Establishment and Partial Characterization of Canine Mammary Tumor Cell Lines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Samples
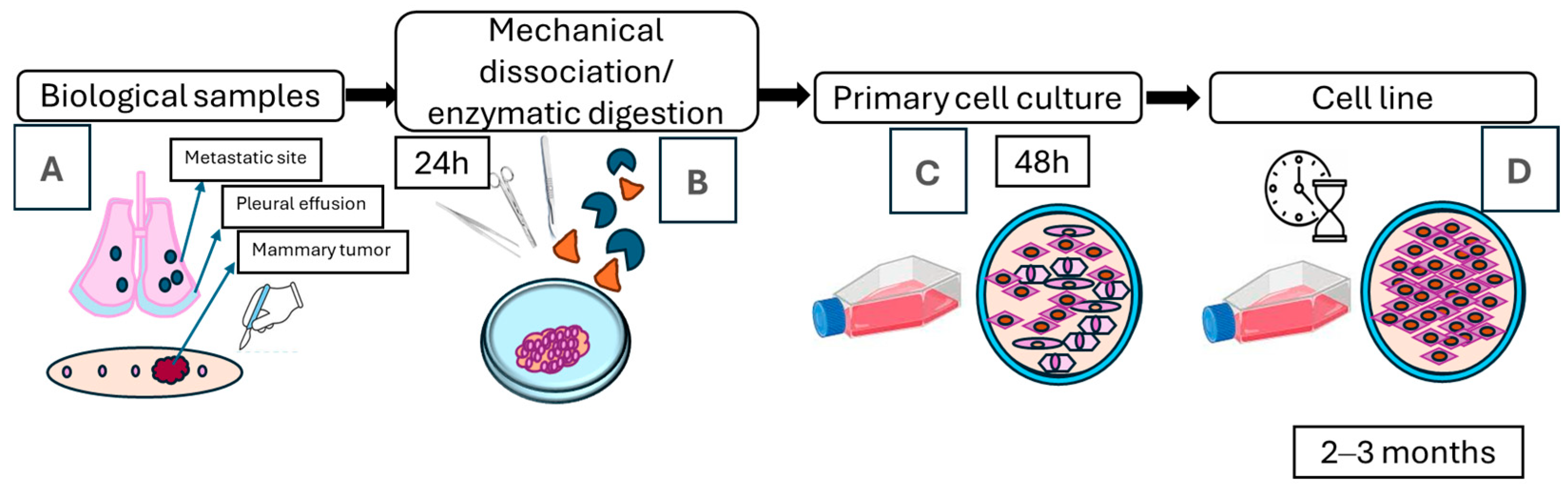
2.2. Establishment of Primary Cultures from Canine Mammary Tumors
2.3. Cell Doubling Time
2.4. Soft Agar Assay
2.5. Immunocytochemistry
2.6. Evaluation of Susceptibility of Cell Lines to Different Cancer Drugs
3. Results
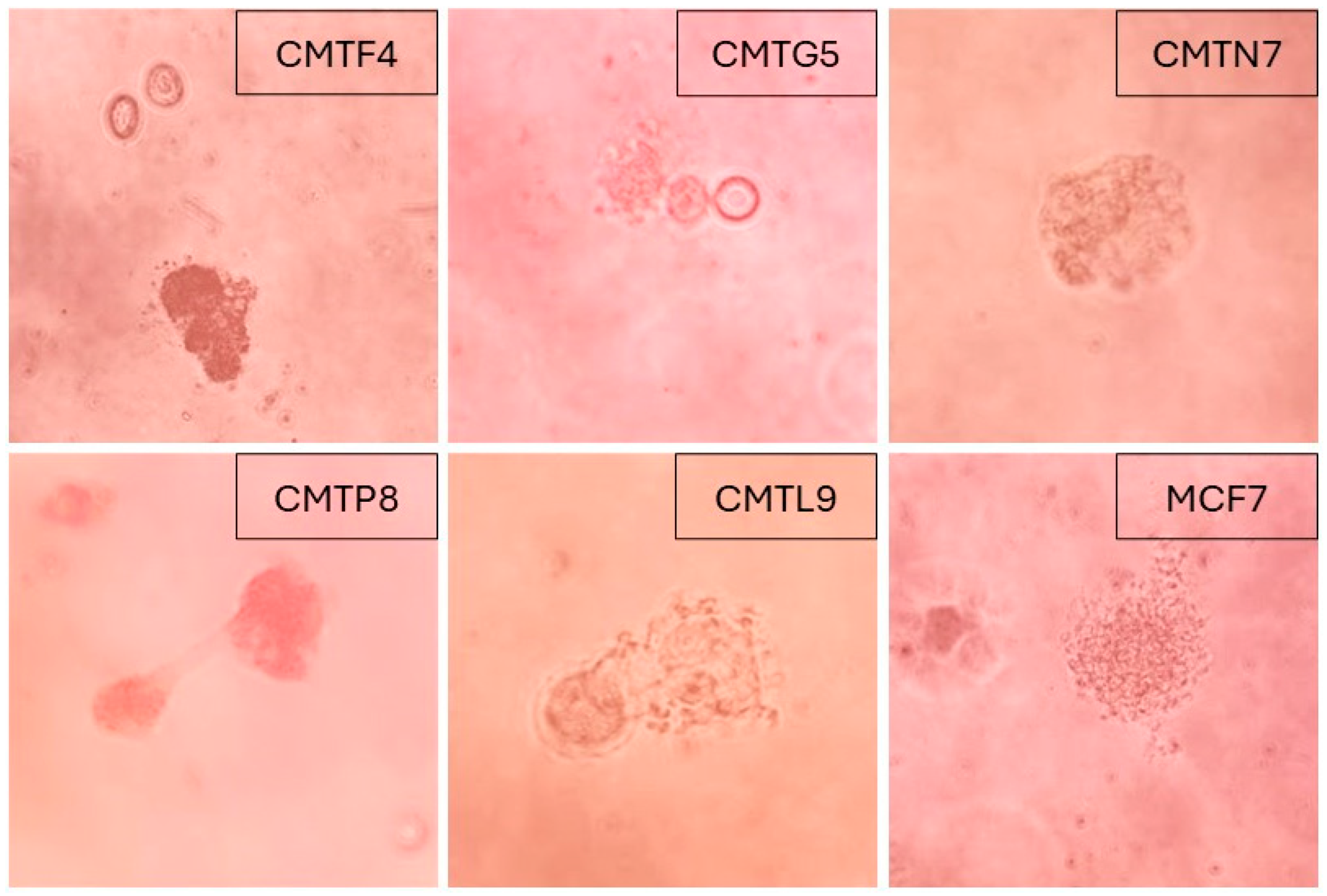
3.1. Establishment of Cell Lines from Canine Mammary Tumors
3.2. Doubling Time Determination
3.3. In Vitro Tumorigenicity
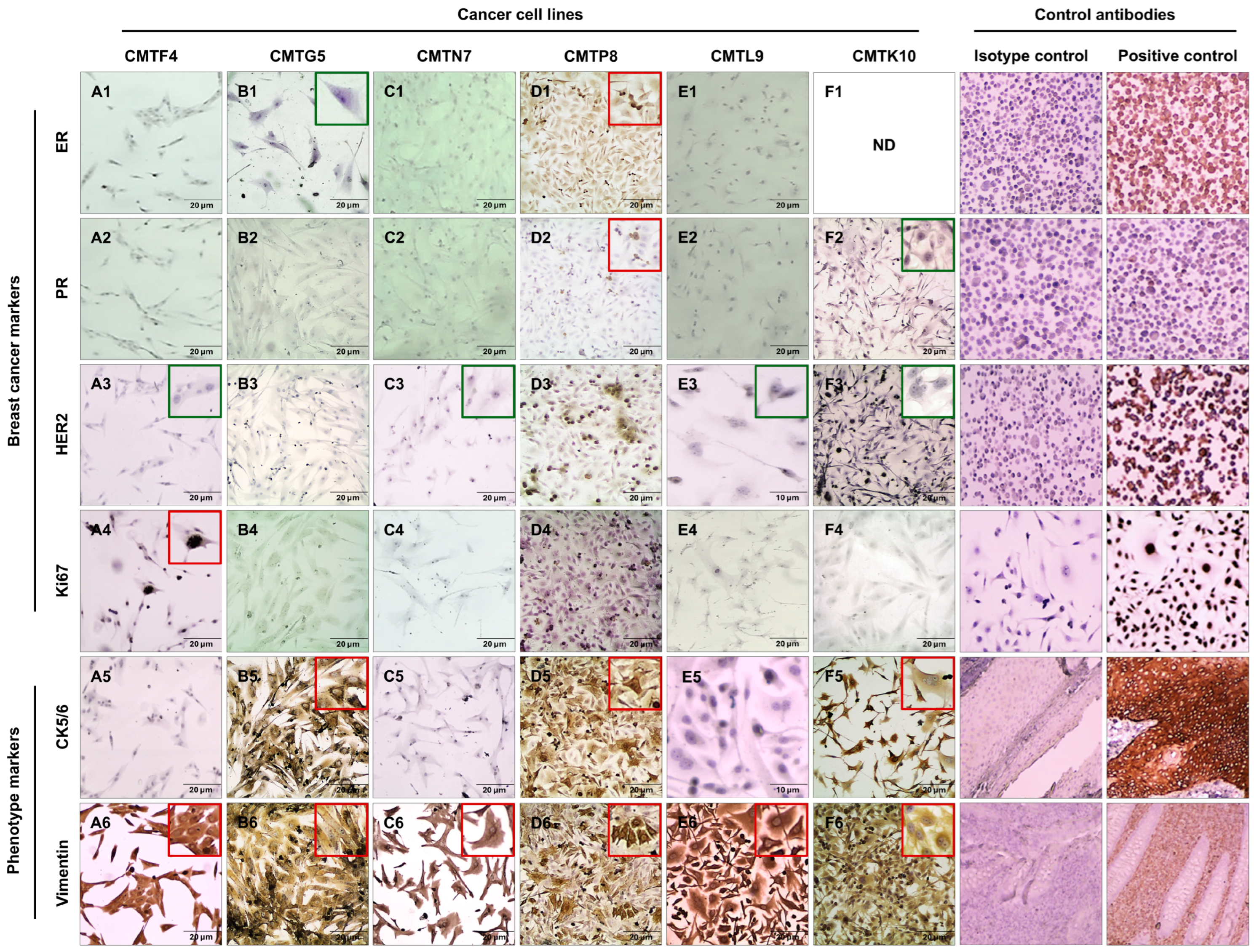
3.4. Immunocytochemistry Characterization of Cell Lines
3.5. Antiproliferative Effects of Different Drugs in Canine Mammary Tumor Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, B.W.; Ostrander, E.A. Domestic dogs and cancer research: A breed-based genomics approach. ILAR J. 2014, 55, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L.; Seagroves, T. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002–2012: A growing animal health problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.P.; Arias-Pulido, H.; Chaher, N.; Fiering, S.N.; Argyle, D.J.; Prada, J.; Pires, I.; Queiroga, F.L. Comparative aspects of canine and human inflammatory breast cancer. Semin. Oncol. 2017, 44, 288–300. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, S.; Zhou, B.; Wang, H.; Du, H.; Zhang, D.; Lin, D. Establishment and characterization of a new triple-negative canine mammary cancer cell line. Tissue Cell 2018, 54, 10–19. [Google Scholar] [CrossRef]
- Pawłowski, K.M.; Maciejewski, H.; Majchrzak, K.; Dolka, I.; Mol, J.A.; Motyl, T.; Król, M. Five markers useful for the distinction of canine mammary malignancy. BMC Vet. Res. 2013, 9, 138. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Munson, L.; Moresco, A.; Cardiff, R.D. Comparative Pathology of Mammary Gland Cancers in Domestic and Wild Animals. Breast Dis. 2007, 28, 7–21. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Raposo, T.P.; Silva-Carvalho, R.; Pires, I.; Prada, J.; Gregório, H.; Queiroga, F.L. The Dog as a Model to Study the Tumor Microenvironment; Springer: Cham, Switzerland, 2021; pp. 123–152. [Google Scholar] [CrossRef]
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: Natural history and prognostic factors. Breast Cancer Res. Treat. 2017, 167, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res. Treat. 2017, 167, 459–468. [Google Scholar] [CrossRef]
- Raposo, T.P.; Pires, I.; Prada, J.; Queiroga, F.L.; Argyle, D.J. Exploring new biomarkers in the tumour microenvironment of canine inflammatory mammary tumours. Vet. Comp. Oncol. 2016, 15, 655–666. [Google Scholar] [CrossRef]
- Schneider, R.; Dorn, C.R.; Taylor, D.O.N. Factors influencing canine mammary cancer development and postsurgical survival. JNCI J. Natl. Cancer Inst. 1969, 43, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Tesi, M.; Millanta, F.; Poli, A.; Mazzetti, G.; Pasquini, A.; Panzani, D.; Rota, A.; Vannozzi, I. Role of body condition score and adiponectin expression in the progression of canine mammary carcinomas. Vet. Med. Sci. 2020, 6, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, E.G.; Glickman, L.T.; Goldschmidt, M.H.; McKee, L.J. Body Conformation, Diet, and Risk of Breast Cancer in Pet Dogs: A Case-Control Study. Am. J. Epidemiol. 1991, 133, 694–703. [Google Scholar] [CrossRef]
