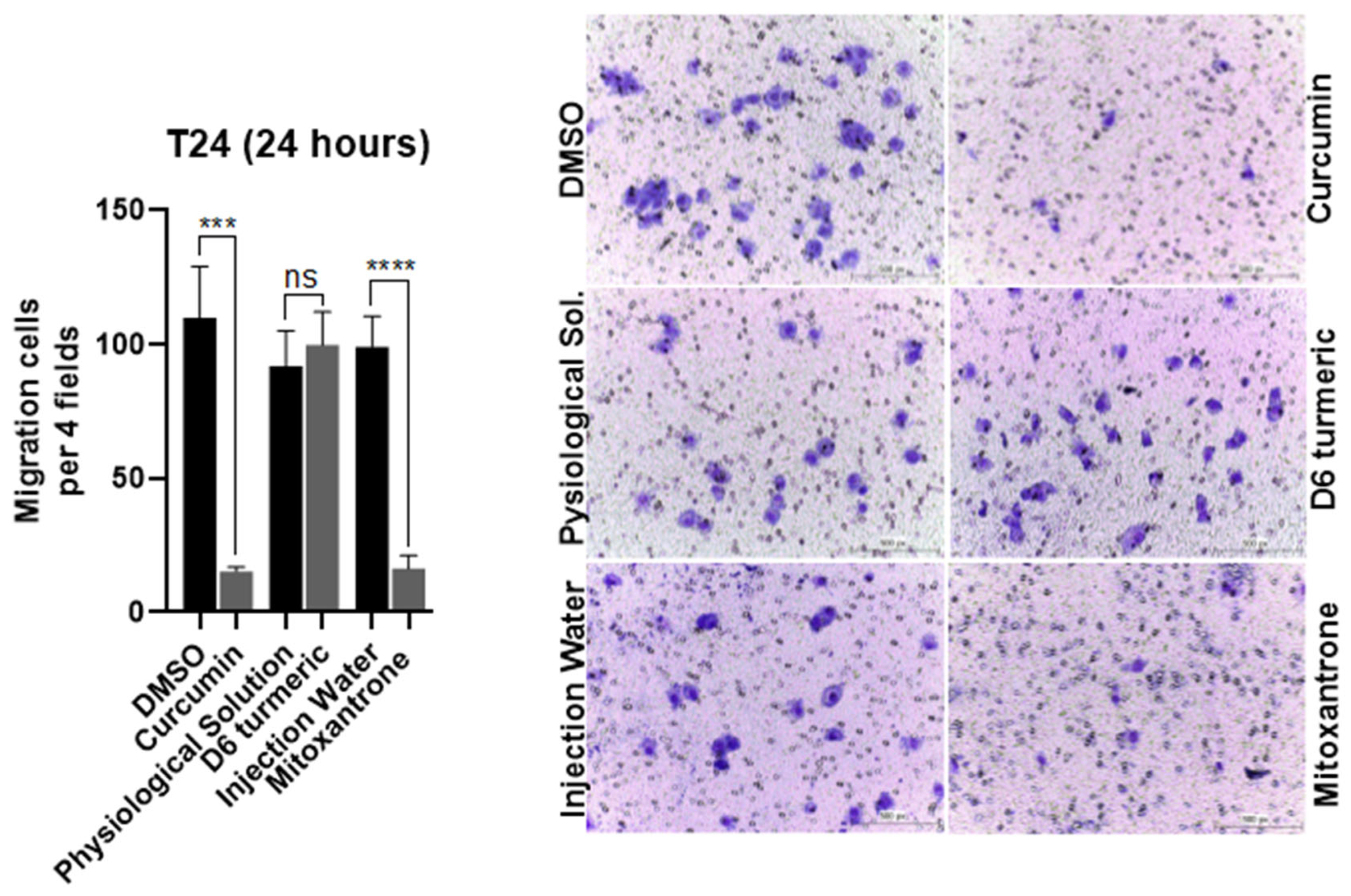
3.3. Effects of Treatment on Cell Migration
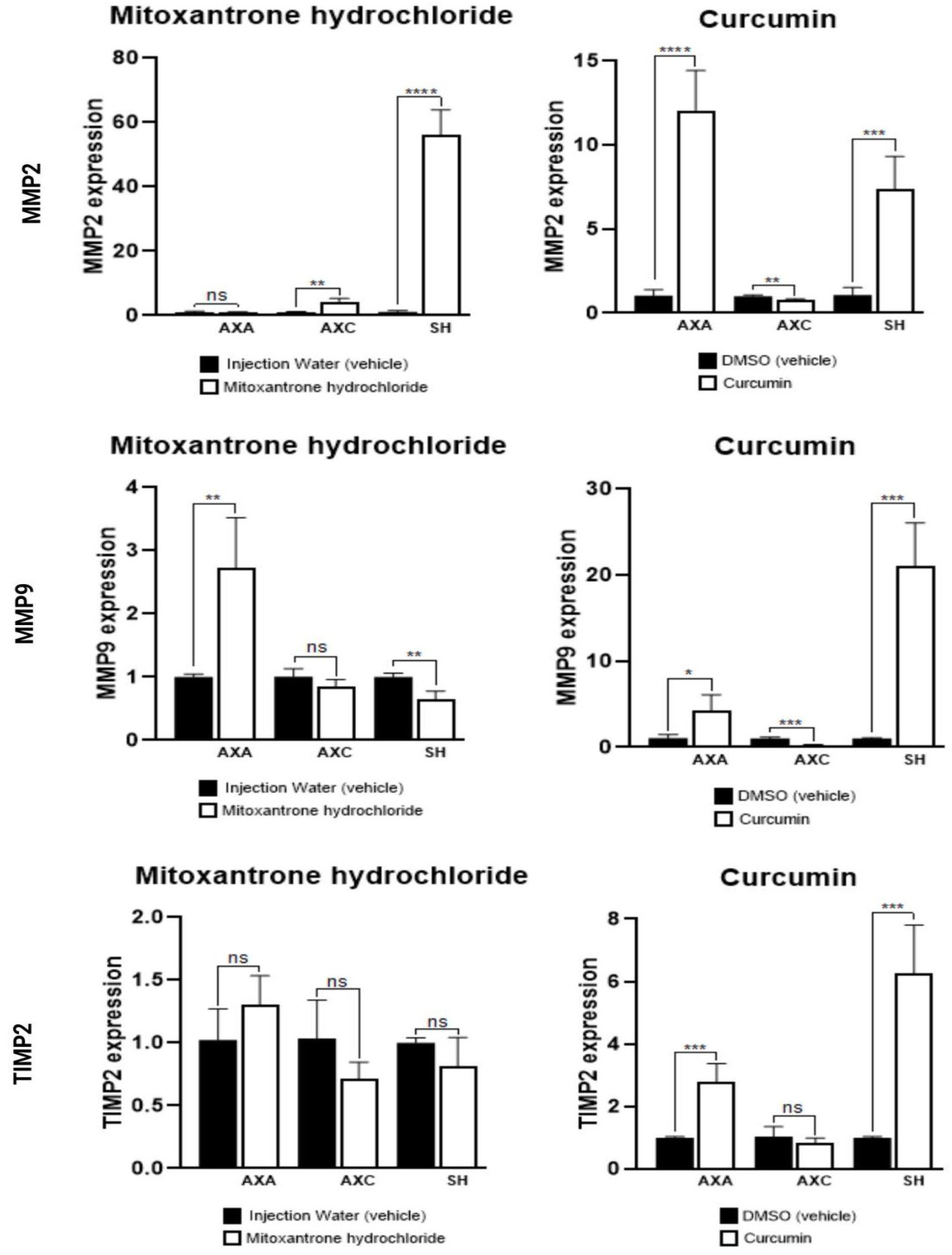
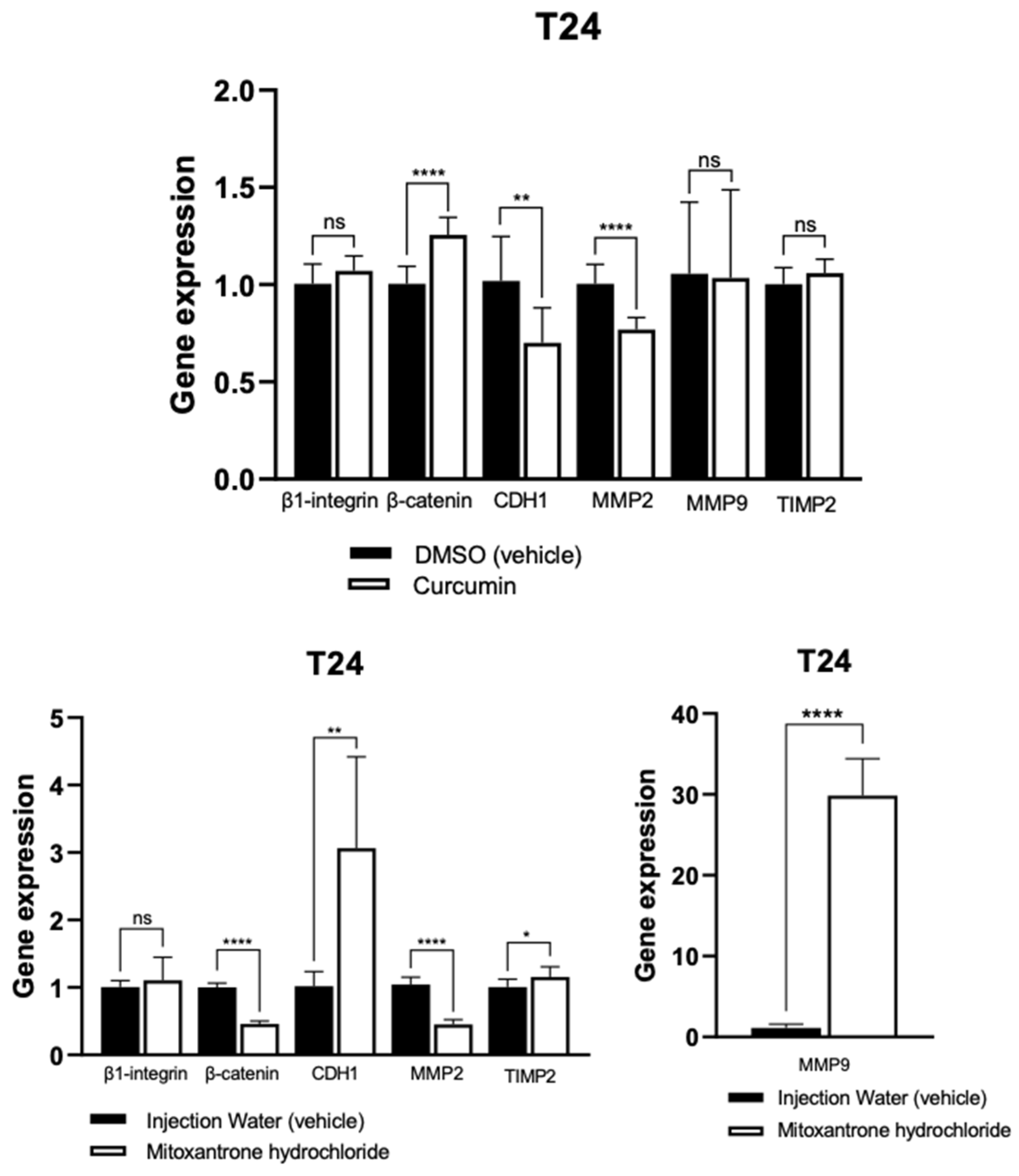
3.4. Quantitative Polymerase Chain Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, C.M.; Knapp, D.W. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet. J. 2015, 205, 217–225. [Google Scholar] [CrossRef]
- Knapp, D.W.; McMillan, S.K. Tumors of the Urinary System. In Withrow and MacEwen’s Small Animal Clinical Oncology, 5th ed.; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 572–582. [Google Scholar] [CrossRef]
- Sakai, K.; Maeda, S.; Saeki, K.; Nakagawa, T.; Murakami, M.; Endo, Y.; Yonezawa, T.; Kadosawa, T.; Mori, T.; Nishimura, R.; et al. Anti-tumour effect of lapatinib in canine transitional cell carcinoma cell lines. Vet. Comp. Oncol. 2018, 16, 642–649. [Google Scholar] [CrossRef]
- Knapp, D.W.; Glickman, N.W.; DeNicola, D.B.; Bonney, P.L.; Lin, T.L.; Glickman, L.T. Naturally-occurring canine transitional cell carcinoma of the urinary bladder: A relevant model of human invasive bladder cancer. Urol. Oncol. 2000, 5, 47–59. [Google Scholar] [CrossRef] [PubMed]
- de Brot, S.; Robinson, B.D.; Scase, T.; Grau-Roma, L.; Wilkinson, E.; Boorjian, S.A.; Gardner, D.; Mongan, N.P. The dog as an animal model for bladder and urethral urothelial carcinoma: Comparative epidemiology and histology. Oncol. Lett. 2018, 16, 1641–1649. [Google Scholar] [CrossRef]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014, 55, 100–118. [Google Scholar] [CrossRef]
- Carvalho, M.B.; Brum, A.M.; Vasconcellos, A.L.; Alves, M.A.M.K. Neoplasias do sistema urinário. In Oncologia em Cães e Gatos; Roca: São Paulo, Brasil, 2009; pp. 392–396. [Google Scholar]
- Sommer, B.C.; Dhawan, D.; Ratliff, T.L.; Knapp, D.W. Naturally-occurring canine invasive urothelial carcinoma: A model for emerging therapies. Bladder Cancer 2018, 4, 149–159. [Google Scholar] [CrossRef]