- Zheng, H.H.; Du, C.T.; Yu, C.; Zhang, Y.Z.; Huang, R.L.; Tang, X.Y.; Xie, G.H. Epidemiological investigation of canine mammary tumors in mainland China between 2017 and 2021. Front. Vet. Sci. 2022, 9, 843390. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.; Alves, A.; Pires, I.; Lopes, C. Expression of Cox-1 and Cox-2 in Canine Mammary Tumours. J. Comp. Pathol. 2007, 136, 177–185. [Google Scholar] [CrossRef]
- de Andrés, P.J.; Cáceres, S.; Illera, J.C.; Crespo, B.; Silván, G.; Queiroga, F.L.; Illera, M.J.; Pérez-Alenza, M.D.; Peña, L. Hormonal Homologies between Canine Mammary Cancer and Human Breast Cancer in a Series of Cases. Vet. Sci. 2022, 9, 395. [Google Scholar] [CrossRef]
- Guimarães, M.; Carvalho, M.; Pires, I.; Prada, J.; Gil, A.G.; Lopes, C.; Queiroga, F. Concurrent expression of cyclo-oxygenase-2 and epidermal growth factor receptor in canine malignant mammary tumours. J. Comp. Pathol. 2014, 150, 27–34. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Perez-Alenza, M.D.; González-Gil, A.; Silván, G.; Peña, L.; Illera, J.C. Quantification of epidermal growth factor receptor (EGFR) in canine mammary tumours by ELISA assay: Clinical and prognostic implications. Vet. Comp. Oncol. 2015, 15, 383–390. [Google Scholar] [CrossRef]
- Nieto, A.; Peña, L.; Pérez-Alenza, M.D.; Sánchez, M.A.; Flores, J.M.; Castaño, M. Immunohistologic Detection of Estrogen Receptor Alpha in Canine Mammary Tumors: Clinical and Pathologic Associations and Prognostic Significance. Vet. Pathol. 2000, 37, 239–247. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Lobo, L.; Queiroga, F.L. Ki-67 and PCNA Expression in Canine Mammary Tumors and Adjacent Nonneoplastic Mammary Glands. Vet. Pathol. 2016, 53, 1138–1146. [Google Scholar] [CrossRef]
- Mei, C.; Xin, L.; Liu, Y.; Lin, J.; Xian, H.; Zhang, X.; Hu, W.; Xia, Z.; Wang, H.; Lyu, Y. Establishment of a New Cell Line of Canine Mammary Tumor CMT-1026. Front. Vet. Sci. 2021, 8, 744032. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Baek, Y.-B.; Kim, H.-J.; Kim, H.-P.; Jeon, Y.-J.; Song, J.E.; Jung, S.-B.; Moon, K.-S.; Park, S.-I.; Lee, C.-M.; et al. Establishment of canine mammary gland tumor cell lines harboring PI3K/Akt activation as a therapeutic target. BMC Vet. Res. 2024, 20, 233. [Google Scholar] [CrossRef] [PubMed]
- Lainetti, P.d.F.; Brandi, A.; Filho, A.F.L.; Prado, M.C.M.; Kobayashi, P.E.; Laufer-Amorim, R.; Fonseca-Alves, C.E. Establishment and Characterization of Canine Mammary Gland Carcinoma Cell Lines With Vasculogenic Mimicry Ability In Vitro and In Vivo. Front. Vet. Sci. 2020, 7, 583874. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, H.; Gao, Y.; He, L.; Li, W.; Xu, M.; Feng, H.; Feng, X.; Qiu, C. Establishment of a new cell line of canine inflammatory mammary cancer: IMC-118. Vet. Comp. Oncol. 2022, 20, 679–687. [Google Scholar] [CrossRef]
- Li, R.; Wu, H.; Sun, Y.; Zhu, J.; Tang, J.; Kuang, Y.; Li, G. A Novel Canine Mammary Cancer Cell Line: Preliminary Identification and Utilization for Drug Screening Studies. Front. Vet. Sci. 2021, 8, 665906. [Google Scholar] [CrossRef]
- Ardicli, S.; Samli, H.; Mecitoglu, G.; Vatansever, B.; Mutlu, A.M. The establishment of primary cell culture from canine mammary gland tumor. J. Cell. Biotechnol. 2021, 7, 57–65. [Google Scholar] [CrossRef]
- Chen, A.; Ye, S.; Zheng, J.; Li, J.; Chen, Z.; Zhang, Y.; Li, S. Establishment and characterization of a HER2-enriched canine mammary cancerous myoepithelial cell line. BMC Vet. Res. 2023, 19, 22. [Google Scholar] [CrossRef]
- Itoh, H.; Naruse, R.; Tani, K.; Sunahara, H.; Nemoto, Y.; Nakaichi, M.; Iseri, T.; Horikirizono, H.; Itamoto, K. Establishment of a new canine inflammatory mammary carcinoma cell line and analysis of its cystine-glutamate transporter subunit expression. J. Vet. Res. 2022, 66, 273–279. [Google Scholar] [CrossRef]
- Gentile, L.B.; Nagamine, M.K.; Biondi, L.R.; Sanches, D.S.; Toyota, F.; Giovani, T.M.; de Jesus, I.P.; da Fonseca, I.I.M.; Queiroz-Hazarbassanov, N.; Diaz, B.L.; et al. Establishment of primary mixed cell cultures from spontaneous canine mammary tumors: Characterization of classic and new cancer-associated molecules. PLoS ONE 2017, 12, e0184228. [Google Scholar] [CrossRef]
- Camacho, L.; Peña, L.; Gil, A.G.; Cáceres, S.; Díez, L.; Illera, J. Establishment and characterization of a canine xenograft model of inflammatory mammary carcinoma. Res. Vet. Sci. 2013, 95, 1068–1075. [Google Scholar] [CrossRef]
- Zamani-Ahmadmahmudi, M.; Jajarmi, M.; Talebipour, S. Molecular phenotyping of malignant canine mammary tumours: Detection of high-risk group and its relationship with clinicomolecular characteristics. Vet. Comp. Oncol. 2022, 21, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Caceres, S.; Peña, L.; Lacerda, L.; Illera, M.J.; de Andres, P.J.; Larson, R.A.; Gao, H.; Debeb, B.G.; Woodward, W.A.; Reuben, J.M.; et al. Canine cell line, IPC-366, as a good model for the study of inflammatory breast cancer. Vet. Comp. Oncol. 2016, 15, 980–995. [Google Scholar] [CrossRef]
- Wolfe, L.G.; Smith, B.B.; Toivio-Kinnucan, M.A.; Sartin, E.A.; Kwapien, R.P.; Henderson, R.A.; Barnes, S. Biologic Properties of Cell Lines Derived From Canine Mammary Carcinomas. J. Natl. Cancer Inst. 1986, 77, 783–792. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, B.; van Selm-Miltenburg, A.J.P.; van Maurik, P.; Rutteman, G.R.; Misdorp, W.; de Laat, S.W.; van Zoelen, E.J.J. Isolation of Autonomously Growing Dog Mammary Tumor Cell Lines Cultured in Medium Supplemented with Serum Treated To Inactivate Growth Factors. JNCI J. Natl. Cancer Inst. 1989, 81, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Sunden, Y.; Sugiyama, A.; Azuma, K.; Murahata, Y.; Tsuka, T.; Ito, N.; Imagawa, T.; Okamoto, Y. Establishment of a canine mammary gland tumor cell line and characterization of its miRNA expression. J. Vet. Sci. 2016, 17, 385–390. [Google Scholar] [CrossRef]
- Else, R.W.; Norval, M.; Neill Fromti, W.A. Short Communication the characteristics of a canine mammary carcinoma cell line, REM 134. Br. J. Cancer 1982, 46, 675–681. [Google Scholar] [CrossRef]
- Hellmén, E. Characterization of four in vitro established canine mammary carcinoma and one atypical benign mixed tumor cell lines. In Vitr. Cell. Dev. Biol.-Anim. 1992, 28, 309–319. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, D.; Pei, S.M.; Zhang, H.; Du, H.C.; Jin, Y.P.; Lin, D.G. Establishment of 5-Fluorouracil-resistant canine mammary tumor cell line. Pol. J. Vet. Sci. 2017, 20, 103–110. [Google Scholar] [CrossRef]
- Hsiao, Y.-L.; Hsieh, T.-Z.; Liou, C.-J.; Cheng, Y.-H.; Lin, C.-T.; Chang, C.-Y.; Lai, Y.-S. Characterization of protein marker expression, tumorigenicity, and doxorubicin chemoresistance in two new canine mammary tumor cell lines. BMC Vet. Res. 2014, 10, 229. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Chen, Z.; Anderson, G.L.; Rohan, T.; Aragaki, A.; Lane, D.; Dolan, N.C.; Paskett, E.D.; McTiernan, A.; Hubbell, F.A.; et al. Ethnicity and breast cancer: Factors influencing differences in incidence and outcome. JNCI J. Natl. Cancer Inst. 2005, 97, 439–448. [Google Scholar] [CrossRef]