- d’Avila, G.F.L.; da Silveira, E.; Baier, M.E.; Gouvêa, A.S.; Fagundes, N.; de Castro Beck, C.A. Anastomose ureterocolônica em um cão com carcinoma de células de transição no trígono vesical. Acta Sci. Vet. 2016, 44, 159. Available online: https://www.redalyc.org/articulo.oa?id=289043698068 (accessed on 20 December 2024).
- Telles, S.A.; Monteiro, R.C.P.; Corrêa, F.M.; Calvo, P.Z.U.; de Moraes Oliveira, A.P.L. Carcinoma de células de transição de bexiga em cão: Relato de caso. Pubvet 2016, 11, 82–86. [Google Scholar] [CrossRef]
- Antonio, G.D.; Tesser, C.D.; Moretti-Pires, R.O. Fitoterapia na atenção primária à saúde. Rev. Saude Publica 2014, 48, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; Saturnino, K.; Cruz, V.; Araújo, E. Ação Antioxidante da curcumina (Curcuma longa L.) na injúria de isquemia e reperfusão tecidual. Enciclopédia Biosf. 2019, 16, 48–62. [Google Scholar] [CrossRef]
- Marchi, J.P.; Tedesco, L.; da Cruz Melo, A.; Frasson, A.C.; França, V.F.; Sato, S.W.; Wietzikoski, E.C. Curcuma longa L., o açafrão da terra, e seus benefícios medicinais. Arq. Cienc. Saude UNIPAR 2016, 20. [Google Scholar] [CrossRef][Green Version]
- Silva, T.O.; Calheiros, L.G.R.M.; Amorim, R.L. Efeito antitumoral invitro da curcumina no carcinoma urotelial de bexiga canino e humano—Revisão de literatura. Veterinária E Zootec. 2023, 30, 1–9. Available online: https://rvz.emnuvens.com.br/rvz/article/view/1053 (accessed on 20 December 2024). [CrossRef]
- Ozawa, H.; Imaizumi, A.; Sumi, Y.; Hashimoto, T.; Kanai, M.; Makino, Y.; Tsuda, T.; Takahashi, N.; Kakeya, H. Curcumin β-D-glucuronide plays an important role to keep high levels of free-form curcumin in the blood. Biol. Pharm. Bull. 2017, 40, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Fuselier, C.; Quemener, S.; Dufay, E.; Bour, C.; Boulagnon-Rombi, C.; Bouland, N.; Djermoune, E.-H.; Devy, J.; Martiny, L.; Schneider, C. Anti-tumoral and anti-angiogenic effects of low-diluted phenacetinum on melanoma. Front. Oncol. 2021, 11, 597503. [Google Scholar] [CrossRef]
- Sunila, E.S.; Kuttan, R.; Preethi, K.C.; Kuttan, G. Dynamized preparations in cell culture. Evid. Based Complement Altern. Med. 2009, 6, 257–263. [Google Scholar] [CrossRef]
- Dhawan, D.; Ramos-Vara, J.A.; Stewart, J.C.; Zheng, R.; Knapp, D.W. Canine invasive transitional cell carcinoma cell lines: In vitro tools to complement a relevant animal model of invasive urinary bladder cancer. Urol. Oncol. 2009, 27, 284–292. [Google Scholar] [CrossRef]
- Bubenik, J.; Barešová, M.; Viklický, V.; Jakoubkova, J.; Sainerova, H.; Donner, J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int. J. Cancer 1973, 11, 765–773. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Allstadt, S.D.; Rodriguez Jr, C.O.; Boostrom, B.; Rebhun, R.B.; Skorupski, K.A. Randomized phase III trial of piroxicam in combination with mitoxantrone or carboplatin for first-line treatment of urogenital tract transitional cell carcinoma in dogs. J. Vet. Intern. Med. 2015, 29, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Korec, D.I.; Louke, D.S.; Breitbach, J.T.; Geisler, J.A.; Husbands, B.D.; Fenger, J.M. Characterization of receptor tyrosine kinase activation and biological activity of toceranib phosphate in canine urothelial carcinoma cell lines. BMC Vet. Res. 2021, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Inkol, J.M.; Hocker, S.E.; Mutsaers, A.J. Combination therapy with cannabidiol and chemotherapeutics in canine urothelial carcinoma cells. PLoS ONE 2021, 16, e0255591. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Liu, Z.; Law, S.; Tian, H.; Xu, C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front. Pharmacol. 2018, 9, 408641. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Aşici, G.S.E.; Kiral, F.; Bayar, I.; Bildik, A.; Ulutaş, P.A. Cytotoxic and apoptotic effects of curcumin on D-17 canine osteosarcoma cell line. Kafkas Univ. Vet. Fak. Derg. 2021, 27, 465–473. [Google Scholar] [CrossRef]