- Knapp, D.W.; Peer, W.A.; Conteh, A.; Diggs, A.R.; Cooper, B.R.; Glickman, N.W.; Bonney, P.L.; Stewart, J.C.; Glickman, L.T.; Murphy, A.S. Detection of herbicides in the urine of pet dogs following home lawn chemical application. Sci. Total. Environ. 2013, 456–457, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Defourny, S.V.; Caioni, G.; Bellocci, M.; Melai, V.; Scortichini, G.; Salini, R.; Martino, M.; Di Teodoro, G.; Cocco, A.; Cantelmi, M.C.; et al. Domestic dogs as environmental sentinel in comparative toxicologic pathology: Assessment of metals and rare earth elements concentrations in healthy and neoplastic mammary glands. One Health 2024, 18, 100749. [Google Scholar] [CrossRef]
- Sévère, S.; Marchand, P.; Guiffard, I.; Morio, F.; Venisseau, A.; Veyrand, B.; Le Bizec, B.; Antignac, J.-P.; Abadie, J. Pollutants in pet dogs: A model for environmental links to breast cancer. SpringerPlus 2015, 4, 27. [Google Scholar] [CrossRef]
- Zamani-Ahmadmahmudi, M.; Nassiri, S.M.; Jahanzad, I.; Shirani, D.; Rahbarghazi, R.; Yazdani, B. Isolation and characterization of a canine mammary cell line prepared for proteomics analysis. Tissue Cell 2013, 45, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Culture of Animal Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- LaBarge, M.A.; Garbe, J.C.; Stampfer, M.R. Processing of human reduction mammoplasty and mastectomy tissues for cell culture. J. Vis. Exp. 2013, 71, e50011. [Google Scholar] [CrossRef]
- Korzyńska, A.; Zychowicz, M. A Method of Estimation of the Cell Doubling Time on Basis of the Cell Culture Monitoring Data. Biocybern. Biomed. Eng. 2008, 28, 75–82. [Google Scholar]
- Rajendran, V.; Jain, M.V. In vitro tumorigenic assay: Colony forming assay for cancer stem cells. In Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2018; Volume 1692, pp. 89–95. [Google Scholar] [CrossRef]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Karuppusamy Rathinam, M.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar] [CrossRef]
- Du, F.; Zhao, X.; Fan, D. Soft Agar Colony Formation Assay as a Hallmark of Carcinogenesis. Bio. Protocol. 2017, 7, e2351. [Google Scholar] [CrossRef]
- Yoo, M.-J.; Jang, Y.-J.; Park, S.-Y.; Choi, J.-W.; Seol, J.-W. Synergistic Anti-Cancer Effects of ERB-041 and Genistein through Estrogen Receptor Suppression-Mediated PI3K/AKT Pathway Downregulation in Canine Mammary Gland Tumor Cells. Int. J. Mol. Sci. 2024, 25, 2466. [Google Scholar] [CrossRef]
- Tahir, M.; Reynaud, K.; Mawa, G.; Thoumire, S.; Chastant-Maillard, S.; Saint-Dizier, M. Immunolocalization of Progesterone Receptors in the Canine Oviduct around Ovulation. Reprod. Domest. Anim. 2012, 47, 35–39. [Google Scholar] [CrossRef]
- Toniti, P.; Sirivisoot, S.; Jandee, P.; Srimontri, P.; Puchadapirom, P.; Doungchawee, G.; Kasorndorkbua, C. AE1/AE3, vimentin and p63 immunolocalization in canine mammary gland tumours: Roles in differentiation between luminal epithelial and myoepithelial lineages. Asian Pac. J. Cancer Prev. 2010, 11, 227–230. [Google Scholar] [PubMed]