- Withers, S.S.; York, D.; Johnson, E.; Al-Nadaf, S.; Skorupski, K.A.; Rodriguez, C.O., Jr.; Burton, J.H.; Guerrero, T.; Sein, K.; Wittenburg, L.; et al. In vitro and in vivo activity of liposome-encapsulated curcumin for naturally occurring canine cancers. Vet. Comp. Oncol. 2018, 16, 571–579. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Jia, Z.; Gao, Y.; Zhao, C.; Yao, Y. Curcumin inhibits bladder cancer progression via regulation of β-catenin expression. Tumor Biol. 2017, 39, 1010428317702548. [Google Scholar] [CrossRef]
- Henry, C.J.; McCaw, D.L.; Turnquist, S.E.; Tyler, J.W.; Bravo, L.; Sheafor, S.; Straw, R.C.; Dernell, W.S.; Madewell, B.R.; Jorgensen, L.; et al. Clinical evaluation of mitoxantrone and piroxicam in a canine model of human invasive urinary bladder carcinoma. Clin. Cancer Res. 2003, 9, 906–911. [Google Scholar]
- Ogilvie, G.K.; Obradovich, J.E.; Elmslie, R.E.; Vail, D.M.; Moore, A.S.; Straw, R.C.; Dickinson, K.; Cooper, M.F.; Withrow, S.J. Efficacy of mitoxantrone against various neoplasms in dogs. J. Am. Vet. Med. Assoc. 1991, 198, 1618–1621. [Google Scholar] [CrossRef]
- Govoni, V.M.; Pigoli, C.; Sueiro, F.A.R.; Zuliani, F.; da Silva, T.O.; Quitzan, J.G.; Amorim, R.; Grieco, V.; Alves, C. Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. Open Vet. J. 2021, 11, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Preethi, S.; Kumar, H.I.; Rawal, V.B.; Ajmeer, R.; Jain, V. Overview of mitoxantrone-a potential candidate for treatment of breast cancer. Int. J. Appl. Pharm. 2022, 14, 10–22. [Google Scholar] [CrossRef]
- Soares, N.P.; Nepomuceno, L.L.; de Sousa Cruz, V.; Arnhold, E.; de Souza Vieira, V.; de Almeida Borges, J.C.; Pereira, D.K.S.; Pereira, K.F.; de Araújo, E.G. Curcumin promotes extrinsic apoptosis in canine osteosarcoma cells. Res. Soc. Dev. 2020, 9, e7289109231. [Google Scholar] [CrossRef]
- Bahrami, A.; Majeed, M.; Sahebkar, A. Curcumin: A potent agent to reverse epithelial-to-mesenchymal transition. Cell. Oncol. 2019, 42, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, K.; Borran, S.; Ebrahimi, M.S.; Masoud khooy, M.J.; Seyedi, Z.S.; Amiri, A.; Abbasi-Kolli, M.; Fallah, M.; Khan, H.; Sahebkar, A. Therapeutic effect of curcumin in gastrointestinal cancers: A comprehensive review. Phytother. Res. 2021, 35, 4834–4897. [Google Scholar] [CrossRef]
- Goncharov, A.P.; Vashakidze, N.; Kharaishvili, G. Epithelial-mesenchymal transition: A fundamental cellular and microenvironmental process in benign and malignant prostate pathologies. Biomedicines 2024, 12, 418. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.H.; Zheng, G. E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget. 2017, 8, 33972. [Google Scholar] [CrossRef]
- Yen, H.Y.; Tsao, C.W.; Lin, Y.W.; Kuo, C.C.; Tsao, C.H.; Liu, C.Y. Regulation of carcinogenesis and modulation through Wnt/β-catenin signaling by curcumin in an ovarian cancer cell line. Sci. Rep. 2019, 9, 17267. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; Zhurinsky, J.; Ben-Ze’ev, A. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Investig. 2002, 109, 987–991. [Google Scholar] [CrossRef]
- Sameri, S.; Das, D.; Shahrivari, S.; Milosevic, V.; Sarhadi, S.; Koerkel-Qu, H.; Fisch, C.; Kokal, M.; Schletter, M.; Hahn, P.S.; et al. HER2 Cellular Localization, Cell-Cell Contacts, and Cell Density Regulate Cancer Cell Plasticity in HER2+ Breast Cancer. bioRxiv 2020. [Google Scholar] [CrossRef]
- JPan, B.; Guo, J.; Liao, Q.; Zhao, Y. β1 and β3 integrins in breast, prostate and pancreatic cancer: A novel implication. Oncol. Lett. 2018, 15, 5412–5416. [Google Scholar] [CrossRef]