- Zuo, R.; Kong, L.; Pang, W.; Jiang, S. Halofuginone-Guided Nano-Local Therapy: Nano-Thermosensitive Hydrogels for Postoperative Metastatic Canine Mammary Carcinoma with Scar Removal. Int. J. Pharm. X 2024, 7, 100241. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-González, G.; García-Castillo, V.; Coronel-Hernández, J.; López-Urrutia, E.; León-Cabrera, S.; Arias-Romero, L.E.; Terrazas, L.; Rodríguez-Sosa, M.; Campos-Parra, A.D.; Zúñiga-Calzada, E.; et al. Anti-Inflammatory and Antitumor Activity of a Triple Therapy for a Colitis-Related Colorectal Cancer. J. Cancer 2016, 7, 1632–1644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nunes, F.C.; Bertagnolli, A.C.; Lavalle, G.E.; Silveira, T.L.; Balabram, D.; Cassali, G.D. The Prognostic Significance of Immunophenotypes in Canine Malignant Mammary Tumors. Arq. Bras. Med. Vet. Zootec. 2022, 74, 299–309. [Google Scholar] [CrossRef]
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; et al. Canine Mammary Tumors: A Review and Consensus of Standard Guidelines on Epithelial and Myoepithelial Phenotype Markers, HER2, and Hormone Receptor Assessment Using Immunohistochemistry. Vet. Pathol. 2014, 51, 127–145. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef]
- Simon, D.; Schoenrock, D.; Baumgärtner, W.; Nolte, I.; Dipl, E.D.S.D. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J. Vet. Intern. Med. 2006, 20, 1184–1190. [Google Scholar] [CrossRef]
- LaValle, G.E.; De Campos, C.B.; Bertagnolli, A.; Cassali, G.D. Canine malignant mammary gland neoplasms with advanced clinical staging treated with carboplatin and cyclooxygenase inhibitors. In Vivo 2012, 26, 375–379. [Google Scholar] [PubMed]
- Karayannopoulou, M.; Kaldrymidou, E.; Constantinidis, T.C.; Dessiris, A. Adjuvant Post-operative Chemotherapy in Bitches with Mammary Cancer. J. Vet. Med. Ser. A 2001, 48, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Varallo, G.R.; Gelaleti, G.B.; Maschio-Signorini, L.B.; Moschetta, M.G.; Lopes, J.R.; De Nardi, A.B.; Tinucci-Costa, M.; Rocha, R.M.; De Campos Zuccari, D.A.P. Prognostic phenotypic classification for canine mammary tumors. Oncol. Lett. 2019, 18, 6545–6553. [Google Scholar] [CrossRef] [PubMed]
- Arenas, C.; Peña, L.; Granados-Soler, J.L.; Pérez-Alenza, M.D. Adjuvant therapy for highly malignant canine mammary tumours: Cox-2 inhibitor versus chemotherapy: A case–control prospective study. Vet. Rec. 2016, 179, 125. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Lafionatis, S. Recent advances on canine mammary. Revue Méd. Vét. 2016, 167, 192–200. [Google Scholar]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Martínez-Pérez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef]
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease (Review). Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Guimarães, M.J.; Pires, I.; Prada, J.; Silva-Carvalho, R.; Lopes, C.; Queiroga, F.L. EGFR and microvessel density in canine malignant mammary tumours. Res. Vet. Sci. 2013, 95, 1094–1099. [Google Scholar] [CrossRef]
- Brunetti, B.; Bacci, B.; Angeli, C.; Benazzi, C.; Muscatello, L.V. p53, ER, and Ki67 Expression in Canine Mammary Carcinomas and Correlation With Pathological Variables and Prognosis. Vet. Pathol. 2020, 58, 325–331. [Google Scholar] [CrossRef]
- Horibata, S.; Vo, T.V.; Subramanian, V.; Thompson, P.R.; Coonrod, S.A. Utilization of the soft agar colony formation assay to identify inhibitors of tumorigenicity in breast cancer cells. J. Vis. Exp. 2015, 99, e52727. [Google Scholar] [CrossRef]
- Soule, H.D.; Vazquez, J.; Long, A.; Albert, S.; Brennan, M. A human cell line from a pleural effusion derived from a breast carcinoma. JNCI J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef]
- Osborne, C.K.; Von Hoff, D.D.; Mullins, K. Endocrine therapy testing of human breast cancers in the soft agar clonogenic assay. Breast Cancer Res. Treat. 1985, 6, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.-I.; Yomogida, S.; Tomizawa, A.; Yamazaki, H.; Ukai, K.; Mangindaan, R.E.; Namikoshi, M.; Ishikawa, M. Combined effect of papuamine and doxorubicin in human breast cancer MCF-7 cells. Oncol. Lett. 2014, 8, 547–550. [Google Scholar] [CrossRef][Green Version]
- Parra, E.; Ferreira, J. Knockdown of the c-Jun-N-terminal kinase expression by siRNA inhibits MCF-7 breast carcinoma cell line growth. Oncol. Rep. 2010, 24, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Matsui, C.; Takatani-Nakase, T.; Maeda, S.; Takahashi, K. High-Glucose Conditions Promote Anchorage-Independent Colony Growth in Human Breast Cancer MCF-7 Cells. Biol. Pharm. Bull. 2018, 41, 1379–1383. [Google Scholar] [CrossRef]
- Taddei, M.; Giannoni, E.; Fiaschi, T.; Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2012, 226, 380–393. [Google Scholar] [CrossRef]
- Adeshakin, F.O.; Adeshakin, A.O.; Afolabi, L.O.; Yan, D.; Zhang, G.; Wan, X. Mechanisms for Modulating Anoikis Resistance in Cancer and the Relevance of Metabolic Reprogramming. Front. Oncol. 2021, 11, 626577. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.H.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Kim, N.; Lim, H.; Im, K.; Kim, J.; Sur, J.-H. Identification of Triple-negative and Basal-like Canine Mammary Carcinomas Using Four Basal Markers. J. Comp. Pathol. 2013, 148, 298–306. [Google Scholar] [CrossRef]
- Won, K.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives. Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Benazzi, C.; Castellani, G.; Sarli, G. Molecular-based tumour subtypes of canine mammary carcinomas assessed by immunohistochemistry. BMC Vet. Res. 2010, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Uxa, S.; Castillo-Binder, P.; Kohler, R.; Stangner, K.; Müller, G.A.; Engeland, K. Ki-67 gene expression. Cell Death Differ. 2021, 28, 3357–3370. [Google Scholar] [CrossRef]
- Peña, L.L.; Nieto, A.I.; Pérez-Alenza, D.; Cuesta, P.; Castaño, M. Immunohistochemical detection of Ki-67 and PCNA in canine mammary tumors: Relationship to clinical and pathologic variables. J. Vet. Diagn. Investig. 1998, 10, 237–246. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Guarino, M.; Rubino, B.; Ballabio, G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007, 39, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Ryu, Y.; An, J.; Lee, Y.; Kim, A. Epithelial–mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology 2012, 60, E87–E95. [Google Scholar] [CrossRef]
- Yamashita, N.; Tokunaga, E.; Kitao, H.; Hisamatsu, Y.; Taketani, K.; Akiyoshi, S.; Okada, S.; Aishima, S.; Morita, M.; Maehara, Y. Vimentin as a poor prognostic factor for triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zheng, H.; Liu, X.; Xie, G. The Analysis of E-Cadherin, N-Cadherin, Vimentin, HER-2, CEA, CA15-3 and SF Expression in the Diagnosis of Canine Mammary Tumors. Animals 2022, 12, 3050. [Google Scholar] [CrossRef]