- Loukopoulos, P.; Mungall, B.A.; Straw, R.C.; Thornton, J.R.; Robinson, W.F. Matrix metalloproteinase-2 and-9 involvement in canine tumors. Vet. Pathol. 2003, 40, 382–394. [Google Scholar] [CrossRef]
- Diepenbruck, M.; Christofori, G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bourboulia, D.; Stetler-Stevenson, W.G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2010; Volume 20, pp. 161–168. [Google Scholar]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef]
- Falke, J.; Parkkinen, J.; Vaahtera, L.; Hulsbergen-van de Kaa, C.A.; Oosterwijk, E.; Witjes, J.A. Curcumin as treatment for bladder cancer: A preclinical study of cyclodextrin-curcumin complex and BCG as intravesical treatment in an orthotopic bladder cancer rat model. Biomed. Res. Int. 2018, 2018, 9634902. [Google Scholar] [CrossRef]
- Guarino, M.; Rubino, B.; Ballabio, G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007, 39, 305–318. [Google Scholar] [CrossRef]


| Targets | Primers Sequences (5′–3′) |
|---|---|
| β-catenin canine | Forward: 5′-GATACCCAGCGCCGTACGT-3′ Reverse: 5′-GACCCCCTCCACAAATTGC-3′ |
| β1-integrin canine | Forward: 5′-AGCCATTTGCAAGGTTTTATCC-3′ Reverse: 5′-GGATTCAGGGTTTCTCAGATGTTAA-3′ |
| CDH1 canine | Forward: 5′-CAGGCCTCCGTTTCTGGAA-3′ Reverse: 5′-GGAGAGGAGTTGGGAAATGTG-3′ |
| MMP-2 canine | Forward: 5′-TGAGCTATGGACCTTGGGAGAA-3′ Reverse: 5′-CCATCGGCGTTCCCATAC-3′ |
| MMP-9 canine | Forward: 5′-GGACGATGCCTGCAACGT-3′ Reverse: 5′-CAAATACAGCTGGTTCCCAATCT-3′ |
| TIMP-2 canine | Forward: 5′-GGGCCAAAGCGGTCAGT-3′ Reverse: 5′-TAGGGTTGCCATAAATGTCGTTT-3′ |
| β-catenin human | Forward: 5′-GATACCCAGCGCCGTACGT-3′ Reverse: 5′-GACCCCCTCCACAAATTGC-3′ |
| β1-integrin human | Forward: 5′-AGCCATTTGCAAGGTTTTATCC-3′ Reverse: 5′-GGATTCAGGGTTTCTCAGATGTTAA-3′ |
| CDH1 human | Forward: 5′-CAGGCCTCCGTTTCTGGAA-3′ Reverse: 5′-AGGAGAGGAGTTGGGAAATGTG-3′ |