- Amaral, C.B.; Leite, J.D.; Fonseca, A.B.; Ferreira, A.M. Vimentin, Osteocalcin and Osteonectin Expression in Canine Primary Bone Tumors: Diagnostic and Prognostic Implications. Mol. Biol. Rep. 2018, 45, 1289–1296. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Jassem, J.; Pieńkowski, T.; Płuzańska, A.; Jelic, S.; Gorbunova, V.; Mrsic-Krmpotic, Z.; Berzins, J.; Nagykalnai, T.; Wigler, N.; Renard, J.; et al. Doxorubicin and Paclitaxel Versus Fluorouracil, Doxorubicin, and Cyclophosphamide as First-Line Therapy for Women With Metastatic Breast Cancer: Final Results of a Randomized Phase III Multicenter Trial. J. Clin. Oncol. 2001, 19, 1707–1715. [Google Scholar] [CrossRef]
- National Health Commission of the PRC. National Health Commission of the People’s Republic of China National guidelines for diagnosis and treatment of breast cancer 2022 in China (English Version). Chin. J. Cancer Res. 2022, 34, 151–175. [Google Scholar] [CrossRef]
- Gherman, M.L.; Zanoaga, O.; Budisan, L.; Raduly, L.; Berindan-Neagoe, I. Doxorubicin as a Potential Treatment Option in Canine Mammary Tumors. Vet. Sci. 2023, 10, 654. [Google Scholar] [CrossRef]
- Huang, J.-F.; Wen, C.-J.; Zhao, G.-Z.; Dai, Y.; Li, Y.; Wu, L.-X.; Zhou, H.-H. Overexpression of ABCB4 contributes to acquired doxorubicin resistance in breast cancer cells in vitro. Cancer Chemother. Pharmacol. 2018, 82, 199–210. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef]
- Ganguly, A.; Yang, H.; Cabral, F. Paclitaxel-dependent cell lines reveal a novel drug activity. Mol. Cancer Ther. 2010, 9, 2914–2923. [Google Scholar] [CrossRef]
- Raffo-Romero, A.; Aboulouard, S.; Bouchaert, E.; Rybicka, A.; Tierny, D.; Hajjaji, N.; Fournier, I.; Salzet, M.; Duhamel, M. Establishment and characterization of canine mammary tumoroids for translational research. BMC Biol. 2023, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-K.; Oh, Y.-I.; Lim, G.-H.; Jung, Y.-C.; Park, S.-H.; An, J.-H.; Park, S.-M.; Seo, K.-W.; Chu, S.-N.; Li, Q.; et al. Anti-cancer effects of DHP107 on canine mammary gland cancer examined through in-vitro and in-vivo mouse xenograft models. BMC Vet. Res. 2024, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Dan, V.M.; Raveendran, R.S.; Baby, S. Resistance to Intervention: Paclitaxel in Breast Cancer. Mini-Rev. Med. Chem. 2021, 21, 1237–1268. [Google Scholar] [CrossRef]
- Luo, B.; Wu, X.-H.; Feng, Y.-J.; Zheng, H.-M.; Zhang, Q.; Liang, X.-J.; Huang, D.-F.; Xu, J. Nuclear Her2 contributes to paclitaxel resistance in breast cancer cells. Anti-Cancer Drugs 2021, 32, 709–716. [Google Scholar] [CrossRef]
- Urbaniak, A.; Jousheghany, F.; Piña-Oviedo, S.; Yuan, Y.; Majcher-Uchańska, U.; Klejborowska, G.; Moorjani, A.; Monzavi-Karbassi, B.; Huczyński, A.; Chambers, T.C. Carbamate derivatives of colchicine show potent activity towards primary acute lymphoblastic leukemia and primary breast cancer cells—In vitro and ex vivo study. J. Biochem. Mol. Toxicol. 2020, 34, e22487. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, X.; Chang, H. Proliferation inhibition and apoptosis of breast cancer MCF-7 cells under the influence of colchicine. J. BUON 2016, 21, 570–575. [Google Scholar]
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Zheng, M.; Mei, Z.; Junaid, M.; Tania, M.; Fu, J.; Chen, H.-C.; Khan, A. Synergistic Role of Thymoquinone on Anticancer Activity of 5-Fluorouracil in Triple Negative Breast Cancer Cells. Anti-Cancer Agents Med. Chem. 2022, 22, 1111–1118. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Habara, K.; Ajiki, T.; Kamigaki, T.; Nakamura, T.; Kuroda, Y. High expression of thymidylate synthase leads to resistance to 5-fluorouracil in biliary tract carcinoma in vitro. Jpn. J. Cancer Res. 2001, 92, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, G.F.; Wlodarczyk, S.R.; Monteiro, G. Carboplatin: Molecular mechanisms of action associated with chemoresistance. Braz. J. Pharm. Sci. 2014, 50, 693–701. [Google Scholar] [CrossRef]
- Stewart, D.J. Mechanisms of resistance to cisplatin and carboplatin. Crit. Rev. Oncol. 2007, 63, 12–31. [Google Scholar] [CrossRef] [PubMed]
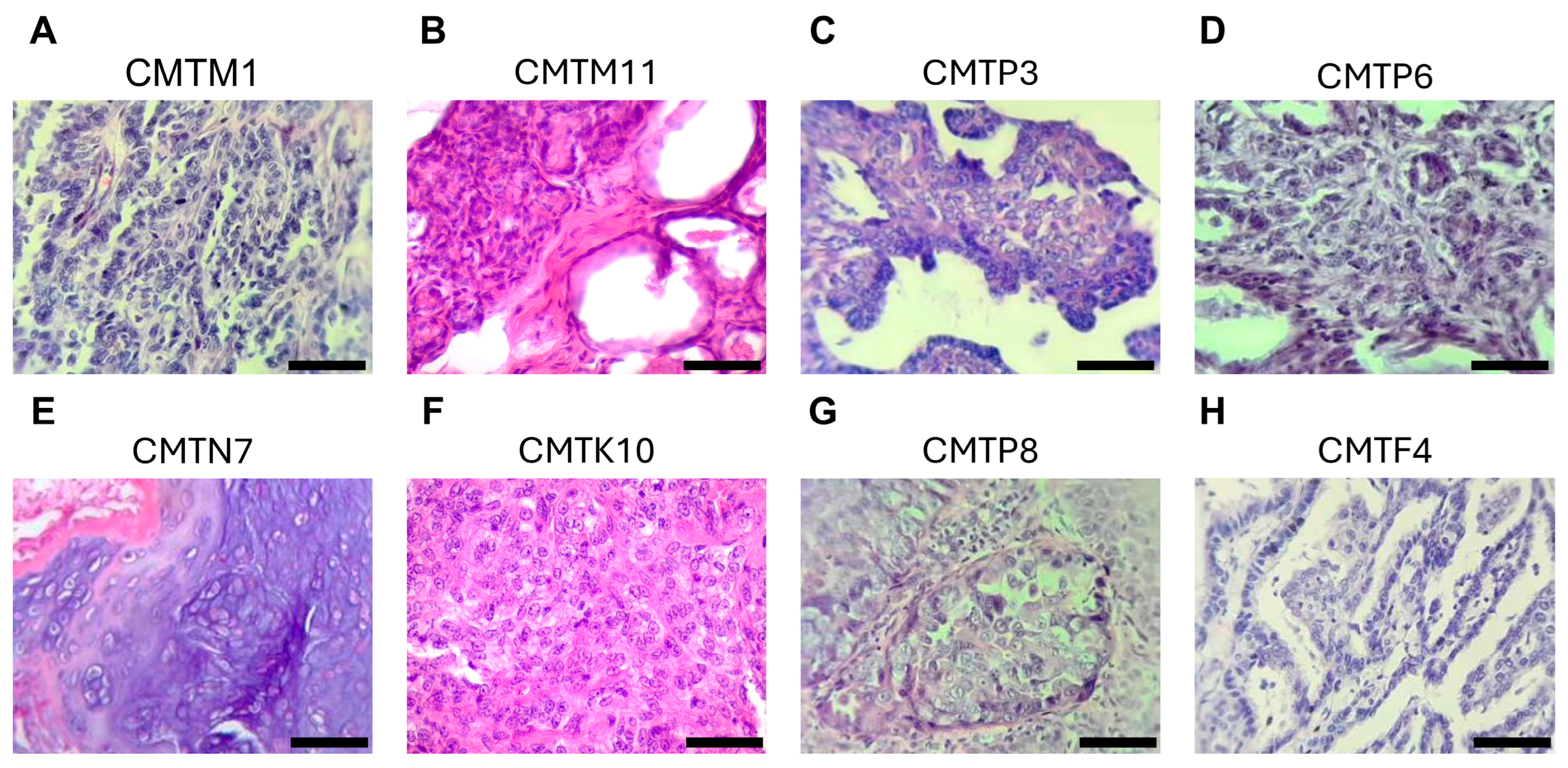
| Number of Cell Line | Name of the Cell Line | Age (Years) | Breed | Reproductive Status | Biological Sample | Histopathology | Grade |
|---|---|---|---|---|---|---|---|
| 1 | CMTM1 | 15 | Chihuahueño | Spayed | Mammary tumor | Intraductal papillary adenoma | Benign tumor |
| 2 | CMTM11 | 12 | Chihuahueño | Unspayed | Mammary tumor | Tubular adenoma with cellular dysplasia | Benign tumor |
| 3 | CMTP3 | 13 | Mixed | Unspayed | Mammary tumor | Benign mixed tumor with intraductal papillary adenoma | Benign tumor |
| 4 | CMTL9 | 15 | Pit bull | Spayed | Mammary tumor | ND | ND |
| 5 | CMTP6 | 11 | Poodle | Spayed | Mammary tumor | Tubular carcinoma | I |
| 6 | CMTN7 | 15 | Chihuahueño | Unspayed | Mammary tumor | Mixed-type adenocarcinoma | I |
| 7 | CMTK10 | 16 | Poodle | Unspayed | Mammary tumor | Solid adenocarcinoma | II |
| 8 | CMTG5 | 13 | Maltes | Spayed | Pleural effusion | Infiltrating tubulopapillary carcinoma | II |
| 9 | CMTP8 | 14 | Mixed | Spayed | Pleural effusion | Intraductal carcinoma | I |
| 10 | CMTF4 | 12 | Golden retriever | Spayed | Pleural effusion/lung metastatic nodule | Infiltrating intraductal papillary carcinoma | I |
| Cell Marker | Epithelial Marker CK5/6 | Mesenchymal Marker Vimentin | ||||
|---|---|---|---|---|---|---|
| Cell Line | ER | PR | HER2 | Ki67 | ||
| CMTF4 | − | − | − | + | − | + |
| CMTN7 | − | − | − | − | − | + |
| CMTL9 | − | − | − | − | − | + |
| CMTP8 | + | + | − | − | + | + |
| CMTG5 | − | − | − | − | + | + |
| CMTK10 | ND | − | − | − | + | + |
| Drug | Cell Lines | ||||
|---|---|---|---|---|---|
| CMTF4 | CMTP8 | CMTK10 | CMTN7 | CMTL9 | |
| IC50 (µM) | |||||
| DOX | 4.37 ± 0.40 | <0.63 | >50 | >50 | >50 |
| Paclitaxel | 0.04 ± 0.0003 | ≥10 | >50 | >100 | >100 |
| Colchicine | 0.19 ± 0.01 | >50 | >100 | >100 | >100 |
| 5-FU | >50 | >200 | >50 | >50 | >50 |
| Carboplatin | >100 | >200 | >200 | >200 | >200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazquez, E.; Dominguez, L.; Silverio, B.; Torres, G.; Garibay-Escobar, A.; Queiroga, F.L.; Velazquez, C. Establishment and Partial Characterization of Canine Mammary Tumor Cell Lines. Animals 2025, 15, 1991. https://doi.org/10.3390/ani15131991
Vazquez E, Dominguez L, Silverio B, Torres G, Garibay-Escobar A, Queiroga FL, Velazquez C. Establishment and Partial Characterization of Canine Mammary Tumor Cell Lines. Animals. 2025; 15(13):1991. https://doi.org/10.3390/ani15131991
Chicago/Turabian StyleVazquez, Eliza, Luis Dominguez, Brian Silverio, Geobanni Torres, Adriana Garibay-Escobar, Felisbina Luisa Queiroga, and Carlos Velazquez. 2025. "Establishment and Partial Characterization of Canine Mammary Tumor Cell Lines" Animals 15, no. 13: 1991. https://doi.org/10.3390/ani15131991
APA StyleVazquez, E., Dominguez, L., Silverio, B., Torres, G., Garibay-Escobar, A., Queiroga, F. L., & Velazquez, C. (2025). Establishment and Partial Characterization of Canine Mammary Tumor Cell Lines. Animals, 15(13), 1991. https://doi.org/10.3390/ani15131991