| MMP-2 human | Forward: 5′-TGAGCTATGGACCTTGGGAGAA-3′ Reverse: 5′-CCATCGGCGTTCCCATAC-3′ |
| MMP-9 human | Forward: 5′-GGACGATGCCTGCAACGT-3′ Reverse: 5′-CAAATACAGCTGGTTCCCAATCT-3′ |
| TIMP-2 human | Forward: 5′-GGGCCAAAGCGGTTCAGT-3′ Reverse: 5′-TAGGGTTGCCATAAATGTCGTTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.O.d.; Calheiros, L.G.R.d.M.; Barbosa, F.; Morrone, F.B.; Rockenbach, L.; Lainetti, P.d.F.; Leis Filho, A.F.; Carvalho, M.d.; Fonseca-Alves, C.E.; Laufer Amorim, R. Antitumor Effect of Curcumin, D6 Turmeric, and Hydrochloride Mitoxantrone on Canine and Human Urothelial Cancer Cells. Animals 2025, 15, 1589. https://doi.org/10.3390/ani15111589
Silva TOd, Calheiros LGRdM, Barbosa F, Morrone FB, Rockenbach L, Lainetti PdF, Leis Filho AF, Carvalho Md, Fonseca-Alves CE, Laufer Amorim R. Antitumor Effect of Curcumin, D6 Turmeric, and Hydrochloride Mitoxantrone on Canine and Human Urothelial Cancer Cells. Animals. 2025; 15(11):1589. https://doi.org/10.3390/ani15111589
Chicago/Turabian StyleSilva, Thayná Oliveira da, Luís Gustavo Ramos de Moraes Calheiros, Felipe Barbosa, Fernanda Bueno Morrone, Liliana Rockenbach, Patrícia de Faria Lainetti, Antonio Fernando Leis Filho, Márcio de Carvalho, Carlos Eduardo Fonseca-Alves, and Renée Laufer Amorim. 2025. "Antitumor Effect of Curcumin, D6 Turmeric, and Hydrochloride Mitoxantrone on Canine and Human Urothelial Cancer Cells" Animals 15, no. 11: 1589. https://doi.org/10.3390/ani15111589
APA StyleSilva, T. O. d., Calheiros, L. G. R. d. M., Barbosa, F., Morrone, F. B., Rockenbach, L., Lainetti, P. d. F., Leis Filho, A. F., Carvalho, M. d., Fonseca-Alves, C. E., & Laufer Amorim, R. (2025). Antitumor Effect of Curcumin, D6 Turmeric, and Hydrochloride Mitoxantrone on Canine and Human Urothelial Cancer Cells. Animals, 15(11), 1589. https://doi.org/10.3390/ani15111589








